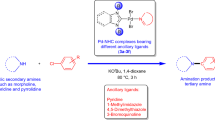
Abstract
An easily available N-heterocyclic carbene–palladium(II) complex was found to be an efficient catalyst for the Buchwald–Hartwig amination of aryl chlorides. Both secondary and primary amines were tolerated under the same reaction conditions. Under the optimal conditions, all reactions proceeded successfully to give the desired products in good to high yields within hours.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The palladium-catalyzed amination of aryl halides (Buchwald–Hartwig amination) is one of the most important and frequently used methods for the formation of C–N bonds and has found widespread applications in organic synthesis [1–12].Footnote 1 Many catalyst systems have been explored in order to extend the scope of the reaction to more substrates, under milder conditions and with lower catalyst loadings. Most published examples concern the use of phosphine palladium complexes as catalysts, providing the corresponding aminated products in good yields [13–20]. Recently, N-heterocyclic carbene (NHC)–palladium complexes [21–27], which have generally higher stabilities toward heat, air and moisture than analogous phosphine palladium complexes, have shown good catalytic activity in the amination of aryl halides. In addition, due to the strong σ-donating ability and sterically demanding character of NHCs, a few NHC-palladium complexes are even very efficient catalysts for the amination of less reactive but more economically attractive aryl chlorides. For example, Wu et al. [28] have developed an air- and moisture-stable carbene–palladacycle complex and found it to be an efficient catalyst for the Buchwald–Hartwig amination of a range of aryl chlorides with amines. The Pd–PEPPSI–NHC complexes [29–33] acting as highly effective pre-catalysts can perform the amination of aryl chlorides under mild conditions. Tu et al. have explored the application of acenaphthoimidazolylidene [34] and benzimidazolylidene [35]. NHC–palladium complexes for the amination of aryl chlorides, and the reaction was found to tolerate a wide range of substrates at low catalyst loadings. Meanwhile, [Pd(NHC)(acac)Cl] complexes [36] readily catalyzed the Buchwald–Hartwig coupling reaction of aryl chlorides with various anilines in excellent yields. The catalytic activities of functionalizable alkoxy-tethered N-heterocyclic carbene–palladium complexes were evaluated for more challenging Buchwald–Hartwig aminations [37]. Additionally, a variety of other N-heterocyclic carbene–palladium complexes have been developed for this purpose, including N-heterocyclic carbene–palladium(II) 4,5-dihydrooxazole complexes [NHC–Pd(II)–Ox] [38], and dinuclear NHC–palladium complexes containing imidazolidin-2-ylidene derivatives as supporting ligands and diphosphine ligands as linkers [39]. During preparation of this manuscript, Lu et al. [40] have reported a new type of N-heterocyclic carbene–PdCl2–(iso)quinoline complex, which proved to be an efficient catalyst for C–N coupling of primary and secondary amines with aryl chlorides at low catalyst loadings. Although these species have been found to be particularly useful as catalysts in the Buchwald–Hartwig amination of aryl chlorides, the development of easily prepared, highly reactive, and stable NHC–Pd(II) complexes still constitutes a challenging endeavor in current organometallic chemistry. Very recently, we reported the first synthesis of N-heterocyclic carbene–palladium(II) complexes 1–4 with benzoxazole or benzothiazole coligands (Scheme 1) [41]. The potential of these complexes in the catalytic Suzuki–Miyaura coupling of aryl chlorides and benzyl chlorides with arylboronic acids was evaluated. Under the optimal reaction conditions, the expected biaryl products were obtained in high yields of up to 99 %. Encouraged by these results, we have extended our investigations of the catalytic potential of these complexes. Herein, we report their application in the Buchwald–Hartwig amination of aryl chlorides with amines.
Experimental
General remarks
Solvents were dried by standard methods and freshly distilled prior to use if needed. All other chemicals were used as purchased. The N-heterocyclic carbene–palladium(II) complexes were synthesized according to our previous report [41]. Preparations of the complexes as well as all the catalytic reactions were carried out under a nitrogen atmosphere. NMR spectra were recorded on a Bruker DPX 400 instrument using TMS as an internal standard.
General procedure for the catalytic amination
A Schlenk flask was charged with the required aryl chloride (0.25 mmol), amine (0.30 mmol), N-heterocyclic carbene–palladium(II) complex (2 mol%), KOtBu (1.3 equiv), and toluene (0.5 mL). The mixture was stirred at 110 °C for 15 h under N2. After cooling, the mixture was evaporated and the product was isolated by preparative TLC on silica gel plates. The purified products were identified by 1H NMR spectra, and their analytical data are given in the Supporting Information.
