Abstract
To investigate the effect of the sites of S-atoms in thiophene carboxylates on the structures of coordination polymers, two thiophene-mono-carboxylic acids (2-Htpc = thiophene-2-carboxylic acid and 3-Htpc = thiophene-3-carboxylic acid) and a fluorescent active semi-rigid amide [N,N′-bis(3-methyl pyridine-3-yl)-2,6-naphthalenediamide (L)] were selected to combine with electrochemically active metal ions of Ni(II), and two new coordination polymers (CPs), namely [Ni0.5(L)0.5(2-tpc)](H2O)]∙1.5H2O (1) and [Ni0.5(L)0.5(3-tpc)](H2O)]∙1.5H2O (2), were obtained through traditional hydrothermal methods. The single-crystal X-ray diffraction analyses of the two CPs show that there are similar zigzag chains with the crossed-stacking modes. The two CPs can act as multifunctional electrochemical sensors to detect NO2−, chloramphenicol, and L-ascorbic acid (AA) and fluorescent recognition of Fe3+ and Cr2O72−. The detection limits of 1 were 1.08 × 10−6, 1.18 × 10−4, and 1.06 × 10−4 for AA, Fe3+, and Cr2O72−. The corresponding values of 2 were 1.43 × 10−6, 2.06 × 10−4, and 2.06 × 10−4.
Graphical abstract

Two Ni(II) coordination polymers showing the same crossed-stacking modes display electrochemical and fluorescent sensing properties for metal ions and anions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid development of industry and agriculture has caused different degrees of damage to the environment [1,2,3,4,5]. Although people's awareness of environmental protection has been obviously improved, pollutants such as nitrite (NO2−), dichromate (Cr2O72−), and antibiotics constantly destroy the balance of the ecosystem [6,7,8,9,10]. The above different pollutants can be successfully detected by different detection methods of spectrophotometry, chromatography, mass spectrometry, etc. [11,12,13,14,15,16]. However, the simultaneous detection of multiple pollutants is still a great challenge at present [17, 18]. Therefore, it is of great academic significance and application value to develop multifunctional detection materials to realize the simultaneous detection of the above pollutants.
At present, coordination polymer materials are used to realize electrochemical detection and fluorescence sensing detection which are the two effective detection methods [19,20,21,22,23]. Generally speaking, coordination polymer materials based on iron, cobalt, nickel, and copper have good electrochemical detection ability because of their reversible redox activity [24,25,26,27]. Coordination polymer materials constructed by organic ligands with appropriate conjugate volume can realize fluorescence detection of pollutants [28, 29]. Therefore, it will be an effective method to construct multifunctional electrochemical and fluorescent detection materials by reasonably utilizing the above two components to construct coordination polymers [30, 31].
Based on the above considerations, Ni2+ and [N,N′-bis(3-methylpyridin-3-yl)-2,6-naphthalenediamide (L)] were selected to combine with two thiophene-mono-carboxylic acids (2-Htpc = thiophene-2-carboxylic acid and 3-Htpc = thiophene-3-carboxylic acid). (i) Nitrogen-containing ligands with amide groups are not only stable in structure and strong in space expansion but also have multiple hydrogen bonding sites, which provide opportunities for the formation of supramolecular structures. (ii) Nickel can be coordinated and is electrochemically active. (iii) S-heterocarboxylic acid can be used as O-donating ligand, and its better hydrophilicity is more conducive to the realization of electrocatalytic performance [32,33,34]. As a result, two one-dimensional (1D) CPs with the crossed-stacking modes, namely [Ni0.5(L)0.5(2-tpc)](H2O)]∙1.5H2O (1) and [Ni0.5(L)0.5(3-tpc)](H2O)]∙1.5H2O (2), were obtained and structurally characterized. The good electrochemical sensing properties for KNO2, chloramphenicol (CAP), and ascorbic acid (AA) were investigated by 1 − 2 modified carbon paste electrode (1–2-CPEs). In addition, the fluorescence sensing properties of the 1 − 2 for Fe3+ and Cr2O72− were also studied.
