Abstract
Platinum (Pt), palladium (Pd/PdO), and rhodium (Rh) decorated carbon nanotube (CNT) based catalysts were prepared, characterized and their activity was tested in ammonia (NH3) and ethanol (EtOH) assisted selective catalytic reduction (SCR) of nitric oxide (NO) at low temperatures (30–300 °C). In addition, the influence of sulphur on NH3-SCR activity was investigated. The catalysts were characterized by transmission and scanning electron microscopy, energy-dispersive X-ray analysis, and X-ray diffraction techniques. In addition, IR measurements were done to determine the adsorbed species on the catalyst surface. The maximum NO conversions over the catalysts were as high as 85 % for Pt/CNT (at 192 °C), 54 % for Pd/PdO/CNT (at 291 °C), and 48 % for Rh/CNT (at 292 °C) with NH3 as the reducing agent. The SO2 deactivation was the most severe in the case of Pd/PdO/CNTs. In the EtOH assisted SCR the maximum NO conversions were 100 % for Pt/CNT, 98 % for Pd/PdO/CNT and 85 % for Rh/CNT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Since nitrogen oxides (NOx) are considered to cause global environmental problems, there is a demand for developing novel and more efficient catalytic materials for the removal of NOx components from flue and exhaust gases. NOx emissions originating e.g. from power plants, engines and vehicles are recognized as a global environmental problem [1, 2]. As NOx emissions are reported to cause e.g. photochemical smog, acidic rain, ozone depletion, and to affect the global warming, there is an urgent need for developing novel, and more efficient and environmental friendly catalysts that can be used e.g. to convert NOx to non-harmful N2 [3–5]. Selective catalytic reduction of NOx by ammonia (NH3-SCR) is considered as a commercial, well proven, and mature technique for NOx abatement in stationary sources and usable also in vehicles [3, 4] as these types of systems/catalysts have been used commercially in NOx reduction since the 1970s. Besides ammonia, also hydrocarbons have been shown to be able to reduce nitrogen oxides in lean-burn conditions [6]. Since the use of biobased fuels, like ethanol, is increasing due to the legislation, ethanol is one possible reducing agent also in the SCR process of passenger cars [7].
Typical catalyst materials used in industrial stationary SCR applications are vanadium pentoxide (V2O5) combined with WO3 or MoO3 oxides on titanium dioxide (TiO2) support [8, 9]. Vanadium based catalysts are the most often used catalyst materials in SCR despite their known toxicity and a possible carcinogenic impact on human beings [10, 11]. In addition, vanadium pentoxide is active mainly at high temperatures and at those it is also possible that V2O5 may evaporate. Besides TiO2 also e.g. alumina (Al2O3), silica and various forms of zeolites are recognized as suitable support materials for catalysts used in NH3-SCR of NO [9]. Nowadays, also carbon based materials such as activated carbon (AC) and carbon nanotubes (CNTs) are considered as interesting catalyst support materials for ammonia assisted SCR of NO at low temperatures below 300 °C [8, 12–14]. In addition, the development of catalysts operating at low temperatures is important for at least two reasons. In stationary sources, the SCR unit can be located far after the electrostatic precipitator where temperature is low and thus necessities to have new catalyst materials. Noble metal based catalysts are widely utilized in SCR reaction at low temperatures. The “tail end” applications operating at low temperatures enable the avoidance of reheating and, in addition, are improving the energy efficiency of the system. On the other hand, catalysis at lower temperatures always implies a better durability of catalytic converters due to limited surface diffusion, agglomeration and surface mediated Oswald ripening of the catalyst particles on the support. Therefore, targeting to low temperature applications is nowadays an important research topic worldwide. [8, 12, 15, 16].
The attraction of using carbonaceous materials as catalyst supports in heterogeneous catalysis is due to the possibility of customizing the surface properties (both chemical and physical) in defined limits. In addition, carbonaceous materials are resistant to acidic or basic media and their environmental impacts are relatively low due to the recovery of active metal particles by burning away the support material. [17, 18] Several advantageous properties of CNTs such as mechanical strength, corrosion resistance, flexibility, low density, tunable porosity, good electrical- and thermal conductivity and large specific surface area [8, 12, 18–22] are making this family of carbons superior compared to the well-established AC support materials. CNTs decorated with metal nanoparticles thus are in the centre of interest in the field and were demonstrated as excellent catalysts e.g. in hydrogenation/dehydrogenation of alkenes/alkanes, hydrogen and methanol oxidation in fuel cells and in Fischer–Tropsch synthesis of hydrocarbons [18, 22–25]. Good catalytic activity of CNT based V2O5 catalysts in NH3-SCR in a low temperature range (<300 °C) has been reported e.g. by Bai et al. [8] and Huang et al. [12].
