Abstract
Potato is an important crop in terms of its nutritional value, cost-effective production, versatile utilization, and starch production. To produce disease-free potato starting material, the production of microtubers in vitro is a viable solution. The process of tuberization is a multifaceted physiological phenomenon that is regulated by a multitude of factors, including environmental conditions, nitrogen availability, plant growth regulators (PGRs), genetic makeup, nutrient availability, photoperiodic, temperature, explant source, potato cultivar, and sucrose. In this study, we evaluated the impact of diverse light qualities and photoperiods on in vitro microtuberzation of potato single node explant. Based on our finding, in comparison with white (W: control), the inclusion of the far-red wavelength in a red-blue light (RBF) in prolonged photoperiod significantly increased both tuberization percentage (18%) and tuberization degree (60%) in Sante® cultivar. Additionally, in comparison with W light at 16 h photoperiod, the application of RBF led to significant increment in various parameters, such as explant fresh weight (23%), microtuber diameter (40%), microtuber number (47%), and microtuber fresh weight (121%), microtuber dry weight (166%), and microtuber yield (FW and DW; 227%). Similarly, the utilization of the far-red spectrum in combination with the red-blue spectrum resulted in a 27% increase in protein content compared to W (control).
Key message
Potato in vitro microtuberzation was significantly improved when subjected to prolonged photoperiod and the application of far-red spectrum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Potato (Solanum tuberosum L.), boasting a cultivated area of 16 million hectares and a production of over 360 million tons, is a critical staple crop for ensuring food security worldwide (Askari et al. 2023). The demand for this tuberous plant is steadily increasing due to its nutritional value, cost-effective production, versatile utilization, and starch production (Plantenga et al. 2019; Gong et al. 2021).This plant is propagated both sexually (through the acquisition of true seeds) and asexually (via tuber formation). However, for commercial purposes, seed tubers are primarily utilized (Plantenga et al. 2019). Despite all precautions this propagation technique, however, contributes to the spread of pests and diseases, consequently decreasing yield. Although the production of mini-tubers from disease-free plants (i.e., tissue culture plantlets) may be an appropriate approach, this method requires modification due to low multiplication rates and acclimatization challenges faced by the plantlets after transplantation (Yagiz et al. 2020). On the other hand, microtubers (≤ 8 mm) provide several advantages, including germplasm conservation, high storage potential, year-round production, decontaminated propagation, easier acclimatization and handling, rapid and economical multiplication procedures, and maximum yield productivity, making them a suitable option for potato production (Uchendu et al. 2016; Teng et al. 2019; Herrera-Isidron et al. 2021).
The induction of potato microtubers is a subject of interest in research due to the impressive number of factors that can impact this process. Among these factors are light, as noted by Rehman et al. (2020), sucrose, as observed by Herrera-Isidron et al. (2021), genotype, as demonstrated by Mohamed et al. (2018), plant growth regulators, which has been explored by Sauer et al. (2013) and Salem and Hassanein (2017), explant type, as studied by Yagiz et al. (2020), gelling agent, as reported by Arregui et al. (2003), and nutrition, as highlighted by Rahman et al. (2015). Light, as a crucial source of energy, represents one of the most significant environmental factors that elicit morphological and physiological responses in potatoes (Jiang et al. 2019). The quality and quantity of light exert a considerable influence on plant growth and morphology (Brini et al. 2022). Notably, red-blue mixed light has been documented as the most effective spectral composition for promoting the growth and tuberization of potato plantlets (Xu et al. 2022), although certain evidence suggests that white light can enhance tuberization and plantlet growth (Chen et al. 2018). Furthermore, the quantity of light, known as the photoperiod, plays a crucial role in potato growth (Raigond et al. 2019), tuberization (Kondhare et al. 2021), photosynthesis, stomatal activity (Wheeler et al. 2019), and hormonal responses (Chincinska et al. 2013). This research aimed to investigate the effects of light spectra and photoperiod on in vitro microtuberization of potato, for determining the optimum light and photoperiod combination to enhance microtuberzation processes.