Experimental
Synthetic procedure of 1–2
Preparation of [Ni 0.5 (L) 0.5 (2-tpc)](H 2 O)]∙1.5H 2 O (1)
A mixture of NiCl2·6H2O (0.0475 g), L (0.0393 g), 2-Htpc (0.0321 g), NaOH solution (1.8 mL, 0.1 mol/L), and H2O (7.5 mL) was sealed in a 25-mL Teflon-lined autoclave and heated at 120 °C for 4 days. The obtained crystals in the autoclave were cooled in the air. After reaching room temperature, light blue crystals of 1 can be collected with a yield of 25% based on L. Calcd for C34H36N4NiO11S2: C, 51.08; H, 4.54; N, 7.01%. Found: C, 51.10; H, 4.50; N, 7.03%. IR(KBr, cm−1): 3384 m, 3327 m, 3073 m, 2925 m, 1928 w, 1852 w, 1641 s, 1531 s, 1422 s, 1268 s, 1194 m, 928 m, 801 m, 776 s, 714 s, 660 m, 614 w, 537 w.
Preparation of [Ni 0.5 (L) 0.5 (3-tpc)](H 2 O)]∙1.5H 2 O (2)
The preparation procedure of complex 2 was similar to that of 1, but the difference was that 3-Htpc was used instead of 2-Htpc. The obtained crystals in the autoclave were cooled in the air. After reaching room temperature, light blue crystals of 2 can be collected with a yield of 20% based on L. Calcd for C34H36N4NiO11S2: C, 51.08; H, 4.54; N, 7.01%. Found: C, 51.12; H, 4.46; N, 7.05%. IR (KBr, cm−1): 3371 m, 3324 m, 3100 m, 3069 m, 1929 w, 1855 w, 1639 s, 1530 s, 1416 s, 1264 s, 1191 m, 933 m, 801 m, 769 s, 706 s, 650 m, 624 w, 545 w.
Results and discussion
Description of the crystal structure characteristics of 1–2
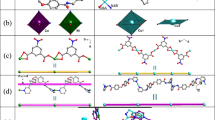
[Ni0.5(L)0.5(2-tpc)](H2O)]∙1.5H2O (1) and [Ni0.5(L)0.5(3-tpc)](H2O)]∙1.5H2O (2)
To explore the effect of S-sites of thiophene carboxylic acid, 2-tpc (the S-site is in ortho position of the carboxyl group) and 3-tpc (the S-site is in ortho position of the carboxyl group) were selected. However, two isostructural one-dimensional structures of 1 and 2 with subtle structural differences were received. In both structures, Ni atoms and one of the water molecules are located in a special position on a twofold axis. They are both in the monoclinic system with C2/c and contain half Ni2+, half L, one coordinated water molecule, one and a half non-coordinated water molecules, and one tpc anion (2-tpc in 1 and 3-tpc in 2) (Fig. 1a). Each Ni2+ has a representative hexacoordination configuration, which is in contact with two pyridine N atoms from two L [Ni − N are 2.106(18) Å in 1 and 2.104(3) Å in 2] and two carboxylic O atoms [Ni − O are 2.048(15) Å in 1 and 2.041(3) Å in 2] and two H2O [Ni − O are 2.102(14) Å in 1 and 2.097(2) Å in 2] (Table S1 and S2). In 1 and 2, each Ni2+ coordinated with two 2-tpc/3-tpc anions and two H2O to form monometallic subunits [Ni(L)(2-tpc)2](H2O)2] for 1 and [Ni(L)(3-tpc)2](H2O)2] for 2. Each subunit is bridged by adjacent μ2-L to generate a 1D wave-like chain (Fig. 1b). The above chains were stacked in a crossed manner forming 3D supramolecular frameworks extended by H-bonds (Fig. 1c and d). The details of H-bonds are listed in Table S3 and Fig. S1.
In addition, several subtle differences in the structural details of 1 and 2 were found by deep analysis. The Ni∙∙∙Ni distances linked by L are 15.925(8) and 15.854(12) Å. The dihedral angles between pyridine and naphthalene rings are 49.11 and 47.96°. The dihedral angles between the thiophene and pyridine rings are 86.45° and 87.34°, respectively. If the 1D title complexes are considered as one-dimensional chains, the torsion angles of the two crossed CPs are 43.649(1)° and 43.657(1)°. The above structural subtle differences can be attributed to the different S-sites of thiophene carboxylic acid used in the synthesis of the complexes. Although the structures of the title complexes show the above subtle differences, they are not significant. The possible main reason is that the S-atoms of 2-tpc in 1 and 3-tpc in 2 are not coordinated with metal ions. In addition, the corresponding thiophene rings have similar steric hindrance and conjugate volume, which cannot have a significant effect on the coordination of the title complexes. Nickel ion has a typical and relatively fixed hexacoordination mode. Based on the above analyses, the similar 1D wave-type structures of 1 and 2 are directed by center metal ions.