Sulphur dioxide (SO2) has been found to have a deactivation effect on the catalysts’ performance in the SCR applications [26, 27]. Bai et al. [8, 28] have reported that over a V2O5/CNT catalyst at low temperature NH3-SCR, the NO conversion remained high also in the presence of SO2. They have explained that the reason why the catalyst did not deactivate was due to the formation of sulphate species on the catalyst surface, and the ammonium ions exist on the catalysts surface mainly in the NH4 + adsorption state. According to Huang et al. [12], besides V2O5 also other metal oxides such as those of Fe, Mn, Cu supported on carbon materials (AC, AFC) have shown very high activity and resistance to the remaining SO2 (sulphate species on the carbon surface increase the surface acidity and hence the activity of the catalyst) [29–36]. However, the catalytic activity of the MnOx/MWNT catalyst decreases in NH3-SCR reaction when 100 ppm of SO2 was in the fluid stream [37]. In addition, the temperature and gas velocity have been found to have a negative effect on the V2O5–CeOX/TiO2–CNT composite catalyst when SO2 is present [38]. According to our knowledge there exist no studies about the effect of SO2 on noble metal/CNTs in the NH3-SCR reactions. In other applications, especially Pt has shown to have a high resistance against SO2 and it is well proven and used active metal in SCR applications [27, 29].
The objective of the work was to gain new knowledge on the feasibility of the noble metal loaded CNT catalysts in the abatement of nitric oxide by the SCR method using NH3 or EtOH as reducing agents. In addition, this study aims to find out the effect of SO2 poisoning on CNT based PGM catalysts at low temperatures. CNTs were chosen as the catalyst support since it may be an excellent material due to structural and surface properties [8, 12, 18–22]. The multi-walled carbon nanotube (MWCNT) supported noble metal catalysts prepared by a wet impregnation method were characterized by transmission and scanning electron microscopy (TEM and SEM), energy-dispersive X-ray analysis (EDX) and X-ray diffraction (XRD). In addition, transmission IR measurements were done to find out the surface compounds and sulphur adsorption during the manufacturing and testing. The activity of the prepared noble metal based CNT catalysts in the NH3 and EtOH assisted model SCR reactions was investigated at low temperatures (<300 °C) by using FTIR spectrometry to analyse the outlet compositions of the reaction gases.
2 Experimental
2.1 Catalyst Preparation and Characterization
MWCNTs provided by Sigma-Aldrich (5 g, outer diameter 10–15 nm, inner diameter 2–6 nm, length 0.1–10 μm) were carboxyl functionalized (marked as CNT-COOH) first to help the better anchoring of metal precursors on the surface of CNTs [12]. In a typical process, 5 g of a powder form CNT was sonicated in 250 mL of 65 % nitric acid (HNO3) for 1 h and refluxed for 8 h. The as-obtained slurry was split to 4 parts and centrifuged (at 3000 rpm for 10 min). After the process, the acidic liquid was removed and the solid product (i.e. carboxylated CNTs) was dispersed again in water (~80 mL). In order to help precipitation of the carboxyl functionalized CNTs, ~0.5 mL of HCl was added to the dispersion before the next centrifugation. The washing in water and centrifuging cycle was repeated 7 times (HCl was added only to the first cycle). The dried product (overnight at 100 °C) was then suspended in acetone, mortared and dried in an oven at 70–80 °C for around 6 h.
Platinum (Pt), palladium (Pd/PdO) and rhodium (Rh) were deposited on the support by a wet impregnation method. The desired noble metal content for each sample was 4 wt%. 1.5 g of carboxylated MWCNT was used for each sample (Pt/CNT, Pd/PdO/CNT and Rh/CNT) preparation. The precursors used in the catalyst preparation were Pt(II) acetylacetonate (Pt(acac)2, 99.99 % trace metal basis), Pd/PdO(II) acetate (Pd(OAc)2, ≥99.9 % trace metals basis), and Rh(III) acetylacetonate (Rh(acac)3, 97 %), all provided by Sigma-Aldrich. First, the precursor was dissolved in 300 mL of toluene and the carboxylated MWCNT was added to the solution. Then the mixtures were sonicated for 4 h and stirred overnight, after which the solvent was evaporated at 80 °C under continuous stirring with a gentle air flow. The sample was dried overnight. Finally, the samples were treated under reducing (H2/Ar for Pt) and oxidizing conditions (air for Pd/PdO and Rh) with specific temperature procedures.
Catalyst morphology and particle size distribution were assessed by energy-filtered transmission electron microscopy (EFTEM, LEO 912 Omega) analysis of several micrographs taken from the samples counting at least 100 particles in each case. XRD patterns showing the crystal phase of catalyst particles were obtained by a Philips PW1380 diffractometer (Cu Kα radiation at a scanning rate of 0.125°/min). The metal content of the catalyst materials was determined by energy-dispersive X-ray analysis (FESEM–EDX, Zeiss ULTRA plus) averaged from at least 5 different locations in each specimen. In addition, FESEM–EDX was used to determine the sulphur content of the SO2 poisoned catalysts (average of 3 different locations). The FTIR spectra of the catalyst materials were determined with Bruker Vertex 80v using KBr pellets at room temperature.
