Abstract
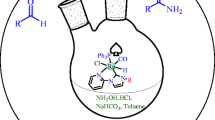
New ruthenium(II) complexes with N-heterocyclic carbene ligand were synthesized by transmetalation reactions between silver(I) N-heterocyclic carbene complexes and [RuCl2(p-cymene)]2. The complexes were characterized by physicochemical and spectroscopic methods. These ruthenium complexes were applied to the N-monoalkylation of aromatic amines with a wide range of primary alcohols under solvent-free conditions using the hydrogen borrowing strategy. The catalytic reactions using all ruthenium complexes resulted in N-monoalkylated products with high selectivities using furfuryl alcohol as the alkylating agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alkylation of amines has received much attention by synthetic chemists, as alkylated amines are important compounds widely found in natural products, pharmaceuticals, bulk and fine chemicals [1]. Traditional methods for alkylation of amines involve substitution reactions with alkyl halides [2] or reductive aminations using stoichiometric reducing agents [3]. However, these reactions have significant drawbacks, such as toxicity of alkylating and reducing agents, the formation of large amounts of waste, acidic reaction conditions and undesired side products. Due to the importance of arylated and alkylated amines in the chemical industry, during the last decades, several catalytic methods have been developed for the arylation and alkylation of amines, including Buchwald–Hartwig amination [4], hydroamination [5], hydroaminomethylation [6] and hydrogen borrowing methodology [7]. Among the carbon–nitrogen bond-forming reactions, the hydrogen borrowing methodology also called hydrogen auto-transfer constitutes one of the most important synthetic methods for the preparation of alkylated amines, which involves a simple operation process and mild conditions [8]. This process involves three steps but carried out in one pot. In the initial step, hydrogen is temporarily removed from alcohol with a transition metal catalyst to form a carbonyl compound, and then, the carbonyl compound reacts with a nucleophile to form an unsaturated intermediate. In the last step, the unsaturated molecule is reduced by the metal hydride complex to obtain the final product (Scheme 1). Particularly, the use of alcohols instead of alkyl halides as alkylating agents in the alkylation of amines is highly attractive, because they are readily available, relatively cheap and less toxic than the corresponding alkyl halide, and water is the only by-product in the overall process [9]. Besides alcohols, amines can also be used as alkylating agents in this process, but the use of amines for the alkylation of amines is much more limited [10].
The hydrogen borrowing methodology has been successfully applied for the formation of new carbon–heteroatom and carbon–carbon bonds under catalytic conditions [11]. The first N-alkylation of amines with alcohols was described independently by Grigg [12] and Watanabe [13] using rhodium- and ruthenium-phosphine complexes as catalysts, respectively, in 1981. Since then, a number of homogeneous and heterogeneous catalytic systems based on ruthenium [14], iridium [15], palladium [16], silver [17], cobalt [18], copper [19] and rhodium [20] have been developed by Williams and other groups for alkylation of amines with alcohols. The most important results for the preparation of the secondary amines have largely been achieved using ruthenium and iridium complexes. Recently, important progress in hydrogen borrowing reactions was achieved with Au and Zr as the catalysts with excellent results [21]. In addition, alkylation of amines with alcohols has been performed using biocatalysts for the preparation of enantiopure amines via biocatalytic hydrogen methodology [22].
N-Heterocyclic carbenes (NHC) are an important class of widely used and investigated ligands in organometallic chemistry and catalysis because of their readily tunable steric and electronic properties, and their ease of synthesis [23]. Generally, NHCs have good σ-donor and weak π-acceptor properties and are very strong nucleophiles. The strong electron-donating property of N-heterocyclic carbene ligands leads to the formation of stable metal–NHC complexes with strong covalent bonds [24]. This strong metal–carbon bond avoids the decomposition of NHC complexes during the course of catalytic reactions. A variety of NHC complexes have been successfully synthesized and used as antimicrobial, antitumor and anticancer agents [25], photoluminescent materials [26] and catalysts for various transformations [27]. In recent years, NHC complexes have been widely used as catalysts in hydrogen borrowing reactions [28,29,30,31]. In particular, NHC complexes of iridium and ruthenium have demonstrated good activity for these transformations [32,33,34,35]. However, the use of ruthenium(II)-NHC complexes in the alkylation of amines with alcohols is rare [36, 37]. To the best of our knowledge, ruthenium(II) complexes bearing a perhydrobenzimidazol-2-ylidene ligand for N-alkylation reactions have not been reported to date.