Results and discussion
In order to test the catalytic activities of these N-heterocyclic carbene–palladium(II) complexes 1–4, initial experiments were carried out using chlorobenzene (0.25 mmol) and morpholine (0.30 mmol) as the reactants, plus complex 1 (2.0 mol%) as the catalyst, in toluene (0.5 mL) at 110 °C, with the results shown in Table 1. The choice of base proved to have an important influence on the reaction (Table 1, entries 1–6) [42]. The reaction proceeded efficiently only when KOtBu was used as base, giving the corresponding coupling product 5a in 91 % yield in this case (Table 1, entry 1). When 3.0 mol% of complex 1 was tested, the yield was not enhanced further (Table 1, entry 7). However, by prolonging the reaction time to 15 h the product 5a was obtained in a 99 % yield (Table 1, entry 8). Complex 3 also showed good catalytic activity (Table 1, entry 10), although slightly lower than that of complex 1, indicating that bulkier NHC ligands make a more reactive catalyst. Interestingly, complexes 2 and 4 were much less efficient than 1 and 3 under identical conditions (entry 8 vs. entry 9; entry 10 vs. entry 11) which might be ascribed to the different coordination abilities of benzoxazole and benzothiazole. It is worth mentioning that a related complex, (IPr)Pd(acac)Cl when used as a catalyst for these reaction at 50 °C in dimethyl ether gave similar yields after 30 min [43] (Scheme 2).
With the optimized conditions in hand, a series of aryl chlorides were first used as reactants with morpholine to test the generality of the reaction. As shown in Scheme 3, most of the coupling reactions proceeded efficiently to give the corresponding aminated products 5a–m in good to excellent yields. Both electron-donating and withdrawing substituents on the aryl chlorides were tolerated and yields of 71–99 % were obtained for 5a–i. The position of the aryl substituted did not seem to affect the product yields. In particular, the reaction was quite feasible with sterically encumbered ortho-substituted aryl chlorides (5d and 5h), while the very sterically hindered 2-chloro-m-xylene reacted with morpholine giving 71 % isolated yield (5e). Subsequently, when heteroaromatic aryl chlorides such as 2-chloropyridine and 3-chloropyridine were used as the substrates, high yields of the corresponding products were observed (5j and 5k).
We next studied the reactions of 1-chloro-2-methylbenzene with piperidine or N-methylaniline. Unlike the corresponding reaction with morpholine, the yields of 5l and 5m were somewhat lower in these experiments (Scheme 3).
We then turned our attention to the reaction of aryl chlorides with primary amines, giving the results presented in Scheme 4. Each of these reactions gave the corresponding monoaminated products 6a–o in moderate to good yields. In particular, the reaction of 1-chloro-2-methylbenzene with aniline proceeded in excellent yield (6d). This may be due to the ortho-substituent of the aryl chloride preventing formation of the triarylamine. The results showed that 1-chloro-2-methylbenzene could be arylated with electronically diverse primary amines. Highly sterically hindered amines such as 2,4,6-trimethylaniline and 2,6-diisopropylaniline were also tolerated, giving the corresponding aminated products 6k–l in high yields. Electron-donating substituents in the phenyl ring of the primary amine showed some beneficial effect on the yields (6h–m vs. 6n–o). Overall, the results indicated that complex 1 is an efficient catalyst for these reactions.
Conclusion
In summary, the easily available, well-defined N-heterocyclic carbene–palladium(II) complexes 1–4, being derived from the corresponding imidazolium salts, palladium chloride and benzoxazole or benzothiazole, showed good catalytic activity in the Buchwald–Hartwig amination of aryl chlorides. In addition to secondary amines, a series of primary amines performed very well with aryl chlorides using the same catalytic system. Further exploration of these N-heterocyclic carbene–palladium(II) complexes and their catalytic applications in other reactions is in progress.
Notes
For early references on amination reactions see Negishi [9].