Materials and methods
Plant materials and culture conditions
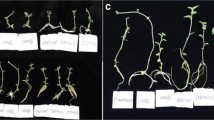
In this study, the high-yielding cultivar of potato (Solanum tuberosum cv. Sante) was selected (Askari et al. 2023). A single nodal segment (1.5 cm) was excised from greenhouse-grown plants (Fig. 1. I) and used as an explant. The explants were subjected to decontamination procedures involving the use of 1% sodium hypochlorite and tween® 20 (a few drops), followed by triple rinsing with sterile distilled water (Askari and De Klerk 2020; Askari et al. 2022; Askari and Visser 2022). The ex vitro explants (5 explants vessel-1) were cultured on free hormone MS medium (Murashige and Skoog 1962) containing 30 g L− 1 sucrose, 0.4 mg L− 1 thiamine; 100 mg L− 1 myo-inositol; 7 g L− 1 microagar. The MS medium was dispensed (30 ml) into a glass vessel (10 × 5 cm) and sealed with transparent lids then maintained at a temperature of 25 ± 2 ºC for two weeks at 30 µmol m− 2 s− 1 light (Philips TL 33) for 16 h d-1. The obtained in vitro potato shoots from ex vitro explants were cut to single node (1 cm) and subcultured (once per two weeks) at the same medium and conditions till enough in vitro explants were produced (Fig. 1. II). For the microtuberzation experiment the in vitro single node explants were cultured on MS medium (Murashige and Skoog 1962) containing 80 g L− 1 sucrose, 0.4 mg L− 1 thiamine; 100 mg L− 1 myo-inositol; 7 g L− 1 microagar and 2 mgl-1 BA. MS medium (30 ml) was poured into glass vessel (10 × 5 cm) and sealed with transparent lids (Fig. 1. III and IV) then maintained at a temperature of 25 ± 2 ºC for two months under different light treatments including blue (B; 400–500 nm), white [W; 41% blue (400–500 nm), 39% intermediate (500–600 nm), and 20% red (600–700 nm)], red and blue [RB; red (80%) and blue (20%)], red (R; 600–700 nm), Red-Blue-Far-red [RBF; 70% R, 20% B, and 10% F] plus darkness (Fig. 2). The photon flux density of LED lamps (Guangzhou Grow Light Company Model IGL-158‐18R17B7‐DC; Input voltage: 220–240 V; 18 W; 0.09 A) was adjusted to 100 µmol m-2 s-1 at the explant level, for 16 h d-1 and 8 h d-1 for all light qualities.
The steps involved in in vitro microtuberization of Potato (Solanum tuberosum cv. Sante) I) Potato cultivated in a greenhouse to supply ex vitro explants II) Shoot multiplication in MS medium (free-hormone) for providing in vitro explants (III) The nodal explants were cultured on MS medium within a glass vessel and sealed with a transparent lid and incubated under light and photoperiod treatments (IV) Nodal explants produced microtuber after eight weeks (V) Single node explant induced microtuber on stolon (VI) Microtubers ready for cultivation
Explant and microtuberization traits
Two months subsequent to the commencement of the experiment, an assessment was conducted on the factors pertaining to explant and induced microtubers (Fig. 1.V and VI). The parameters considered were the fresh weight (FW) of the explant, as well as the fresh and dry weights (DW) of the microtubers, microtuber diameter, and the number of induced microtubers. All the fresh weights were measured with analytical balance (± 0.01 g; MXX-412; Denver Instruments, Bohemia, NY, USA). To obtain the dry weights, the microtubers and explants were subjected to a drying process in a forced-air drying oven. Initially, they were dried at 105 °C for duration of 3 h, followed by further drying at 80 °C for over 72 h until no further reduction in weight was observed. The microtuber diameter was measured with digital caliper (Mitutoyo).
The percentage of tuberization under in vitro conditions was derived by applying this formula:
Tuberization percent (%) = (number of explants with microtuber / Total number of explants) x 100.
For determination of tuberization degree, the method of Veramendi et al. (2000) was employed, wherein the microtubers were assessed on a range of 0 to 4, taking into account the status of their induction on a single node explant. 0, representing non-stolon or microtuber non-induction; 1, indicating stolon development; 2, representing microtuber induction on shoot; 3, indicating microtuber induction on stolon; and 4, representing sessile microtuber.
The microtuber yield (Chen et al. 2018) was measured by sum of fresh weight and dry weight of total microtubers produced in each tissue culture vessel.