In this study, the new [RuCl2(NHC)(p-cymene)] (NHC = perhydrobenzimidazol-2-ylidene) complexes were readily synthesized by treating the [RuCl2(p-cymene)]2 dimer directly with the corresponding Ag(I)-NHC complexes, which was prepared in situ by reaction of Ag2O with symmetrical 1,3-dialkylperhydrobenzimidazolium salts in dichloromethane. The catalytic activity of these complexes was evaluated in N-alkylation reactions of aniline with arylmethyl alcohols.
Experimental
All reactions for the preparation of the ruthenium(II)-NHC complexes were carried out under argon in flame-dried glassware using standard Schlenk techniques. The solvents were purified by distillation over the drying agents indicated and were transferred under Ar: THF, Et2O (Na/K alloy), CH2Cl2 (P4O10), hexane, toluene (Na). All reagents were purchased from Sigma-Aldrich, Merck and Fluka. 1H-NMR and 13C-NMR spectra were recorded with a Varian AS 400 Merkur spectrometer operating at 400 MHz (1H), 100 MHz (13C) in CDCl3 with tetramethylsilane as an internal reference. Coupling constants (J values) are given in Hertz. NMR multiplicities are abbreviated as follows: s = singlet, d = doublet, t = triplet, q = quartet, sept = septet and m = multiplet signal. FTIR spectra were recorded on ATR unit in the range 400–4000 cm−1 on PerkinElmer Spectrum 100. GC was measured by GC-FID on an Agilent 6890 N gas chromatograph equipped with an HP-5 column of 30 m length, 0.32 mm diameter and 0.25 μm film thickness. Melting points were measured in open capillary tubes with an Electrothermal-9200 melting point apparatus and uncorrected. Elemental analyses were performed at Inönü University research center.
Synthesis of perhydrobenzimidazolium salts 1
A mixture of N,N′-dialkyl-1,2-diaminocyclohexane (4.0 mmol), NH4Cl (4.0 mmol) and triethyl orthoformate (10 mL) was heated for 12 h at 110 °C. Upon cooling to room temperature, colorless crystals were obtained. These were filtered off, washed with diethyl ether (3 × 15 mL) and dried under vacuum. The crude product was recrystallized from EtOH/Et2O.
1,3-Bis(4-diethylaminobenzyl)perhydrobenzimidazolium chloride 1c
Yield: 1.79 g, 93%, m.p. 172–174 °C. IR, υ: 1521 cm−1 (NCN). 1H NMR (CDCI3) δ: 1.15 (t, 12H, J = 6.9 Hz, N(CH2CH3)2), 1.17–1.36 (m, 4H, NCHCH2CH2CH2CH2CHN), 1.80–2.25 (m, 4H, NCHCH2CH2CH2CH2CHN), 3.10–3.12 (m, 2H, NCHCH2CH2CH2CH2CHN), 3.36 (d, 8H, J = 7.2 Hz, N(CH2CH3)2), 4.45 and 5.05 (d, 4H, J = 14.4 Hz, NCH2Ar), 6.61 and 7.22 (d, 8H, J = 8.7 Hz, CH2C6H4N(CH2CH3)2-4), 10.75 (s, 1H, NCHN). 13C NMR (CDCI3) δ: 12.5 (N(CH2CH3)2), 23.6, 27.3 and 50.3 (NCH(CH2)4CHN), 44.2 (N(CH2CH3)2), 66.0 (NCH2Ar), 111.7, 118.8, 129.9 and 147.9 (CH2C6H4N(CH2CH3)2-4), 161.5 (NCHN); Anal. Calcd. for C29H43N4CI: C, 72.12; H, 8.91; N, 11.60. Found C, 72.16; H, 8.87; N, 11.62%.
Synthesis of ruthenium(II)-NHC complexes 2
Ag2O (0.54 mmol) was added to a solution of the appropriate perhydrobenzimidazolium chloride (1.08 mmol) in dichloromethane (25 mL). The mixture was stirred for 24 h at room temperature in the dark conditions, covered with aluminum foil under argon and then filtered through Celite to remove the AgCl formed. [RuCl2(p-cymene)]2 (0.43 mmol) was added to the colorless solution, and the reaction mixture was stirred for 24 h at room temperature. The resulting mixture was filtered through Celite, and the solvent was removed under vacuum to afford the product. The crude product was recrystallized from dichloromethane/diethyl ether (1:2) at room temperature. The orange-brown crystals were filtered off, washed with diethyl ether (3 × 10 mL) and dried under vacuum.