Electrocatalytic activities of the prepared electrodes
With the improvement of people's awareness of environmental protection, water environment has attracted widespread attention [35, 36]. Therefore, a fast, simple, and convenient sensor is needed to detect some analytes in water. Because of its reversible single-electron redox process, CP based on Ni2+ is very attractive for electrochemical research [37]. Because 1–2 has the characteristics of water stability and clear composition (Fig. S2), it also has good crystallinity (Fig. S3), successfully fabricated 1–2 bulk modified carbon paste electrodes (n-CPE, n = 1–2) based on the previous literature [38, 39]. Their electrochemical sensing studies were carried out in 0.1 M H2SO4 + 0.5 M Na2SO4 aqueous solution. As can be seen in Fig. S4, the cyclic voltammograms of 1–2-CPE in the same potential range of − 200 ~ + 400 mV under different scan rates were displayed. One pair of obvious reversible redox peaks was observed. The oxidation peaks were at 220 mV and 233 mV, and the corresponding reduction peaks of 1–2-CPE were at − 19 and − 53 mV. With 40 mV s−1 as the scan rate, the measured relative mean peak potentials of the pair of peaks [E1/2 = (Epa + Epc)/2] were 100.5 mV and 90 mV, respectively, which should be due to the redox between NiIII and NiII [40, 41]. When the scan rates were increased from 20 mV s−1 to 100 mV s−1, the anodic oxidation peak and the corresponding cathodic reduction peak potential moved in the positive and negative directions, respectively. The corresponding peak-to-peak currents of the cathode and anode increased linearly with the increase in the scan rate, and the average peak potential did not change much. This phenomenon expresses that the redox process of 1–2-CPEs was controlled by the surface [42].
The electrocatalytic reduction of KNO2, chloramphenicol (CAP), and oxidation of ascorbic acid (AA) for 1–2-CPEs in the 0.5 M Na2SO4 + 0.1 M H2SO4 aqueous solution was also investigated. As we all know, bare CPE has no obvious response to KNO2, CAP, or AA in an aqueous solution of 0.5 M Na2SO4 + 0.1 M H2SO4 [43]. Figure 2 shows the cyclic voltammograms of the electrocatalytic reduction of KNO2 under 1–2-CPEs. After adding a certain amount of KNO2, it can be seen that all the currents of reduction peak and the corresponding currents of oxidation peak decreased and increased, respectively. It can be concluded that 1–2-CPEs had electrocatalytic reduction ability for NO2– [44]. It can be seen from Fig. 3 that the reduction peak current of 1–2-CPEs increased significantly after adding CAP, which can indicate that 1 and 2 had good electrocatalytic activities for the reduction of CAP. In order to study the relationship between concentrations and peak currents, differential pulse voltammetry (DPV) was used. With the addition of a certain amount of CAP, the peak currents of 1–2-CPEs increased linearly. This result indicated that 1 and 2 can also be used as effective electrochemical catalysts for CAP [43]. As shown in Fig. 4a and b, after a certain amount of AA was added, the currents of oxidation peak and reduction peak of 1–2-CPEs gradually increased and remained unchanged, respectively, which proved that 1–2-CPEs exhibited excellent electrocatalytic activities for the oxidation of AA [45]. There was a good linear relationship between the peak current intensity and the concentration of AA, which made them be a potential material for AA electrochemical analysis and detection. The above results display that the 1–2-CPEs are multifunctional detection materials with excellent electrocatalytic activities, which can not only reduce KNO2 and CAP but also oxidize AA. In addition, to evaluate the analytic performance of 1 and 2 for AA determination quantitatively, the 1–2-CPEs were used as the AA amperometric sensors, respectively [46]. Under continuous stirring, add a certain concentration and volume of AA in 0.5 M Na2SO4 + 0.1 M H2SO4 aqueous solution every 30 s. As shown in Fig. 4c and d, the response time of 1 and 2 was almost 2 s and 1 s, respectively, and it was pointed out that the rapid electroreduction activities of these CPs can prevent the accumulation of reducing intermediates on the electrode surface. In addition, within a certain concentration range, the detection response of 1–2-CPEs to AA was linear. The detection limit of 1–2 was 1.08 × 10−6 and 1.43 × 10−6 (signal-to-noise ratio: 3), respectively. All in all, the 1–2-CPEs can be used as multifunctional electrochemical sensors, not only can detect KNO2 and CAP, but also can detect AA, which may expand the application of CPs-based complexes.