Biochemical properties
To determine the starch content of microtubers, 200 mg of the dried microtubers were ground and transferred into a test tube. Subsequently, 100 µl of distilled water (this step was carried out on ice) was added. Following this, 130 µl of 52% perchloric acid was introduced into the mixture. The test tubes were then placed in an ice bath for duration of 15 min. Afterward, the supernatant liquid was carefully separated using a 5000 rpm centrifuge for a period of 10 min, and subsequently transferred to another test tube. The pelleted sediments were treated with 250 µl distilled water and 750 µl of 52% perchloric acid. The resulting mixture was subjected to centrifugation at 5000 rpm for 10 min. After centrifugation, 2 ml of the supernatant liquid were mixed with 3 ml of anthrone reagent. This mixture was then placed in a Bain-Marie device and incubated at a temperature of 100 °C for 20 min. After cooling, the solution was analyzed using a spectrophotometer (UV-1601) with a wavelength of 620. This method was adapted from the work of Fairbairn (1953).
To determine the concentration of soluble sugar, 100 mg dried microtubers were ground. Subsequently, one ml of double distilled water was added to the grinded microtubers, then the mixture was transferred to a small beaker. The beaker was then placed in a bain-marie and heated at a temperature of 100 °C for duration of 20 min. Once cooled, the mixture was transferred to microtubes and subjected to centrifugation at a speed of 10,000 rpm for 15 min. Following centrifugation, 50 ml of the supernatant liquid was taken and mixed with 950 ml of concentrated sulfuric acid on ice. The resulting mixture was then analyzed using a Spectrophotometer (UV-1601) at a wavelength of 315 (Albalasmeh et al. 2013).
To determine the protein content of microtubers, 100 gr dried microtubers were ground. Subsequently, one ml of phosphate buffer (50 mM, pH = 7.8, containing 1 mM EDTA, 2% of polyvinyl polypyrrolidone (PVP) and 0.1% triton X-100) was added to the grinded microtubers. The resulting mixture was then transferred into a test tube and placed on ice. Then the mixture was subjected to centrifugation at a speed of 12,000 rpm for duration of 20 min at a temperature of 4 °C. Following the centrifugation, 10 ml of the supernatant liquid was mixed with 200 ml of bioreagent and 790 ml of phosphate buffer. After allowing the mixture to incubate for 10 to 15 min, its absorbance was measured using a spectrophotometer (UV-1601) at a wavelength of 595 (Bradford 1976).
Statistical analysis
The present investigation was executed as a factorial experiment in accordance with the principles of a completely randomized design (CRD), involving two factors, namely light quality (Fig. 2) and photoperiod (8 and 16 h d-1). Five different light qualities were assessed plus darkness including: {blue (B; having a peak at λ = 460 nm), white [W; consisting of 41% B (400–500 nm), 39% intermediate (500–600 nm), and 20% R (λ = 660 nm)], red-blue [RB; 80% R and 20% B], Red-Blue-Far-red [RBF; 70% R, 20% B, and 10% F], red (R)}. The experimental design was replicated three times with fifteen explants per replicate. For statistical analyses, analysis of variance (ANOVA), normality test and Tukey’s multiple range test (P ≤ 0.01) to compare means were conducted using the SAS software (Version 9.0).
Results
Explant and tuberization traits
The findings presented in Table 1 revealed that the influence of light treatment and photoperiod as well as the interaction between light treatment and photoperiod showed statistically significant differences in various parameters, such as explant fresh weight, microtuber diameter, tuberization percentage, tuberization degree, and microtuber number (Table 1).
Applying of RBF resulted in a significant increase in the fresh weight of explants, in comparison to the other treatments. Specifically, the explants that were exposed to RBF at 16 h photoperiod exhibited the highest level of fresh weight, as indicated by the data presented in Table 2. Extending the photoperiod from 8 to 16 h significantly results in a 44% and 68% increase in the fresh weight of the explant in RB and RBF, respectively. However, the increase in light duration did not show a significant impact on explant fresh weight in R and B. Notably, extending the photoperiod in RBF light led to a 50% increase in the fresh weight of the explant compared to W. It is worth mentioning that all light treatments demonstrated better performance than the control (W) at 8 h photoperiod.
Conversely, at 16 h photoperiod, only RBF exhibited superior performance, with a 23% increase in the fresh weight of the explants compared to W light.
Except W, the diameter of microtuber exhibited a notable augmentation when the photoperiod was extended to 16 h. Specifically, in the presence of RBF light, an impressive 70% increase in microtuber diameter was observed at 16 h photoperiod. It is noteworthy that RBF recorded the largest microtuber diameter (3.85 mm) at 16 h photoperiod, while R resulted in the smallest diameter (1.93 mm) at 8 h photoperiod.