Dichloro-[1,3-bis(4-phenylbenzyl)perhydrobenzimidazol-2-ylidene](p-cymene)ruthenium(II) 2a
Yield: 0.13 g, 81%, m.p = 257–258 °C. IR, υ: 1516 cm−1 (NCN). 1H NMR (CDCI3) δ: 0.95–1.20 (m, 4H, NCHCH2CH2CH2CH2CHN), 1.22 and 1.29 (d, 6H, J = 4 Hz, p-CH3C6H4CH(CH3)2), 1.57–1.79 (m, 4H, NCHCH2CH2CH2CH2CHN), 2.14 (s, 3H, p-CH3C6H4CH(CH3)2), 2.88 (sept, 1H, J = 8 Hz, p-CH3C6H4CH(CH3)2), 3.20–3.35 (m, 2H, NCHCH2CH2CH2CH2CHN), 4.64, 4.90, 5.79 and 6.00 (d, 4H, J = 16 Hz, p-CH3C6H4CH(CH3)2), 5.10, 5.18, 5.34 and 5.49 (d, 4H, J = 8 Hz, NCH2Ar), 7.26–7.61 (m, 18H, CH2C6H4C6H5-4). 13C NMR (CDCI3) δ: 18.7 (p-CH3C6H4CH(CH3)2), 21.8 (p-CH3C6H4CH(CH3)2), 30.6 (p-CH3C6H4CH(CH3)2), 23.4, 24.1, 29.7, 54.1 and 55.7 (NCH(CH2)4CHN), 68.0 and 69.8 (NCH2Ar), 83.0, 84.4, 85.9, 86.3, 97.6 and 108.4 (p-CH3C6H4CH(CH3)2), 126.7, 126.9, 127.1, 127.3, 128.3, 128.7, 128.8, 137.5, 138.0, 140.0, 140.5 and 140.8 (CH2C6H4C6H5-4), 215.2 (Ru-Ccarb). Anal. Calc. for C43H46N2RuCl2: C, 67.71; H, 6.04; N, 3.67. Found: C, 67.74; H, 6.06; N, 3.66%.
Dichloro-[1,3-bis(4-benzyloxybenzyl)perhydrobenzimidazol-2-ylidene](p-cymene)ruthenium(II) 2b
Yield: 0.14 g, 79%, m.p = 214–216 °C. IR, υ: 1511 cm−1 (NCN). 1H NMR (CDCI3) δ: 0.90–1.19 (m, 4H, NCHCH2CH2CH2CH2CHN), 1.21 and 1.27 (d, 6H, J = 8 Hz, p-CH3C6H4CH(CH3)2), 1.57–1.75 (m, 4H, NCHCH2CH2CH2CH2CHN), 2.10 (s, 3H, p-CH3C6H4CH(CH3)2), 2.85 (sept, 1H, J = 8 Hz, p-CH3C6H4CH(CH3)2), 3.10–3.27 (m, 2H, NCHCH2CH2CH2CH2CHN), 4.49, 4.83, 5.57 and 5.88 (d, 4H, J = 16 Hz, p-CH3C6H4CH(CH3)2), 5.04, 5.12, 5.30 and 5.44 (d, 4H, J = 8 Hz, NCH2Ar), 5.05 and 5.06 (s, 4H, OCH2Ar), 6.90–6.99 and 7.26–7.43 (m, 18H, CH2C6H4OCH2C6H5-4). 13C NMR (CDCI3) δ: 18.6 (p-CH3C6H4CH(CH3)2), 21.8 (p-CH3C6H4CH(CH3)2), 30.6 (p-CH3C6H4CH(CH3)2), 23.2, 24.2, 29.6, 53.7 and 55.4 (NCH(CH2)4CHN), 67.8 and 69.7 (NCH2Ar), 70.0 (OCH2Ar), 82.9, 84.2, 85.8, 86.3, 97.5 and 108.4 (p-CH3C6H4CH(CH3)2), 114.4, 114.8, 127.5, 127.8, 128.0, 128.5, 129.1, 131.0, 136.9 and 158.0 (CH2C6H4OCH2C6H5-4), 214.5 (Ru-Ccarb). Anal. Calc. for C45H50N2O2RuCl2: C, 65.69; H, 6.08; N, 3.41. Found: C, 65.66; H, 6.05; N, 3.43%.