2.2 Activity Measurements
Catalytic activity of each prepared catalyst (Pt/CNT, Pd/PdO/CNT, Rh/CNT) as well as the parent CNT-COOH support was measured in the NH3-SCR and EtOH-SCR model reactions by light-off experiments. A horizontal (i.d. 8 mm, length 50 mm) continuous flow fixed-bed tubular reactor at atmospheric pressure was used in the experiments. The catalyst powders (80 mg each) were packed into the quartz tube reactor by alternating layers of quartz wool and the catalyst. The inlet gas feed was set with Brooks mass flow controllers to reach a gas composition of 1100 ppm NO, 1100 ppm NH3, and 10 vol% O2 in the NH3-SCR model reaction and 1000 ppm NO, 2000 ppm C2H5OH, 10 vol% H2O, and 10 vol% O2 in the EtOH-SCR model reaction, respectively. Nitrogen was used as inert carrier gas, balancing the total flow rate to be 1000 ml/min corresponding to the gas hourly space velocity (GHSV) of around 24 000 h−1. Temperature was elevated from room temperature to 300 °C with a rate of 5 °C/min. In the EtOH-SCR, a peristaltic pump was used to feed the ethanol–water solution into the reactor. The feed was started when the temperature in the reactor reached ca. 110 °C. The concentrations of compounds present in the outlet gas were measured on-line by a FTIR gas analyzer (Gasmet™). The concentration of O2 was determined by using a paramagnetic analyzer (ABB Advance Optima).
In addition, the effect of water was tested for the catalyst having the highest NO conversion in dry NH3-SCR activity measurements. The same reactor set up and procedure was utilized in the experiments, and the feed gas contained 1100 ppm NO, 1100 ppm NH3, 10 vol% O2, 10 vol% H2O, and N2 as balance gas. H2O was added to the feed gas at ca. 110 °C.
The activity and selectivity of the studied catalysts was evaluated in terms of NO conversion (XNO) according to Eqs. (1) and (2):
2.3 Catalyst Deactivation Study by Sulphur Treatment
The influence of sulphur on the activity of the catalyst materials in low temperature NH3-SCR was studied. The Pt/CNT, Pd/PdO/CNT and Rh/CNT as well as parent CNT-COOH catalysts were poisoned with gaseous SO2. The catalyst poisoning was done in a horizontal laboratory scale aging equipment consisting of a quartz glass reactor surrounded by a tubular furnace. The catalyst was packed in the reactor loosely between the quartz wool layers. The temperature of the reactor was elevated to 200 °C under N2 flow with a heating rate of 10 °C/min. In the procedure 300 ppm of SO2 balanced with N2 was fed into the reactor for 1 h with a total flow rate of 500 ml/min. After the deactivation, N2 was introduced to the reactor while cooling down the system. The sulphur content of the catalyst materials was analyzed by FESEM–EDX and calculated as an average of three analyses taken from different areas of each sample. After deactivation, activity of the catalyst was studied in NH3-SCR model reaction.
3 Results and Discussion
3.1 Catalyst Characterization
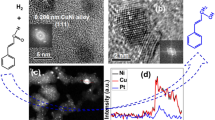
For the particle size assessment at least 100 particles were analysed in each case. Pt, Pd/PdO, or Rh metal particles were observed to have a homogeneous distribution and a good dispersion over the CNT-COOH support. The particle size for each sample follows the log-normal distribution with an average diameter of 3.1 ± 1.6, 2.9 ± 1.9 and 3.2 ± 2.9 nm for the Pt/CNT, Pd/PdO/CNT and Rh/CNT catalysts, respectively (Fig. 1). It is noteworthy to mention that the small metal particles obtained showed a rather uniform distribution on the surface. Despite the desired equal metal loadings (i.e. 4 wt% of metal) the observed metal content was measured to be different between the samples having values of 6.2 ± 1.2 wt% for Pt/CNT, 5.3 ± 1.5 wt% for Pd/PdO/CNT, and only 2.8 ± 0.3 wt% for Rh/CNT. In the latter case, the significantly reduced amount of Rh may probably be due to evaporation/sublimation of the precursor, while the enriched Pt and Pd content of the other samples could be caused by the partial catalytic oxidation of the support, when the samples were calcined during the catalyst preparation [39–41].