a-b Cyclic voltammograms of 1–2-CPEs at different AA concentrations at a sweep speed of 40 mV s–1; c-d amperometric current responses of 1–2-CPEs upon addition of AA, respectively. The inset: plots of the concentrations of AA vs the oxidation currents for 1–2-CPEs, respectively (applied potential: 400 mV for 1-CPE, 320 mV for 2-CPE)
Luminescent properties of 1–2
In order to explore the luminescence of 1–2, the solid-state fluorescence properties of 1–2 and the organic L ligand were investigated under the same experimental conditions (slit: 2.5 nm, voltage: 700) at ambient temperature (Fig. S5a). When the excitation at 360 nm was given, it is found that the maximum emission peaks of 1–2 and the L ligand appeared at 379 nm, 380 nm, and 372 nm, respectively. It is well known that there were only weak p* → n transitions, and the carboxylates had very little effect on the photoluminescence of 1–2 [47]. In addition, the solid ultraviolet absorption spectra showed that both the ligand L and the complexes 1–2 had strong absorption peaks in the ultraviolet region of 200–400 nm (Fig. S5b). Thus, the emissions of 1–2 may be assigned to intraligand charge transitions of the organic L ligand [48]. A certain red shift occurred from the L ligand to 1–2, which may be caused by the coordination of organic L ligands to the metal centers [49].
Fluorescence sensing detection of metal cations
Fe3+ played an important role in the process of human metabolism. If the human body contained high content of Fe3+, it will cause some cancers and some organ abnormalities [7, 50]. Therefore, it was necessary to adopt a simple method to determine whether Fe3+ was contained in the aqueous solution and its content. First, according to the method in the literature, the fluorescence sensing abilities of 1–2 to metal cations were explored [51]. Using 1 as an example, disperse the 3 mg fine powder of 1 as evenly as possible in 3 mL M(NO3)x aqueous solution(10−2 M) (M is Ba2+, Cd2+, Co2+, Cu2+, K+, La3+, Na+, Ni2+, Zn2+, and Fe3+). As shown in Fig. 5a, among the added cations, only Fe3+ ion makes the fluorescence quenching effect of 1 remarkable. Fe3+ has a similar quenching effect on polymer 2 (Fig. S6a) (Table 1).
a Fluorescence emission peak intensity of 1 in different metal cation solutions at the same excitation wavelength. b Fe3+ (10−3 M) concentration gradient experiment. c Linear relationship plot between fluorescence intensity and Fe3+ ions concentration. d Comparison of the luminescence emission peak intensity of 1 upon the addition of Fe3+
To explore the sensitivity of Fe3+, a concentration gradient experiment was carried out by adding an appropriate amount of 10− 3 M Fe3+ solution. As can be seen in Fig. 5b, when the concentration of Fe3+ gradually increased, the emission peak intensity of 1 gradually decreased. In addition, the Stern–Volmer constant (KSV, KSV = [I0/I − 1]/C) and the detection limit (3σ/KSV) of 1 were calculated to be 2.8 × 104 and 1.18 × 10−4, respectively. It can be seen that polymer 1 showed a better linear relationship at a lower concentration, which may be caused by the static quenching of Fe3+(Fig. 5c) [52]. The concentration gradient of 2 was similar to that of 1, which can indicate that this phenomenon of fluorescence quenching may be caused by the electron transfer between Fe3+ and the organic ligand L [48]. It was also necessary to explore the anti-interference effect of 1 in the presence of other metal cations. Fe3+ was added to the solution of other cations in 2 at the same time. The result is shown in Fig. 5d. It can be seen that after adding other interfering cations, Fe3+ can still quench 1. Polymer 2 shows the same effect (Fig. S6b–d, and Table 2).