Dichloro-[1,3-bis(4-diethylaminobenzyl)perhydrobenzimidazol-2-ylidene](p-cymene)ruthenium(II) 2c
Yield: 0.12 g, 75%, m.p = 223–224 °C. IR, υ: 1519 cm−1 (NCN). 1H NMR (CDCI3) δ: 0.95–1.06 (m, 4H, NCHCH2CH2CH2CH2CHN), 1.16–1.18 (m, 12H, N(CH2CH3)2), 1.19 and 1.22 (d, 6H, J = 8 Hz, p-CH3C6H4CH(CH3)2), 1.55–1.77 (m, 4H, NCHCH2CH2CH2CH2CHN), 2.06 (s, 3H, p-CH3C6H4CH(CH3)2), 2.80 (sept, 1H, J = 8 Hz, p-CH3C6H4CH(CH3)2), 3.13–3.25 (m, 2H, NCHCH2CH2CH2CH2CHN), 3.33–3.35 (m, 8H, N(CH2CH3)2), 4.52, 4.81, 5.40 and 5.64 (d, 4H, J = 16 Hz, p-CH3C6H4CH(CH3)2), 5.10, 5.20, 5.31 and 5.44 (d, 4H, J = 8 Hz, NCH2Ar), 6.65, 6.70 and 7.19 (d, 8H, J = 8 Hz, CH2C6H4N(CH2CH3)2-4). 13C NMR (CDCI3) δ: 12.6 (N(CH2CH3)2), 18.4 (p-CH3C6H4CH(CH3)2), 21.9 (p-CH3C6H4CH(CH3)2), 30.5 (p-CH3C6H4CH(CH3)2), 23.2, 24.2, 29.2, 29.8, 53.7 and 54.8 (NCH(CH2)4CHN), 44.2 (N(CH2CH3)2), 68.2 and 68.5 (NCH2Ar), 83.4, 84.9, 85.5, 85.6, 96.7 and 107.4 (p-CH3C6H4CH(CH3)2), 111.9 124.7, 126.0, 127.3, 128.8 and 147.0 (CH2C6H4N(CH2CH3)2-4), 213.7 (Ru-Ccarb). Anal. Calc. for C39H56N4RuCI2: C, 62.23; H, 7.45; N, 7.45. Found: C, 62.20; H, 7.43; N, 7.49%.
Dichloro-[1,3-bis(2,4,6-trimethoxybenzyl)perhydrobenzimidazol-2-ylidene](p-cymene)ruthenium(II) 2d
Yield: 0.14 g, 83%, m.p = 200–2001 °C. IR, υ: 1591 cm−1 (NCN). 1H NMR (CDCI3) δ: 0.67–1.05 (m, 4H, NCHCH2CH2CH2CH2CHN), 1.24 and 1.28 (d, 6H, J = 8 Hz, p-CH3C6H4CH(CH3)2), 1.39–1.56 (m, 4H, NCHCH2CH2CH2CH2CHN), 2.08 (s, 3H, p-CH3C6H4CH(CH3)2), 2.62–2.68 (m, 1H, J = 8 Hz, p-CH3C6H4CH(CH3)2), 2.78–3.09 (m, 2H, NCHCH2CH2CH2CH2CHN), 3.83 and 3.85 (s, 18H, CH2C6H2(OCH3)2-2,4,6), 4.98–5.05 (m, 4H, p-CH3C6H4CH(CH3)2), 5.65 and 5.69 (d, 4H, J = 4 Hz, NCH2Ar), 6.13 (s, 4H, CH2C6H2(OCH3)2-2,4,6). 13C NMR (CDCI3) δ: 18.8 (p-CH3C6H4CH(CH3)2), 22.3 (p-CH3C6H4CH(CH3)2), 30.4 (p-CH3C6H4CH(CH3)2), 23.2, 24.5, 29.2, 30.2, 44.7 and 45.5 (NCH(CH2)4CHN), 55.3, 55.8 (CH2C6H2(OCH3)2-2,4,6), 65.8 and 66.3 (NCH2Ar), 83.5, 84.1, 87.9, 90.6, 92.9 and 104.8 (p-CH3C6H4CH(CH3)2), 159.8, 160.0, 160.7 and 160.8 (CH2C6H2(OCH3)2-2,4,6), 213.9 (Ru-Ccarb). Anal. Calc. for C37H50N2O6RuCl2: C, 56.20; H, 6.33; N, 3.54. Found: C, 56.24; H, 6.30; N, 3.56%.