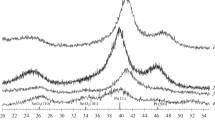
According to the XRD results, after the decomposition of the Pd-precursor, the formed catalyst nanoparticles were mainly in the form of PdO, however the weak reflection at 2θ–40° indicates the presence of some metallic Pd/PdO as well in the catalyst. In the other two prepared catalysts, only metallic Pt and Rh were found to be present. In Fig. 2, the most intensive reflections assigned to the crystal planes of the corresponding phases were labelled in the diffractograms (along with the C(100) reflection of the support at 2θ–43°). The particle sizes determined from the broadening of reflections were calculated to be 10.1 ± 0.2 nm for PdO(101), 11.9 ± 0.1 nm for Pt(111), and 9.5 ± 0.7 nm for Rh(111) by the Scherrer’s equation [42]. The reflection from Pd(111) was very broad and of low intensity, which indicates a low amount of Pd and/or very small, poorly crystallized Pd particles.
It is commonly accepted that TEM analysis gives a true particle size, and the results match well with those obtained from the broadening of XRD patterns, when the particle size distribution is narrow. [43] TEM however can slightly under or overestimate the crystal size by not resolving agglomerated crystals or polycrystalline particles, particularly in multi-phase specimens having nonuniform image backgrounds. In complex samples, such as powders of supported catalyst materials, evaluation of the particle size is often difficult with computer-assisted image recognition thus the assessment done by the operator/researcher can add also further errors to the results. [44] Our XRD results show about threefold crystal size as compared to that assessed by TEM, which is often observed when analyzing supported catalyst nanoparticles. [22, 40, 45, 46] Such a large difference is explained by missing some very large catalyst nanoparticles when analyzing with TEM. [45] Since the Scherrer equation gives an estimate for the volume averaged crystal size, a few very large crystals (that are unnoticed by TEM) can shift the results considerably to larger XRD crystal diameter values. (Note: Even if all the large particles would be noticed and counted for in the TEM images, the diameter average would be significantly smaller than the volumetric average calculated from XRD.)
According to the EDX results in Table 1, sulphur was found to adsorb on all catalysts in quite high amounts (0.13–0.65 wt%) except on –COOH functionalized CNTs (only 0.01 wt%). Bai et al. [28] have reported SO2 adsorbed as sulphates (SO4 2−) on the surface of V2O5/CNT and most probably also in our case sulphates on noble metals have been formed.
The transmission IR spectra of the Pt/CNT-catalysts without and with SO2 poisoning after the activity tests were measured (Fig. 3). In the measured spectra, the sharp peak detected at 1600-1700 cm−1 might be corresponding to carbonyl and carboxyl groups [47, 48]. The broad bands at the range of 3200–3600 cm−1 are assigned to O–H vibration modes [48, 49]. The broad band at around 1100 cm−1 can be assigned as C=O stretching and O–H bending modes of carboxylic acids [50]. After the sulphur poisoning new peaks found in the range of 65–850 cm−1 can be assigned e.g. as sulphur on carbon, C=S [51] and in the range 950–1170 cm−1 several peaks are SO4 2− vibration modes [52–54].
3.2 Catalytic Activity
The activities of each prepared catalyst and also the parent HNO3 pre-treated MWCNT support (CNT-COOH) in the model reaction of NH3-SCR differed much as presented in Fig. 4. Over the parent CNT-COOH- catalyst support, NO conversion remained low in the entire temperature range with a maximum of only ~9 % at 125 °C. In the study by Huang et al. [12] the NO conversion in NH3-SCR over the parent CNTs was also observed at low temperatures (<200 °C). One reason for the NO conversion already at low temperatures might be the adsorption of NO on the CNTs surface [12, 55]. This phenomenon has also been reported by Ahmed et al. [56] for AC and in addition, they found that the adsorption of NO was enhanced in the presence of oxygen. Over the sulphur-treated CNT-COOH the NO conversion was detected to be 5 % at maximum in NH3-SCR.
Pt, Pd and Rh additions increased remarkably the activity of the catalysts in NO conversion compared to the parent CNT-COOH (Fig. 4). In the NH3-SCR the maximum NO conversions were 85, 54, and 48 % over the Pt/CNT, Pd/PdO/CNT and Rh/CNT catalysts, respectively (Table 2; Fig. 4). The high NO conversion (>80 %) was achieved only in a quite narrow temperature window (~185 to ~210 °C) over the Pt/CNT. The sulphur-treatment had a significant decreasing effect on the NO conversions in the cases of Pd and Rh decorated CNTs (19 and 30 %), however, with the Pt/CNT sulphur was found to have a minor promoting effect on the maximum NO conversion (87 %). It is worth to be noted that the amount of sulphur in the Pt/CNTs was much lower than in the PdO/Pd and Rh/CNTs which thus might have an effect on the NO conversions.