Fluorescence sensing detection of inorganic anions
Some industry wastewater contains a large amount of heavy metal ions such as Cr2O72−, so it is very important to detect it quickly [53]. Use the same experimental procedure as cations to measure 10 anions, namely Cl−, Br−, I−, CH3COO−, CO32−, HCO3−, NO3−, OH−, SO42−, and Cr2O72−. As shown in Fig. 6a, it can be found that Cr2O72− can significantly quench the fluorescence intensity of 1 suspension. Polymer 2 shows similar effects (Fig. S7a). From Fig. 6b and c, it can be found that when the concentration of Cr2O72− ion slowly increased, the luminescence intensity of 1 gradually decreased. From the SV plot, it can be obtained that the KSV value of Cr2O72− of 1 was calculated to be 3.1 × 104. The detection limits were 1.06 × 10−4. In addition, the anti-interference ability of other inorganic anions to Cr2O72− anion by 1 was also explored. From Fig. 6d, it can be found that the peak intensity dropped sharply when Cr2O72− was introduced into the prepared aqueous solution of 1 of different anions (Cl−, Br−, I−, CH3COO−, CO32−, HCO3−, NO3−, OH−, and SO42−), so the quenching effects of other anions on Cr2O72− were hardly affected, which showed that 1 had good anti-interference to Cr2O72− in aqueous solution. Polymer 2 shows the same effect (Fig. S7b–d and Table 2).
a Fluorescence emission peak intensity of 1 in different anion solutions at the same excitation wavelength. b Cr2O72− (10−3 M) concentration gradient experiment. c Linear relationship plot between fluorescence intensity and Cr2O72− anions concentration. d Comparison of the luminescence emission peak intensity of 1 upon the addition of Cr2O72−
Conclusions
In summary, two 1D CPs with crossed-stacking modes were successfully constructed under hydrothermal conditions. The title CPs exhibited good electrochemical sensing activities for ascorbic acid, chloramphenicol, and KNO2, which provided the possibility to become potential electrochemical sensing material. The 1 − 2-CPEs can act as AA amperometric sensors. Furthermore, 1 − 2 had similar multifunctional luminescence sensing properties to metal cations (Fe3+) and inorganic anions (Cr2O72−). In short, the title CPs can be used as multifunctional electrochemical and fluorescent sensors to detect different pollutants. This work not only broadens the scope of application of CPs but also has greater significance in the field of environmental monitoring and protection. In addition, more CP-based multifunctional chemical sensors to determine environmentally harmful pollutants are further studied and explored.
References
Ma DY, Zhang SY, Zhan SH, Feng LT (2019) Ind Eng Chem Res 58:20090
Richardson JR, Fitsanakis V, Westerink RHS, Kanthasamy AG (2019) Acta Neuropathol 138:343–362
Feng DD, Zhao YD, Wang XQ, Fang DD (2019) Dalton Trans 48:10892–10900
Yu XP, Yang C, Song P, Peng J (2020) Tungsten 2:194–202
Bolisetty S, Peydayesh M, Mezzenga R (2019) Chem Soc Rev 48:463–487
Tian AX, Tian Y, Ning YL, Hou X (2016) Dalton Trans 45:13925–13936
Zhang J, Peh SB, Wang J, Du YH (2019) Chem Commun 55:4727–4730
Fu HR, Zhao Y, Xie T, Han ML (2018) J Mater Chem 6:6440
Tajik S, Beitollahi H, Nejad FG, Dourandish Z (2021) Ind Eng Chem Res 60:1112–1136
Bieber VS, Ozcelik E, Cox HJ, Ottley CJ (2020) ACS Appl Mater Interfaces 12:52136–52145
Sandford RC, Exenberger A, Worsfold PJ (2007) Environ Sci Technol 41:8420–8425
Shang XN, Kang HH, Chen YQ, Abdumutallip M (2021) Environ Sci Technol 55:9794–9804