General procedure for the N-alkylation of amines with alcohols
Under an inert atmosphere, KOBut (1 mmol), aromatic amine (1 mmol), alcohol derivative (1.5 mmol) and Ru-NHC complex (2.5 mol%) were added to Schlenk tube. The sealed Schlenk tube was stirred at 120 °C for 24 h. At the end of the reaction, the reaction mixture was cooled to room temperature, and CH2Cl2 (2 ml) was added and filtered through a short pad of SiO2. The filtrate was analyzed by GC–MS with the calibrations based on dodecane.
Results and discussion
Synthesis of perhydrobenzimidazolium salts
The symmetrical 1,3-dialkylperhydrobenzimidazolium salts 1 as NHC precursors were synthesized from the N,N′-dialkylcyclohexan-1,2-diamines, triethyl orthoformate and ammonium chloride. Perhydrobenzimidazolium salts are colorless solids and were obtained in good yields. The salts were characterized by 1H and 13C NMR spectroscopy, FTIR and elemental analysis. The spectral properties of 1c are similar to those of other reported perhydrobenzimidazolium salts. In the 1H NMR spectra of 1c, NCHN proton appeared as a singlet at 10.75 ppm and benzylic protons appeared as two doublets at 4.45 and 5.05 ppm. The NCHN carbon resonance of 1c was observed at 161.5 ppm in the 13C NMR spectra. The appearances of these downfield signals indicate the formation of 1c. The other perhydrobenzimidazolium salts used in this study (1a, 1b and 1d) were previously reported by our group [38,39,40].
Synthesis of Ru(II)-NHC complexes
There are several methods in the literature that describe the synthesis of Ru(II)-NHC complexes. One of these is the generation of silver(I)-NHC complex, followed by transfer of the carbene unit to ruthenium metal. The new [RuCl2(NHC)(p-cymene)] complexes were prepared by transmetalation from the corresponding silver N-heterocyclic carbene complexes in two steps (Scheme 2). In the first step, the Ag(I)-NHC complexes were synthesized according to the general method described by Wang and Lin [41] by reaction of Ag2O with 2 equiv. of 1,3-dialkylperhydrobenzimidazolium salts in dichloromethane at ambient temperature in the dark. In the second step, in situ prepared Ag(I)-NHC complexes were converted into the orange-brown monocarbene Ru(II)-NHC complexes at ambient temperature. Ruthenium(II)-NHC complexes were purified by crystallization. The air- and moisture-stable ruthenium–carbene complexes in the solid state were soluble in solvents such as dichloromethane, chloroform and toluene and insoluble in non-polar solvents. Single crystals of these complexes suitable for X-ray diffraction studies could not be obtained. Therefore, all of the Ru(II)-NHC complexes were characterized by 1H NMR, 13C NMR, IR spectroscopy and elemental analysis, which all supported the proposed structures. They exhibit characteristic υ(C = N) band typically at 1516, 1511, 1519 and 1591 cm−1, respectively, for 2a–d. In the NMR spectrum of ruthenium complexes 2a–d, the characteristic downfield NCHN proton and NCHN carbon resonances of perhydrobenzimidazolium salts 1a–d were disappeared upon complexation with ruthenium. The 13C chemical shifts provide a useful diagnostic tool for this type of metal–carbene complexes and show that Ccarbene is substantially deshielded. The chemical shifts for the carbene carbon atom of 2a–d complexes were observed at 215.2, 214.5, 213.7 and 213.9 ppm, respectively, for 2a–d, and these values were similar to those found for other (p-cymene)-ruthenium(II)-NHC complexes. These new complexes show typical spectroscopic signatures which are in line with those recently reported for other [RuCl2(NHC)(p-cymene)] complexes [42]. The results obtained from the elemental analysis of complexes 2a–d are in agreement with the theoretical requirements of their structures.