It is well known that Pd and Rh are very sensitive elements towards sulphur and S deactivates the active sites effectively forming sulphates and sulphites on the surface. In addition, Pt has been reported to promote less the SO4 2−/SO3 2− compounds formation than Pd/PdO. [e.g. 26, 27] Bai et al. [28] have also found that sulphur has a promoting effect on NH3-SCR over the V2O5/CNT when the V content was low (<1 wt% V2O5). In the case of the EtOH-SCR, the NO conversions of 100, 98, and 85 % for Pt/CNT, Pd/PdO/CNT and Rh/CNT, respectively, were achieved. (Table 2).
Besides the low NO conversion over the parent CNT-COOH the NH3 conversions remained at a low level, the maximum NH3 conversions were around 15 % for both fresh and S-treated catalysts (Table 2). At the low temperatures (<100 °C) a small NH3 conversion was detected over all the tested catalysts (Figure not shown). Huang et al. [57] have found that nitric acid treated AC adsorbs ammonia, which is plausible considering the acidic surface. In addition, Bai et al. [8] have reported that even the surface of pristine CNTs has the ability to adsorb ammonia. This phenomenon may thus explain the non-zero conversion of NH3 at the beginning of the experiment over CNT-COOH. Over the fresh Pt/CNT, Pd/PdO/CNT, and Rh/CNT catalysts the ammonia conversion is 100 %. The SO2 poisoning was observed to have only a slight or none decreasing effect on the NH3 conversions (Table 2). The ethanol conversion in the EtOH assisted SCR reached values higher than 95 % over all the noble metal decorated catalysts.
Based on the results (Table 2) in the NH3-SCR experiments the light-off temperature (T50, i.e. the temperatures that correspond to 50 % conversion) over the Pt/CNT catalyst for NO and the reductant (NH3 or EtOH) were observed to be close to each other (around 170 ± 8 °C). On the sulphur-treated Pt/CNT the T50 of NO was observed to be lower (147 °C) than over the fresh one (167 °C). With the Pd/PdO/CNT the S-treatment decreased the NO maximum conversion significantly, and T50 was not achieved while with the fresh catalyst it was 285 °C. Over Rh/CNT and CNT-COOH the T50 of NO was not achieved at all. The T50 values for NH3 were achieved over all the fresh and S-treated Me/CNT catalysts but not over CNT-COOH. (Table 2) In the EtOH-SCR over the Pd/PdO/CNT the T50 of NO and EtOH decreased by ~130 and 104 °C, respectively when compared to fresh one in NH3-SCR. In the case of Rh/CNT the T50 of NO was 249 °C.
It can be concluded that ethanol is a more efficient reductant than ammonia especially at low temperatures in the case of Pd/PdO/CNT. Pt/CNT was found to be active with NH3 and EtOH and in addition, sulphur did not affect the maximum conversions of NO or NH3. The Rh/CNT catalyst was observed to be the poorest and the lowest activity for NO abatement was achieved.
In the NH3-SCR over all the noble metal decorated CNT catalysts N2O was formed. The formation was higher with Pt/CNT (up to 500 ppm), and around 200 ppm over Pd/PdO/CNT and Rh/CNT. In addition, the formation started at much lower temperatures with Pt than with Pd and Rh decorated CNTs. Intensive NO2 formation was detected over Pt/CNT (~630 ppm), however, no any or very minor amounts (<50 ppm) of NO2 were observed over Pd/PdO/CNT and Rh/CNT (Figures not shown). Accordingly, in the case of Pt/CNT, considerable activity towards the NO oxidation reaction takes place, however, there is no clear evidence for the same over Pd- and Rh/CNTs. CO2 formation was ~350, <50, and ~200 ppm over Pt/CNT, Pd/PdO/CNT and Rh/CNT, respectively. With each noble metal decorated CNTs, acetaldehyde was found to be the most remarkable side-product (500–1100 ppm) in the EtOH-SCR reactions, while formaldehyde and N2O formed only in much smaller quantities (<100 ppm and <25 ppm, respectively). Over Pt/CNT a high NO2 formation with a maximum at around 250 °C was detected, but no NO2 was formed over the other catalysts. CO2 formation started at around 170 °C (Pt/CNT), 160 °C (Pd/PdO/CNT), and 210 °C (Rh/CNT) and the amount increased with the temperature. The formation of CO and CO2 might be explained by the oxidation of carbonaceous support material in the presence of oxygen at temperatures higher than 230 °C [17, 39–41]. The sulphur treatment does not have an effect on the formed compound distribution or formation rates. Over Pt/CNT the most significant by-product formed was N2O in temperature range of 150–250 °C and NO2 above 250 °C. Over Pd/PdO/CNT formation of by-products was very low and only N2O formation was detected above 250 °C.