References
Bariwal J, Van der Eycken E (2013) Chem Soc Rev 42:9283
Johansson Seechurn CCC, Kitching MO, Colacot TJ, Snieckus V (2012) Angew Chem Int Ed 51:5062
Lundgren RJ, Stradiotto M (2012) Chem Eur J 18:9758
Hartwig JF (2010) Organotransition metal chemistry. University Science Books, Sausalito
Surry DS, Buchwald SL (2008) Angew Chem Int Ed 47:6338
Hartwig JF (2008) Acc Chem Res 41:1534
Lakshman MK (2005) Curr Org Synth 2:83
Beletskaya IP, Averin AD (2004) Pure Appl Chem 76:1605
Negishi EI (ed) (2002) Handbook of organopalladium chemistry for organic synthesis. Wiley-Interscience, New York
Rennels AS, Buchwald SL (1995) Angew Chem Int Ed Engl 34:1348
Louie J, Hartwig JF (1995) Tetrahedron Lett 36:3609
Kosugi M, Kameyama M, Migita T (1983) Chem Lett 12:927
Breitler S, Oldenhuis NJ, Fors BP, Buchwald SL (2011) Org Lett 13:3262
Rodriguez S, Qu B, Haddad N, Reeves DC, Tang W-J, Lee H, Krishnamurthy D, Senanayake CH (2011) Adv Synth Catal 353:533
Lundgren RJ, Sappong-Kumankumah A, Stradiotto M (1983) Chem Eur J 2010:16
Schulz T, Torgorg C, Enthaler S, Schaeffner B, Dumrath A, Spannenberg A, Neumann H, Boerner A, Beller M (2009) Chem Eur J 15:4528
Chen G-S, Lam WH, Fok WS, Lee HW, Kwong FY (2007) Chem Asian J 2:306
Ackermann L, Spatz JH, Gschrei CJ, Born R, Althammer A (2006) Angew Chem Int Ed 45:7627
Shen Q-L, Shekhar S, Stambuli JP, Hartwig JF (2005) Angew Chem Int Ed 44:1371
Liu D, Gao W-Z, Dai Q, Zhang X-M (2005) Org Lett 7:4907
Menon RS, Biju AT, Nair V (2015) Chem Soc Rev 44:5040
Budagumpi S, Haque RA, Salman AW (2012) Coord Chem Rev 256:1787
Yuan D, Huynh HV (2012) Molecules 17:2491
Fortman GC, Nolan SP (2011) Chem Soc Rev 40:5151
Droge T, Glorius F (2010) Angew Chem Int Ed 49:6940
Diez-Gonzalez S, Marion N, Nolan SP (2009) Chem Rev 109:3612
Nair V, Vellalath S, Babu BP (2008) Chem Soc Rev 37:2691
Li J, Cui M, Yu A, Wu Y (2007) J Organomet Chem 692:3732
Sharif S, Rucker RP, Chandrasoma N, Mitchell D, Rodriguez MJ, Froese RDJ, Organ MG (2015) Angew Chem Int Ed 54:9507
Pompeo M, Farmer JL, Froese RDJ, Organ MG (2014) Angew Chem Int Ed 53:3223
Zhang Y, Lavigne G, César V (2015) J Org Chem 80:7666
Zhang Y, César V, Lavigne G (2015) Eur J Org Chem 2015:2042
Zhang Y, César V, Storch G, Lugan N, Lavigne G (2014) Angew Chem Int Ed 53:6482
Tu T, Fang W, Jiang J (2011) Chem Commun 47:12358
Fang W, Jiang J, Xu Y, Zhou J, Tu T (2013) Tetrahedron 69:673
Duc GL, Meiries S, Nolan SP (2013) Organometallics 32:7547
Krinsky JL, Martinez A, Godard C, Castillon S, Claver C (2014) Adv Synth Catal 356:460
Huang P, Wang Y-X, Yu H-F, Lu J-M (2014) Organometallics 33:1587
Yang J, Li P, Zhang Y, Wang L (2014) J Organomet Chem 766:73
Liu F, Zhu Y-R, Song L-G, Lu J-M (2016) Org Biomol Chem 14:2563
Wang T, Xie H, Liu L, Zhao W-X (2016) J Organomet Chem 804:73
Ouyang K, Xi Z (2013) Acta Chim Sin 71:13
Navarro O, Marion N, Scott NM, Gonzάlez J, Amoroso D, Bell A, Nolan SP (2005) Tetrahedron 61:9716
Acknowledgments
We are grateful to the National Natural Sciences Foundation of China (Nos. U1404205 and 21202095), the Program for Science & Technology Innovation Talents in Universities of Henan Province (14HASTIT016) and Innovation Scientists and Technicians Troop Construction Projects of Henan Province for financial support.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, T., Xu, K., Liu, L. et al. An easily available N-heterocyclic carbene–palladium(II) catalyst for Buchwald–Hartwig amination of aryl chlorides. Transit Met Chem 41, 525–529 (2016). https://doi.org/10.1007/s11243-016-0048-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-016-0048-1