Catalytic studies
The catalytic activities of new ruthenium complexes 2a–d for the N-alkylation of aromatic amines with arylmethyl alcohols were evaluated. Initially, the reaction of aniline with benzyl alcohol was selected as a model reaction to determine the optimal catalytic conditions with 2c as a catalyst. The effect of the base, reaction time, solvent and catalyst loading was examined. The results are summarized in Table 1. The bases were crucial to the efficiency of this reaction. Cs2CO3, K2CO3, KOH and KOBut were tested as the base. Among the tested bases, KOBut displayed the highest reactivity (entry 3). KOH was less effective (entry 2), while alkali metal carbonates Cs2CO3 and K2CO3 stopped the reaction completely at the dehydrogenation step. It is noteworthy that, in the absence of base and ruthenium complex, no reaction was observed (entry 11). Toluene and dioxane often used these reactions and gave excellent or poor conversions (entries 2 and 5). However, in the absence of a solvent, an excellent conversion was also observed. Reducing the catalyst loading to 1 mol% decreased the conversion (entry 9). Excellent conversion and selectivity were obtained by the use of 2.5 mol% of 2c (entry 10). Lower temperature (100 °C) or shorter time (15 h) reduced the conversion (entries 2 and 1), whereas full conversion was observed at 120 °C for 24 h (entry 7). The catalytic experiments were carried out using 1 mmol aromatic amine, 1.5 mmol arylmethyl alcohol, 0.01 mmol KOBut and 0.025 mmol 2a–d at 120 °C for 24 h under argon without any solvent or additive. Under these reaction conditions, the ruthenium-catalyzed N-alkylation of aromatic amines (aniline, 2,4-dimethylaniline and 2-aminopyridine) with arylmethyl alcohols (benzyl alcohol, 4-methylbenzyl alcohol, 4-methoxybenzyl alcohol and furfuryl alcohol) was examined. In all cases, only monoalkylated amines and imines were formed, and no bis-alkylated products were detected. The reaction products were characterized by NMR. The conversions and selectivity were screened by GC and GC–MS analysis.

With the determined optimal conditions in hand, we first investigated complexes 2a–d as catalysts the N-alkylation of aniline with benzyl alcohol, 4-methylbenzyl alcohol, 4-methoxybenzyl alcohol and furfuryl alcohol. In all reactions, alkylation of aniline with high selectivity was achieved. The reaction of aniline with benzyl alcohol gave the N-benzylaniline in 96–99% conversions (Table 2, entry 1). The formation of N-benzylamine with high selectivity was obtained with complex 2c (Table 2, entry 1). The treatment of aniline with 4-methylbenzyl alcohol and 4-methoxybenzyl alcohol using complexes 2a–d as catalysts gave also corresponding monoalkylated amines in conversions between 95–100% and 85–91%, respectively, under these conditions. 4-Methoxybenzyl alcohol afforded the corresponding monoalkylated product N-(4-methoxybenzyl)aniline with excellent selectivities for all complexes 2a–d (Table 2, entry 3). Complete selectivity was achieved with catalysts 2b–d (Table 2, entry 2). High yields were obtained with complexes 2c and 2d (Table 2, entry 1–4). These results show that the electron-donating substituent (4-methyl and 4-methoxy) on benzyl alcohol gave higher selectivity when compared to benzyl alcohol itself. When using furfuryl alcohol as the alkylating agent, N-(furan-2-ylmethyl)aniline with 100% selectivity was obtained for all complexes 2a–d, but conversions of reactions were slightly lower than others (Table 2, entry 4).
We next examined the reactions of 2,4-dimethylaniline with benzyl alcohol, 4-methylbenzyl alcohol, 4-methoxybenzyl alcohol and furfuryl alcohol in the presence of complexes 2a–d as catalysts under the same reaction conditions (Table 2, entries 5–8). Thus, 2,4-dimethylaniline was treated with benzyl alcohol for all complexes 2a–d under optimized reaction conditions to obtain the corresponding N-benzyl-2,4-dimethylaniline in 70–84% conversions with 74–87% selectivity (Table 2, entry 5). The reaction of 2,4-dimethylaniline with 4-methylbenzyl alcohol and 4-methoxybenzyl alcohol also afforded corresponding monoalkylated amines in conversions between 75–92% and 70–87% with 80–100% and 55–77% selectivity, respectively. The above results show that the electron-donating substituents such as Me and OMe on both benzyl alcohol and aniline slightly increased the selectivity of monoalkylated amine products under the same conditions (Table 2, entries 1–3, and Table 2, entries 5–7). Similar trends have been observed for the other Ru(II) systems bearing NHC ligands [36]. When the furfuryl alcohol was used as an alkylating agent, only the corresponding monoalkylated amine was obtained with 100% selectivity for all complexes 2a–d (Table 2, entry 8). No imine or bis-alkylated products were detected.