The selectivity of the catalysts tested with dry conditions are estimated with Eq. (2). Due to high formation amounts of by-products such as N2O and NO2, the selectivity is estimated to be around 30 % for Pt/CNT. In the case of Pd/PdO/CNT and Rh/CNT, the low selectivity (~30 %) might be due to high amount of unreacted NO present in the product gas.
Since Pt/CNT reached the highest NO conversion under dry conditions, it was selected to further investigations done in the presence of H2O in the feed gas. According to the results, the effect of water was insignificant on the NO and NH3 maximum conversions. The product gas distribution in the experiments containing H2O in the feed gas was following the same trend than the one in dry condition carried out over Pt/CNT; high amounts of N2O is formed at the same temperature where the maximum NO conversion is reached, and in the experiment strong NO2 formation is observed.
The CNT based noble metal decorated catalyst materials were tested for low temperature applications of NO reduction also by ammonia assisted SCR. By comparing the activity of the used Pt/CNT, Pd/PdO/CNT and Rh/CNT catalysts with other CNT-based catalysts studied at low temperatures, the maximum NO conversions reached in this study are in good agreement with the results reported by other research groups. Huang et al. [12] have observed conversions higher than 90 % for NO (CNTs with an outer diameter of 60–100 nm) in NH3-SCR at around 190 °C over V2O5/CNT (metal loading 2.35 wt%) using 800 ppm NO, 800 ppm NH3 and 5 % O2 and GHSV 35 000 h−1. However, in this study the outer diameter of the CNTs used was remarkably smaller (10–15 nm) and the NO conversion reached was 85 %. According to Huang et al. [12] the outer diameter of the CNT has influenced the activity as the wider the diameter the better the conversion. With the prepared vanadium-free Pt/CNT catalysts the NO conversion achieved was higher already over the smaller outer diameter CNTs at 190 °C. Our results with Pt/CNT prepared and tested in this study are also in agreement with those achieved by Bai et al. [8] with a 0.5 wt% loaded V2O5/CNT (XNO ≈ 80 %) at 250 °C in reaction conditions of 450 ppm NO, 500 ppm NH3 and 5 % O2. Also the activity of MnOx–CNTs [13] and Fe–Cu–Ox/CNTs–TiO2 [58] have been studied at low temperatures (<300 °C). Su et al. [13] have reported that the 3 wt% MnOx/CNTs reached the maximum NO conversion (>95 %) at 250 °C. They have found that the active metal location and length of the CNTs have a significant role in the SCR reaction. Ma et al. [58] have found that the addition of CNTs to the catalyst material lowered the temperature of maximum NO conversion significantly. In their study the maximum NO conversions over the CNT containing catalysts were achieved at 150–250 °C. This is in good agreement with the results gained in our study over Pt/CNTs in NH3-SCR. However, in this study, the NO conversions over Pt/CNT and Pd/PdO/CNT achieved ~100 % with ethanol as a reductant.
Zhu et al. [59] studied the activity of AC decorated with various active phases such as V2O5, V–W, V–Mo and V–Zr. At 250 °C, they obtained NO conversions in the range from 53 to 88 %, the V2O5/AC catalyst being the best one. The NO conversions achieved in this study are in good accordance with the results above.
In comparison to the non-carbonaceous catalyst materials, such as e.g. Ti0.9Fe0.1O2−δ [60] but also FexTiOy–TiCl4 and Fe2O3/TiO2 [61], the NO conversion achieved over the prepared Pt/CNT is higher at 200 °C than over the titanium based catalysts [60, 61] for both EtOH and NH3 assisted SCR. The NO conversions over the Ti0.9Fe0.1O2−δ, FexTiOy–TiCl4 and Fe2O3/TiO2 catalysts at 200 °C are ~50, ~30, and ~50 %, respectively, but in this study the NO conversion as high as 85 % is reached over Pt/CNT at the same temperature. Thus, the Pt decorated CNT is equally or even more active at low temperatures than the oxide based catalysts mentioned above.
4 Conclusions
Pt, Pd/PdO and Rh nanoparticles supported on carboxyl functionalized CNT catalysts were studied in the model reaction of SCR of nitric oxide by ammonia and ethanol at low temperatures (<300 °C). The active metal particles decorated on the CNT support were very small in size (average value of around 3 nm). The results of the activity measurements clearly show quite high NO conversions with NH3 (85, 54 and 49 %) and with EtOH (100, 98 and 85 %) over the studied Pt, Pd/PdO and Rh/CNT catalysts, respectively. Though the NO conversion in NH3-SCR was relatively high over Pt/CNT, the formation of undesired by-products such as N2O and NO2 was found to be high. Sulphur was not found to have effect on Pt/CNT, however the NO reduction activity decreased over Pd/PdO and Rh decorated CNTs. Measurements done by IR indicated that sulphur compounds most probably as the form of SO4 2− on the Pt/CNT catalyst surface are present. Although it is a well-known fact that Pd/PdO is the most sensitive material towards sulphur poisoning and Rh and Pt have some tolerance in this study no significant effects were detected caused by sulphur.