Cabello NF, González PR, Castillo Á, Malherbe J (2012) Environ Sci Technol 46:12542–12549
Shakya R, Navarre DA (2006) J Agric Food Chem 54:5253–5260
Frenich AG, Torres MEH, Vega AB, Vidal JLM (2005) J Agric Food Chem 53:7371–7376
Hamilton EM, Young SD, Bailey EH, Humphrey OS (2021) Environ Sci Technol 55:2422–2429
Yang SL, Liu WS, Li G, Bu R (2020) Inorg Chem 59:15421–15429
Cui JW, Hou SX, Li YH, Cui GH (2017) Dalton Trans 46:16911–16924
Wu Y, Gu ZJ, Luo W, Wu L (2018) Transition Met Chem 43:673–681
Chen C, Xiong DK, Gu ML, Lu CX (2020) ACS Appl Mater Interfaces 12:35365–35374
Chai HM, Zhang GQ, Jiao CX, Ren YX (2020) ACS Omega 5:33039–33046
JindalS, Maka VK, Moorthy JN (2020) J Mater Chem C 8: 11449−11456
Wu XQ, Feng PQ, Guo ZQ, Wei XH (2020) Langmuir 36:14123–14129
Song YP, Duan FH, Zhang S, Tian JY (2017) J Mater Chem A 5:19378–19389
Sheta SM, El-Sheikh SM, Osman DI, Salem AM (2020) Dalton Trans 49:8918–8926
Saraf M, Rajakb R, Mobin SM (2016) J Mater Chem A 4:16432–16445
Wang XY, Zhang J, Wei YA, Xing TY (2020) Analyst 145:1933–1942
Nagarkar SS, Desai AV, Samanta P, Ghosh SK (2015) Dalton Trans 44:15175–15180
Bhowal S, Ghosh A (2021) RSC Adv 11:27787–27800
Tian AX, Yang ML, Fu YB, Ying J (2019) Inorg Chem 58:4190–4200
Wang C, Ying J, Mou HC, Tian AX (2020) Inorg Chem Front 7:3882–3894
Yazigi FJ, Wilson C, Long DL, Forgan RS (2017) Cryst Growth Des 17:4739–4748
Jürgens E, Back O, Mayer JJ, Heinze K (2016) Z Naturforsch 71:1011–1018
Gurudevaru C, Gopalakrishnan M, Senthilkumar K, Hemachandran H (2018) Applied Organometallic Chem 32:3998
Subudhi S, Mansingh S, Swain G, Behera A (2019) Inorg Chem 58:4921–4934
Xu MZ, Li Q, Lv YY, Yuan ZM (2020) Tungsten 2:203–213
Buschbaum KM, Beuerle F, Feldmann C (2015) Micropor Mesopor Mat 216:171–199
Xue Z, Jia L, Zhu RR, Du L (2020) J Electroanal Chem 858:113783
Wang XL, Hu HL, Liu GC, Lin HY (2010) Chem Commun 46:6485–6487
Zhou Y, Hu Q, Yu F, Ran GY (2020) J Am Chem Soc 142:20313–20317
Du HJ, Wang CH, Li Y, Niu YY (2015) RSC Adv 5:74065–74074
Tian AX, Ni HP, Ji XB, Tian Y (2017) RSC Adv 7:5774–5781
Wang XL, Xiong Y, Liu GC, Lin HY (2018) Dalton Trans 47:9903–9911
Zheng YP, Tan Y, Zhou WL, Hao XR (2021) Inorg Chem 60:12323–12330
Argoubi W, Rabti A, Aoun SB, Raouafi N (2019) RSC Adv 9:37384–37390
Su CH, Sun CL, Liao YC (2017) ACS Omega 2:4245–4252
Lin HY, Wang XL, Hu HL, Chen BK (2009) Solid State Sci 11:643–650
Liu GC, Chen YQ, Wang XL, Chen BK (2009) J Solid State Chem 182:566–573
Huang QY, Tang WP, Yang Y, Liu W (2014) Z Naturforsch 69b:423–431
Wang KM, Du L, Ma YL, Zhao QH (2016) Transition Met Chem 41:573–580
Gao LL, Zhao QN, Li MM, Fan LM (2017) CrystEngComm 19:6651–6659
Xia YP, Li YW, Li DC, Yao QX (2015) CrystEngComm 17:2459–2463
Xiao Y, Li B, You ZX, Xing YH (2021) J Mater Chem C 9:3193–3203
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21401010, 21901018), Education Department, and the Natural Science Foundations of Liaoning province (LJ2020008, 2021-MS-312). We thank Professor Ninghai Hu (Changchun Institute of Applied Chemistry) for refining the crystal data structures.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Mu, Y., Zhao, J. et al. Metal-directed thiophene-carboxylate-based nickel(II) complexes as multifunctional electrochemical and fluorescent sensors for detecting different analytes. Transit Met Chem 46, 613–621 (2021). https://doi.org/10.1007/s11243-021-00479-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-021-00479-z