The percentage of tuberization was significantly influenced by both light treatment and the photoperiod. All light treatments resulted in a significant increase in tuberization percentage when the photoperiod was extended to 16 h. The highest tuberization percentage was observed in B and RBF at 16 h photoperiod with values of 75% and 73% respectively. In contrast, at 8 h photoperiod, RBF exhibited the highest tuberization percentage (51.9%), while R showed the lowest tuberization percentage (41%). RBF recorded the highest tuberization percentage (90%), whereas RB presented the lowest tuberization percentage (52%) at 16 h photoperiod. Furthermore, RBF increased the tuberization percentage by 18% compared to W at 16 h photoperiod.
The tuberization degree remained unaffected by the light treatment at 8 h photoperiod. However, when the explants were exposed to a 16 h photoperiod, the highest tuberization degree was observed in the presence of B (37%) and RBF (41%).
The microtuber number increased significantly while photoperiod extended to 16 h across all light treatments. The most notable improvement was observed in B (97%) and RBF (106%). Additionally, the highest microtuber number was produced in RBF at 16 h photoperiod, with 5.46 microtubers vessel-1 (Table 2).
The data presented in Table 3 revealed that the influence of light treatment and the photoperiod as well as the interaction between light treatment and photoperiod exert a substantial statistically significant impact in various parameters, such as microtuber fresh weight, microtuber dry weight, yield fresh weight and yield dry weight (Table 3).
The fresh weight of microtubers was significantly influenced by both light treatment and photoperiod. All light treatments resulted in a significant increase in the fresh weight of microtubers when the photoperiod was extended to 16 h. The most notable improvement, with a 115%, 70% and 60% increase, was observed in the B and RB and RBF respectively.
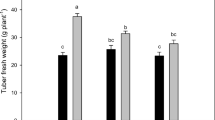
Effect of light treatments including blue (B; 400–500 nm), white [W; 41% blue (400–500 nm), 39% intermediate (500–600 nm), and 20% red (600–700 nm)], red and blue [RB; red (80%) and blue (20%)], red (R; 600–700 nm), Red-Blue-Far-red [RBF; 70% R, 20% B, and 10% F] and photoperiods (0 [darkness], 8 and 16 h) on microtuber fresh weight (A), dry weight (B), yield (fresh weight) (C) and yield (dry weight) (D) in potato in vitro microtuberzation
The highest fresh weight of microtubers, reaching 58.6 mg per microtuber, was achieved in the RBF at 16 h photoperiod. Additionally, the RBF resulted in a 60% and 121% increase in the fresh weight of microtubers compared to the W at 8 and 16 h photoperiod respectively (Fig. 3A).
The highest dry weight of microtubers (13.6 mg microtuber-1) was observed under RBF at 16 h photoperiod. This weight was 166% higher than that observed under W for the same photoperiod. Furthermore, across all light treatments, extending the photoperiod from 8 to 16 h resulted in a significant increase in the dry weight of microtubers. The most substantial increases were observed under B and RBF, with a 127% and 88% increase, respectively (Fig. 3B).
The yield (FW) of microtubers vessel-1 was significantly influenced by the light treatment and photoperiod. The RBF produced the highest yield (FW) with 320.57 mg vessel-1 when exposed to 16 h photoperiod. All light treatments resulted in an increase in yield (FW) when the photoperiod was extended to 16 h. The greatest improvement was observed in B (324%) and RBF (235%). In both photoperiods, RBF increased the yield (FW) by 65% and 227% in comparison to W at 8 and 16 h photoperiod, respectively (Fig. 3 C). The yield (DW) showed similar trends as the yield (FW). Extending the photoperiod to 16 h resulted in a 324% and 232% increase in yield (DW) in B and RBF, respectively. The highest yield (DW) was achieved in RBF at 16 h photoperiod with 64.11 mg DW vessel-1 (Fig. 3D).
Biochemical properties
The data presented in Table 3 revealed that the influence of light treatment and the photoperiod as well as the interaction between light treatment and photoperiod exert a substantial statistically significant impact on sucrose and protein content of the induced microtubers (Table 3). In the realm of starch, light treatment and photoperiod exhibited a statistically significant impact on the accumulation of starch in the induced microtubers. However, the interaction between light treatment and photoperiod did not yield a significant effect on the accumulation of starch in the microtubers (Table 3).