Finally, we also investigated the N-alkylation of 2-aminopyridine with the same alcohols (benzyl alcohol, 4-methylbenzyl alcohol, 4-methoxybenzyl alcohol and furfuryl alcohol) by using ruthenium(II)-NHC 2a–d catalysts in order to obtain N-alkylated amines under the same reaction conditions. 2-(N-alkylamino)pyridines were obtained in good-to-excellent selectivities in the presence of 2.5 mol% catalysts. Moreover, the heteroaromatic moiety in 2-aminopyridine was also well tolerated under this catalytic system. In all reactions, only the nitrogen atom of the amino group of 2-aminopyridine was alkylated; the products with N-alkylpyridine were not detected. 2-Aminopyridine was efficiently arylated with benzyl alcohol and furfuryl alcohol for all complexes 2a–d with 100% selectivity (Table 3, entries 1 and 4). The reaction of 2-aminopyridine with 4-methylbenzyl alcohol and 4-methoxybenzyl alcohol also gave corresponding products in conversions between 98–100% and 98–99% with 71–100% and 96–98% selectivity, respectively. As shown in Tables 2 and 3, ruthenium complexes (2c, 2 d) bearing NHC ligands with diethylamino or methoxy substituents on benzyl group exhibited better catalytic activity in some cases than the others for the N-alkylation of aromatic amines with arylmethyl alcohols.
To the best of our knowledge, studies of Ru-NHC complexes for the alkylation of amines with alcohols are rare in the literature. Recently, our group has investigated ruthenium–carbene complexes in the alkylation of amines with alcohols [37]. In this work, we described the synthesis and characterization of benzimidazole–ruthenium(II) complexes of the general formula [RuCl2(NHC)(η6-p-cymene)]. These complexes were tested as catalysts for the N-alkylation of aniline with arylmethyl alcohols. In this work we described the synthesis and characterization of perhydrobenzimidazole-ruthenium(II) complexes of the general formula [RuCl2(NHC)(η6-p-cymene)]. By comparison, reported for related Ru catalysts such as N-heterocyclic carbene ruthenium(II) complexes, similar results were obtained. The present catalytic system using NHC–ruthenium(II) complexes is particularly effective and selective for amines formation (A) despite low-temperature and solvent-free condition [37]. For example in the case of furfuryl alcohol, the amine product (A) was obtained in 84–93% when benzimidazole–ruthenium(II) complexes were employed, whereas the perhydrobenzimidazole–ruthenium(II) complexes (2a–d) afforded full conversion and the amine product (A) was favored (Table 2, entry 4).
Conclusion
In summary, ruthenium(II)-NHC complexes 2a–d have been easily prepared by the reaction of silver(I)-NHC complexes as a carbene transfer reagent with [RuCl2(p-cymene)]2 in dichloromethane at room temperature in good yields. The catalytic activity of these complexes was investigated in N-alkylation reactions of aniline, 2,4-dimethylaniline and 2-aminopyridine with arylmethyl alcohols including benzyl alcohol, 4-methyl benzyl alcohol, 4-methoxybenzyl alcohol and furfuryl alcohol. The N-alkylation of amines was performed in solvent-free conditions for 24 h at 120 °C using 2.5 mol% of complexes 2a–d. All of these complexes were found to be suitable for N-alkylation of aromatic amines with arylmethyl alcohols via hydrogen borrowing reactions. In this study, high selectivity was obtained. In all cases only monoalkylated amines were formed and no bis-alkylated products were detected.