In the EtOH-SCR the conversions (NO and EtOH) were high already at low temperatures but the operation temperature should be below 250 °C. The advantage of Pd/PdO/CNT and Rh/CNT is that intensive NO2 formation was not observed in NH3 or EtOH assisted SCRs. The maximum NO conversions over the prepared Pt catalyst were similar to maximum NO in the case of Pd/PdO catalyst.
According to the results gained in this study, the CNT based noble metal decorated catalysts are promising materials for the EtOH assisted SCR of NO at low temperatures. The formation of N2O and NO2 was low and the NO removal efficiency high over Pt/CNT. Until now, there is no knowledge on the nature of the active sites of the prepared catalysts. Therefore, further experimental work is still needed to investigate the materials in more details.
References
Zhao Y, Wang S, Duan L, Lei Y, Cao P, Hao J (2008) Atmos Environ 42:8442–8452
European Parliament and Council (2007) Regulation (EC) 715/2007. PDF-document. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:171:0001:0016:EN:PDF. Accessed 12 June 2014
Bosch H, Janssen F (1988) Catal Today 2:369–532
Roy S, Hedge MS, Madras G (2009) Appl Energy 86:2283–2297
Sloss L (1991) NOx emissions from coal combustion. IEA Coal Research, London
Burch R, Breen JP, Meunier FC (2002) Appl Catal B 39:283–303
European Parliament and Council (2009) Directive 2009/28/EC. [Online]. PDF-document. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:140:0016:0062:EN:PDF. Accessed 6 April 2010
Bai S-L, Zhao J-H, Wang L, Zhu Z-P (2009) J Fuel Chem Technol 37:583–587
Busca G, Lietti L, Ramis G, Berti F (1998) Appl Catal B 18:1–36
Lagerkvist BJ, Oskarsson A (2007) In: Nordberg G, Fowler BA, Nordberg M, Friberg LT (eds) Handbook on the toxicology of metals, 3rd edn. Academic Press, New York, pp. 905–923
Moura de Oliveira ML, Monteiro Silva C, Moreno-Tost R, Lopes Farias T, Jiménez-López A, Rodríguez-Castellón E (2009) Appl Catal B 88:420–429
Huang B, Huang R, Jin D, Ye D (2007) Catal Today 126:279–283
Su Y, Fan B, Wang L, Liu Y, Huang B, Fu M, Chen L, Ye D (2013) Catal Today 201:115–121
Tang X, Hao J, Yi H, Li J (2007) Catal Today 126:406–411
Heck RM (1999) Catal Today 53:519–523
Ouyang R, Liu J-X, Li W-X (2013) J Am Chem Soc 135:1760–1771
Rodríguez-Reinoso F (1998) Carbon 36:159–175
Serp P, Corrias M, Kalck P (2003) Appl Catal A 253:337–358
Endo M, Strano MS, Ajayan PM (2008) In: Jorio A, Dresselhaus MS, Dresselhaus G (eds) Topics in applied physics 111: carbon nanotubes. Springer-Verlag, Berlin, pp 13–62
Halonen N, Kordás K, Tóth G, Mustonen T, Mäklin J, Vähäkangas J, Ajayan PM, Vajtai R (2008) J Phys Chem C 112:6723–6728
Kordás K, Tóth G, Moilanen P, Kumpumäki M, Vähäkangas J, Uusimäki A, Vajtai R, Ajayan PM (2007) Appl Phys Lett 90:123105
Sápi A, Rémiás R, Kónya Z, Kukovecz A, Kordás K, Kiricsi I (2009) React Kinet Catal Lett 96:379–389
Tessonnier J-P, Pesant L, Pham-Huu C, Ehret G, Ledoux MJ (2000) Stud Surf Sci Catal 143:697–704
Pham-Huu C, Keller N, Ehret G, Charbonniere LJ, Ziessel R, Ledoux MJ (2001) J Mol Catal A Chem 170:155–163
Solhy A, Machado BF, Beausoleil J, Kihn Y, Gonçalves F, Pereira MFR, Órfão JJM, Figueiredo JL, Faria JL, Serp P (2008) Carbon 46:1194–1207
Ersson A (2003) Materials for high-temperature catalytic combustion. Doctoral dissertation. KTH-Kunglika tekniska högskolan, Stockholm
Meeyoo V, Trimm DL, Cant NW (1998) Appl Catal B 16:L101–L104