The impact of photoperiod on sugar accumulation was found to be dependent on the quality of light, as evidenced by the results in Table 4. Red light was determined to be the most effective in promoting the accumulation of sugar by 35% in potato microtubers at 16 h photoperiod. Moreover, in comparison to the control (W), the R light led to an augmentation in the accumulation of sugar, attaining a rise of 20% at 16 h photoperiod (Table 4).
According to the findings presented in Table 4, both the light treatment and photoperiod presented a significant impact on the protein content of the microtubers. Across all light treatments, there was a significant increase in protein content when the photoperiod was extended to 16 h. Notably, RB light exhibited a 52% improvement, while RBF light showed an even greater improvement of 65% when the photoperiod was extended. The highest protein content of 3.3 (mg g FW− 1) was observed in B and RBF at 16 h photoperiod. In comparison to W (control), B and RBF resulted in a 27% increase in protein content at 16 h photoperiod (Table 4).
The microtubers induced under W light demonstrated the highest starch content, measuring at 20.68 (mg g FW− 1), while the microtubers induced under RBF displayed the lowest starch content, recording a value of 19.17 (mg g FW− 1). Additionally, the microtubers induced under a 16 h photoperiod exhibited the greatest quantity of starch content, reaching 20.95 (mg g FW− 1) (Fig. 4).
Effect of light treatments including blue (B; 400–500 nm), white [W; 41% blue (400–500 nm), 39% intermediate (500–600 nm), and 20% red (600–700 nm)], red and blue [RB; red (80%) and blue (20%)], red (R; 600–700 nm), Red-Blue-Far-red [RBF; 70% R, 20% B, and 10% F] (A) and photoperiod (0 [darkness], 8 and 16 h) (B) on microtuber starch content
Discussion
The activity of plant life is stimulated by environmental signals in the form of light wavelengths. Furthermore, the quantity of light of varying wavelengths, acting as primary sources of energy, exerts a significant impact on photosynthesis, which ultimately leads to alterations in plant growth (Yang et al. 2021). The process of tuberization is a multifaceted physiological phenomenon that is regulated by a multitude of factors, including environmental conditions, nitrogen availability, growth regulators (PGRs), genetic makeup, nutrient availability, photoperiodic cycles, temperature, explant source, potato cultivar, and sucrose concentration (Ramawat and Merillon 2013). In vitro tuberization of nodal explants was found to vary depending on exposure to different LED lights, which led to the exploration of the pathway reliant upon the light involved in tuberization (Jiang et al. 2019). The photoperiodic response holds significant importance for plants in perceiving environmental symptoms and plays a crucial role in their development, particularly in regulating tuber formation (Sivakumar et al. 2006). In the context of potato plants, phytochromes serve as the primary photoreceptors for sensing day-length, with phytochromes B and F playing pivotal roles in the modulation of photoperiodic tuberization through the regulation of the CO-FT pathway. The perception of day length by photoreceptors in leaves is followed by the transportation of signals to subterranean stolons, which in turn triggers tuber initiation (Ai et al. 2021). It has been reported that there exists a close relationship between the formation of tubers and the presence of gibberellic acid (GA3) and abscisic acid (ABA) (Xu et al. 1998). Additionally, it has been observed that the inhibition of tuber formation is a direct result of the presence of GA3 (Martínez-García et al. 2001). The findings of our investigation indicate that the potato tuberization in vitro is influenced by both the light source and day length. Of the various light regimes tested, the RBF at a 16 h photoperiod resulted in the highest values for several parameters, including fresh weight (FW) explant, FW microtuber, dry weight (DW) microtuber, microtuber diameter, tuberization degree, tuberization percentage, number of tubers vessel-1, and yield vessel-1 (FW and DW). However, the incorporation of the far-red wavelength in a combination of red and blue light spectra significantly enhanced both the tuberization percentage and tuberization degree, resulting in a 73% and 43% increase, respectively when the photoperiod extended to 16 h. The tuberization percentage was notably enhanced by extending the photoperiod across all light regimes. However, it was observed that light regimes B (75%) and RBF (73%) exhibited a more pronounced response to the extension of day length. The extension of photoperiod has led to a significant enhancement in the majority of the measured variables. Furthermore, adding far red to RB spectrum (RBF) led to notable improvements in various parameters including explant fresh weight (50%), tuber number (39%), as well as microtuber fresh (45%) and dry (86%) weight, when compared to the RB at 16 h photoperiod. Furthermore, it was noted that the explants exposed to the RBF spectra demonstrated a significant rise in the percentage of tuberization (18%) and the fresh weight of the explants (23%). Moreover, there was a notable increase in the diameter of microtubers (40%), tuberization degree (60%), the fresh weight of microtubers (121%), the dry weight of microtubers (166%), yield fresh and dry weight (227%) and the number of microtubers (47%) when compared to the control (W) at 16 h photoperiod. The inhibitory effect of gibberellins on tuber formation in potato has been observed (Melis and van Staden 1984; Xu et al. 1998). This effect is elicited through the application of exogenous gibberellins, which hinder tuber formation (Vreugdenhil and Sergeeva 1999). Specifically, exogenous GA4/7 has been shown to promote stolon elongation and inhibit tuber formation, while exogenous ABA stimulates tuberization and reduces stolon length (Xu et al. 1998). Far-red light has the capacity to impede the biosynthesis of gibberellin hormones, which are crucial for plant growth (Li et al. 2020). The precise mechanism underlying the inhibition of gibberellin biosynthesis by far-red light remains incompletely elucidated, but it is hypothesized to involve the modulation of gene expression and enzymatic activity within the biosynthetic pathway (Sun et al. 2023).