References
Ricci A (2008) Amino group chemistry: from synthesis to the life sciences. Wiley-VCH, Weinheim
Salvatore RN, Yoon CH, Jung KW (2001) Tetrahedron 57:7785
Storer RI, Carrera DE, Ni Y, MacMillan DWC (2006) J Am Chem Soc 128:84
Hartwig JF (1998) Angew Chem Int Ed Engl 37:2046
Hannedouche J, Schulz E (2013) Chem Eur J 19:4972
Moballigh A, Seayad A, Jackstell R, Beller M (2003) J Am Chem Soc 125:10311
Guillena G, Ramon DJ, Yus M (2010) Chem Rev 110:1611
Yang Q, Wang Q, Yu Z (2015) Chem Soc Rev 44:2305
Corma A, Navas J, Sabater MJ (2018) Chem Rev 118:1410
Saidi O, Blacker AJ, Farah MM, Marsden SP, Williams JMJ (2009) Angew Chem Int Ed 48:7375
Corma A, Navas J, Rodenas T, Sabater MJ (2013) Chem Eur J 19:17464
Grigg R, Mitchell TRB, Sutthivaiyakit S, Tongpenyai N (1981) J Chem Soc Chem Commun 12:611
Watanabe Y, Tsuji Y, Ohsugi Y (1981) Tetrahedron Lett 22:2667
Hamid MHSA, Allen CL, Lamb GW, Maxwell AC, Maytum HC, Watson AJA, Williams JMJ (2009) J Am Chem Soc 131:1766
Saidi O, Blacker AJ, Farah MM, Marsden SP, Williams JMJ (2010) Chem Commun 46:1541
Llabres-Campaner PJ, Ballesteros-Carrido R, Ballesteros R, Abarde B (2017) Tetrahedron 73:5552
Liu H, Chuah GK, Jaenicke S (2012) J Catal 191:130
Quintrad A, Rodrigez J (2016) Chemsuschem 9:28
Santoro F, Psaro R, Ravasio N, Zaccherio F (2014) RSC Adv 4:2596
Wong MC, Peterson MB, Pernik I, McBurney RT, Messerle BA (2017) Inorg Chem 56:14682
Huang R, Yang Y, Wang DS, Zhang L, Wang D (2018) Org Chem Front 5:203
Montgomery SL, Mangas-Sanchez J, Thompson MP, Aleku GA, Dominguez B, Turner NJ (2017) Angew Chem Int Ed 56:10491
Jansenn-Müller D, Glorius F (2017) Chem Soc Rev 46:4845
De Fremont P, Marion N, Nolan SP (2009) Coord Chem Rev 253:862
Liu W, Gust R (2013) Chem Soc Rev 42:755
Mercs L, Albrecht M (2010) Chem Soc Rev 39:1903
Diez-Gonzalez S (2007) N-heterocyclic carbenes. Royal Society of Chemistry, Cambridge
Buil ML, Esteruelas MA, Herrero J, Izquierdo S, Pastor IM, Yus M (2013) ACS Catal 3:2072
Dam JH, Osztrovszky G, Nordstrom LU, Madsen R (2010) Chem Eur J 16:6820
Fujita K, Furukawa S, Morishima N, Shimizu MS, Yamaguchi R (2018) ChemCatChem 10:1993
Wong CM, McBurney RT, Binding SC, Peterson MB, Goncales VR, Gooding JJ, Messerle BA (2017) Green Chem 19:3142
Prades A, Corberan R, Poyatos M, Peris E (2008) Chem Eur J 14:11474
Bartoszewich A, Marcos R, Sahoo S, Inge AK, Zou X, Martin-Matute B (2012) Chem Eur J 18:14510
Marichev KO, Takacs JM (2016) ACS Catal 6:2205
Li JQ, Andersson PG (2013) Chem Commun 49:6131
Shan SP, Xiaoke X, Gnanaprakasam B, Dang TT, Ramalingam B, Huynh HH, Seayad AM (2015) RSC Adv 5:4434
Kaloğlu M, Gürbüz N, Semeril D, Özdemir İ (2018) Eur J Inorg Chem 1236
Yigit M (2009) Molecules 14:2032
Özdemir İ, Yigit M, Çetinkaya E, Çetinkaya B (2006) Heterocycles 68:1371
Yiğit M, Bayram G, Yiğit B, Özdemir İ (2013) Heterocycles 87:897
Wang HMJ, Lin IJB (1998) Organometallics 17:972
Yiğit B, Yiğit M, Özdemir İ, Çetinkaya E (2012) Transit Met Chem 37:297
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yiğit, M., Karaca, E.Ö., Yiğit, B. et al. Ruthenium(II)-NHC-catalyzed (NHC = perhydrobenzimidazol-2-ylidene) alkylation of amines using the hydrogen borrowing methodology under solvent-free conditions. Transit Met Chem 44, 565–573 (2019). https://doi.org/10.1007/s11243-019-00313-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-019-00313-7