Bai S, Zhao J, Wang L, Zhu Z (2010) Catal Today 158:393–400
Zhu Z, Liu Z, Niu H, Liu S (1999) J Catal 187:245–248
Marbán G, Antuña R, Fuertes AB (2003) Appl Catal B 41:323–338
Marbán G, Valdés-Solís T, Fuertes AB (2004) J Catal 226:138–155
Muñiz J, Marbán G, Fuertes AB (2000) Appl Catal B 27:27–36
Valdés-Solís T, Marbán G, Fuertes AB (2001) Catal Today 69:259–264
Valdés-Solís T, Marbán G, Fuertes AB (2003) Appl Catal B 46:261–271
Yoshikawa M, Yasutake A, Mochida I (1998) Appl Catal A 173:239–245
Zhu Z, Liu Z, Liu S, Niu H (1999) Appl Catal B 23:L229–L233
Pourkhalil M, Moghaddam AZ, Rashidi A, Towfighi J, Mortazavi Y (2013) Appl Surf Sci 279:250–259
Li Q, Hou X, Yang H, Ma Z, Zheng J, Liu F, Zhang X, Yuan Z (2012) J Mol Catal A Chem 356:121–127
Halonen N, Rautio A, Leino A-R, Kyllönen T, Tóth G, Lappalainen J, Kordás K, Huuhtanen M, Keiski RL, Sápi A, Szabó M, Kukovecz A, Kónya Z, Kiricsi I, Ajayan PM, Vajtai R (2010) ACS Nano 4:2003–2008
Leino A-R, Mohl M, Kukkola J, Mäki-Arvela P, Kokkonen T, Shchukarev A, Kordas K (2013) Carbon 57:99–107
Sapi A (2012) Doctoral thesis, University of Szeged
Holzwarth U, Gibson N (2011) Nat Nanotech 6:534
Borchert H, Shevchenko EV, Robert A, Mekis I, Kornowski A, Grübel G, Weller H (2005) Langmuir 21:1931–1936
Pyrz WD, Buttrey DJ (2008) Langmuir 24:11350–11360
Onoe T, Iwamoto S, Inoue M (2007) Catal Commun 8:701–706
Seelam PK, Huuhtanen M, Sápi A, Szabó M, Kordás K, Turpeinen E, Tóth G, Keiski RL (2010) Int J Hydrog Energy 35:12588–12595
Yu R, Chen L, Liu Q, Lin J, Tan K-L, Ng SC, Chan HSO, Xu G-Q, Hor TSA (1998) Chem Mater 10:718–722
Yang C, Hu X, Wang D, Dai C, Zhang L, Jin H, Agathopoulos S (2006) J Power Sources 160:187–193
Chen S, Shen W, Wu G, Chen D, Jiang M (2005) Chem Phys Lett 402:312–317
Karatepe N, Orbak I, Yavuz R, Özyuğuran A (2008) Fuel 87:3207–3215
Hase T (1997) Tables for organic spectrometry, 4th edn. Otatieto Oy, Helsinki, p 78
Ma Z, Yang H, Liu F, Zhang X (2013) Appl Catal A 467:450–455
Jiang L, Wang Y, Liu X, Cao Y, Wei K (2013) Chin J Catal 34:2271–2276
Abdulhamid H, Fridell E, Dawody J, Skoglundh M (2006) J Catal 241:200–210
Mäklin J, Mustonen T, Kordás K, Saukko S, Tóth G, Vähäkangas J (2007) Phys Stat Sol B 244:4298–4302
Ahmed SN, Baldwin R, Derbyshire F, McEnaney B, Stencel J (1993) Fuel 72:287–292
Huang C-C, Li H-S, Chen C-H (2008) J Hazard Mater 159:523–527
Ma Z, Yang H, Li Q, Zheng J, Zhang X (2012) Appl Catal A 427–428:43–48
Zhu Z, Liu Z, Liu S, Niu H (2001) Appl Catal B 30:267–276
Roy S, Viswanath B, Hedge MS, Madras G (2008) J Phys Chem C 112:6002–6012
Liu F, He H, Zhang C, Feng Z, Zheng L, Xie Y, Hu T (2010) Appl Catal B 96:408–420
Acknowledgments
This work has been carried out with the financial support from Fortum Foundation, the Academy of Finland (Project Number 128783), and Graduate School in Chemical Engineering (GSCE). The authors would also like to thank D.Sc.(Tech.) Tao Hu for running the XRD analyses, M.Sc. Elina Pulkkinen for preparing the KBr pellets and helping with the IR measurement, and M.Sc.(Eng.), M.Sc.(Health Sci.) Mari Pietikäinen for help with the EtOH-SCR experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valtanen, A., Huuhtanen, M., Rautio, AR. et al. Noble Metal/CNT Based Catalysts in NH3 and EtOH Assisted SCR of NO. Top Catal 58, 984–992 (2015). https://doi.org/10.1007/s11244-015-0467-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-015-0467-9







 , EtOH-SCR
, EtOH-SCR  , NH3-SCR (S)
, NH3-SCR (S) 