Based on our results, the addition of far-red to a red-blue spectrum (RBF) and blue (B) light resulted in a noteworthy increase of 27% in protein content when compared to the W (control) at 16 h photoperiod. Furthermore, it was observed that the application of W light alleviated the accumulation of starch by 8% and 36% when compared to RBF and darkness respectively. The presence of sugar in microtuber exposed to red (R) light was found to be 21% higher in comparison to the W light at 16 h photoperiod. The regulation of starch, protein and sugar are important factors in determining the growth of tuber tissues in potato plants. The significance of light quality in this process has been recognized (Kang et al. 2013). For instance, it has been reported that blue light induces sugar and starch contents in potato tubers under in vitro conditions (Chen et al. 2020). Additionally, exposure to RBF at longer photoperiod increased the accumulation of protein content (65%) in the microtuber tissues. B and RBF produced the highest amount of protein (3.3 mg g FW-1) when the photoperiod extended to 16 h. On the contrary, the microtubers exhibited the greatest quantity of sugar (11.1 mg g FW-1) when exposed to R light under a 16 h photoperiod, resulting in a 35% increase in sugar content with the extension of the photoperiod to 16 h. These findings are in line with previous research that has demonstrated an enhanced biosynthesis of soluble sugar content under R light (Pundir et al. 2021).
Conclusion
Microtuber production plays a crucial role in the contamination losses, multiplication, and yield of potatoes. The present study aimed to evaluate the impact of photoperiod and light quality on potato (Solanum tuberosum cv. Sante). The findings of this research indicate that incorporating a far red fraction into a RB light significantly improves the fresh and dry weight of microtubers, as well as tuberization traits and protein accumulation in comparison with the other light regimes. This effect is particularly pronounced when the potatoes are exposed to an extended photoperiod. In summary, the inclusion of a far red fraction in the light spectrum can greatly enhance various aspects of microtuber development and yield in potato cultivation. A more in-depth comprehension of the complex molecular pathways affected by the far-red spectrum is necessary in order to elucidate the mechanisms by which it enhances in vitro microtuberzation in potatoes.
Data Availability
The data will be available on a reasonable request.
References
Ai Y, Jing S, Cheng Z et al (2021) DNA methylation affects photoperiodic tuberization in potato (Solanum tuberosum L.) by mediating the expression of genes related to the photoperiod and GA pathways. Hortic Res 8. https://doi.org/10.1038/s41438-021-00619-7
Albalasmeh AA, Berhe AA, Ghezzehei TA (2013) A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr Polym 97:253–261. https://doi.org/10.1016/j.carbpol.2013.04.072
Arregui LM, Veramendi J, Mingo-Castel AM (2003) Effect of gelling agents on in vitro tuberization of six potato cultivars. Am J Potato Res 80:141–144. https://doi.org/10.1007/BF02870213
Askari N, De Klerk GJ (2020) Elimination of epidermal wax from explants increases growth in tissue culture of lily. Sci Hortic (Amsterdam) 274:109637. https://doi.org/10.1016/j.scienta.2020.109637
Askari N, Visser RGF (2022) The role of scale explants in the growth of regenerating lily bulblets in vitro. Plant Cell Tissue Organ Cult 149:589–598. https://doi.org/10.1007/s11240-022-02328-y
Askari N, Aliniaeifard S, Visser RGF (2022) Low CO2 levels are detrimental for in vitro plantlets through disturbance of photosynthetic functionality and accumulation of reactive oxygen species. Horticulturae 8:44. https://doi.org/10.3390/horticulturae8010044
Askari N, Ghahramani R, Reisi A et al (2023) The role of thermal stress on in Vitro Potato Microtuber induction. J Veg Sci 6:73–84. https://doi.org/10.22034/IUVS.2022.562669.1236
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brini F, Mseddi K, Brestic M, Landi M (2022) Hormone-mediated plant responses to light quality and quantity. Environ Exp Bot 202
Chen L, Xue X, Yang Y et al (2018) Effects of red and blue LEDs on in vitro growth and microtuberization of potato single-node cuttings. Front Agric Sci Eng 5:197–205. https://doi.org/10.15302/J-FASE-2018224
Chen L, li ZHANGK, GONG X, chen et al (2020) Effects of different LEDs light spectrum on the growth, leaf anatomy, and chloroplast ultrastructure of potato plantlets in vitro and minituber production after transplanting in the greenhouse. J Integr Agric 19:108–119. https://doi.org/10.1016/S2095-3119(19)62633-X
Chincinska I, Gier K, Krügel U et al (2013) Photoperiodic regulation of the sucrose transporter StSUT4 affects the expression of circadian-regulated genes and ethylene production. Front Plant Sci 4. https://doi.org/10.3389/fpls.2013.00026
Fairbairn NJ (1953) A modified anthrone reagent. Chem Ind 4:86
Gong HL, Dusengemungu L, Igiraneza C, Rukundo P (2021) Molecular regulation of potato tuber dormancy and sprouting: a mini-review. Plant Biotechnol Rep 15:417–434
Herrera-Isidron L, Valencia-Lozano E, Rosiles-Loeza PY et al (2021) Gene expression analysis of microtubers of potato solanum tuberosum l. Induced in cytokinin containing medium and osmotic stress. Plants 10. https://doi.org/10.3390/plants10050876
Jiang L, Wang Z, Jin G et al (2019) Responses of Favorita Potato plantlets cultured in Vitro under fluorescent and light-emitting diode (LED) light sources. Am J Potato Res 96:396–402. https://doi.org/10.1007/s12230-019-09725-8
Kang JH, KrishnaKumar S, Atulba SLS et al (2013) Light intensity and photoperiod influence the growth and development of hydroponically grown leaf lettuce in a closed-type plant factory system. Hortic Environ Biotechnol 54:501–509. https://doi.org/10.1007/s13580-013-0109-8
Kondhare KR, Kumar A, Patil NS et al (2021) Development of aerial and belowground tubers in potato is governed by photoperiod and epigenetic mechanism. Plant Physiol 187:1071–1086
Li W, Liu SW, Ma JJ et al (2020) Gibberellin signaling is required for far-red light-induced shoot elongation in pinus tabuliformis seedlings1. Plant Physiol 182:658–668. https://doi.org/10.1104/pp.19.00954
Martínez-García JF, García-Martínez JL, Bou J, Prat S (2001) The interaction of gibberellins and photoperiod in the control of potato tuberization. J Plant Growth Regul 20:377–386. https://doi.org/10.1007/s003440010036
Melis R, van Staden J (1984) Tuberization and hormones. Z für Pflanzenphysiologie 113:271–283. https://doi.org/10.1016/s0044-328x(84)80008-2
Mohamed F, Omar G, Abd El-Hamed K, El-Safty B (2018) Influence of Plant Density and genotype on Potato Minituber Production from Microshoots and microtubers. Catrina Int J Environ Sci 17:77–84. https://doi.org/10.21608/cat.2018.14317
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with Tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/J.1399-3054.1962.TB08052.X
Plantenga FDM, Bergonzi S, Abelenda JA et al (2019) The tuberization signal StSP6A represses flower bud development in potato. J Exp Bot 70:925–936. https://doi.org/10.1093/jxb/ery420
Pundir RK, Pathak A, Upadhyaya DC et al (2021) Red and blue light-emitting diodes significantly improve in vitro tuberization of potato (Solanum Tuberosum L). J Hortic Res 29:95–108. https://doi.org/10.2478/johr-2021-0010
Rahman MZ, Shahinul Islam SM, Chowdhury AN, Subramaniam S (2015) Efficient microtuber production of potato in modified nutrient spray bioreactor system. Sci Hortic (Amsterdam) 192:369–374. https://doi.org/10.1016/j.scienta.2015.06.014
Raigond P, Buckseth T, Singh B et al (2019) Influence of photoperiod and EDTA salts on endogenous gibberellic acid concentration of tissue culture grown potato microplants. Agric Res 8:176–183. https://doi.org/10.1007/s40003-018-0364-0
Ramawat K, Merillon J (2013) Bulbous plants: biotechnology. CRC Press
Rehman M, Fahad S, Saleem MH et al (2020) Red light optimized physiological traits and enhanced the growth of ramie (Boehmeria nivea L). Photosynthetica 58:922–931. https://doi.org/10.32615/ps.2020.040
Salem J, Hassanein AM (2017) In vitro propagation, microtuberization, and molecular characterization of three potato cultivars. Biol Plant 61:427–437. https://doi.org/10.1007/s10535-017-0715-x
Sauer M, Robert S, Kleine-Vehn J (2013) Auxin: simply complicated. J Exp Bot 64:2565–2577
Sivakumar G, Heo JW, Kozai T, Paek KY (2006) Effect of continuous or intermittent radiation on sweet potato plantlets in vitro. J Hortic Sci Biotechnol 81:546–548. https://doi.org/10.1080/14620316.2006.11512101
Sun R, Okabe M, Miyazaki S et al (2023) Biosynthesis of gibberellin-related compounds modulates far-red light responses in the liverwort Marchantia polymorpha. bioRxiv
Teng Y, Zhang Y, Guo JT et al (2019) Acid pretreatment improves microtuberization of potato plantlets. Vitr Cell Dev Biol - Plant 55:36–43. https://doi.org/10.1007/s11627-018-09950-6
Uchendu EE, Shukla M, Saxena PK, Keller JER (2016) Cryopreservation of potato microtubers: the critical roles of sucrose and desiccation. Plant Cell Tissue Organ Cult 124:649–656. https://doi.org/10.1007/s11240-015-0916-y
Veramendi J, Sota V, Fernandez-San Millan A et al (2000) An in vitro tuberization bioassay to assess maturity class of new potato clones. J Hortic Sci Biotechnol 75:733–738. https://doi.org/10.1080/14620316.2000.11511315
Vreugdenhil D, Sergeeva LI (1999) Gibberellins and tuberization in potato. Potato Res 42:471–481. https://doi.org/10.1007/BF02358163
Wheeler RM, Fitzpatrick AH, Tibbitts TW (2019) Potatoes as a crop for Space Life Support: Effect of CO2, irradiance, and Photoperiod on Leaf Photosynthesis and Stomatal Conductance. Front Plant Sci 10. https://doi.org/10.3389/fpls.2019.01632
Xu X, Van Lammeren AAM, Vermeer E, Vreugdenhil D (1998) The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol 117:575–584. https://doi.org/10.1104/pp.117.2.575
Xu JM, Liu Y, Liu MX, Xu ZG (2022) Proteomic, physiological, and anatomical analyses reveal the effects of Red, Blue, and White Light on the growth of Potato plantlets under in Vitro Culture. Russ J Plant Physiol 69. https://doi.org/10.1134/S1021443722060346
Yagiz AK, Yavuz C, Tarim C et al (2020) Effects of Growth regulators, media and explant types on microtuberization of Potato. Am J Potato Res 97:523–530. https://doi.org/10.1007/s12230-020-09801-4
Yang L, Fanourakis D, Tsaniklidis G et al (2021) Contrary to red, blue monochromatic light improves the bioactive compound content in broccoli sprouts. Agronomy 11. https://doi.org/10.3390/agronomy11112139
Acknowledgements
The authors acknowledge University of Jiroft for their supports, Mehdi Seif and Pegah Behzadi Rad are also appreciated for their scientific cooperation.
Funding
This work was supported by University of Jiroft (Iran).
Author information
Authors and Affiliations
Contributions
N.A. designed and directed the experiments. N.A. and A.R., M.S.H. and B.P. fulfilled the project. N.A analyzed the data and N.A., R.G drafted the manuscript. All authors have perused and consented to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No possible conflict of interest was announced by the authors.
Additional information
Communicated by Jose M. Segui-Simarro.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Reisi, A., Askari, N., Sadat‑Hosseini, M. et al. Far-red spectrum leads to enhanced in vitro microtuberzation in potato (Solanum tuberosum cv. Sante). Plant Cell Tiss Organ Cult 156, 45 (2024). https://doi.org/10.1007/s11240-023-02673-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11240-023-02673-6