Abstract
The light spectrum has a strong effect on potato tuber development. To investigate the effect of varying the spectrum on potato tuber growth and yield, potato plantlets were transplanted under red/white light (RW; red light: 200 μmol m−2 s−1; white light: 100 μmol m−2 s−1), blue/white light (BW; blue light: 200 μmol m−2 s−1; white light: 100 μmol m−2 s−1), and white/red/blue light (WRB; white light: 100 μmol m−2 s−1; red light: 100 μmol m−2 s−1; blue light: 100 μmol m−2 s−1) with a 11/13-h (light/dark) photoperiod. Potato plants grown under RW had the highest mean fresh tuber weight and total yield among all treatments. Plants under RW resulted in the highest shoot dry weight at 30 d and 45 d among all treatments, providing a photosynthetic source for the tubers. Furthermore, the photosynthetic leaves under RW had a significantly higher total chlorophyll content than other treatments at 90 d. Plants under RW significantly increased tuber fresh weight per plant by 57.7% from 60 to 90 d. The spectrum of BW benefited potato bulking. Plants under BW at 40 d resulted in significant sucrose and starch changes between day and night compared with those at 20 d. BW had a positive effect on the activities of ADP-glucose pyrophosphorylase at 40 d and sucrose synthase at 60 d compared with RW and WRB during the day. Thus, the ratio of tuber (fresh weight of tuber ≥2.0 g) under BW was significantly higher than that under RW and WRB. The total yield of tubers under WRB was the lowest among all treatments.
Resumen
El espectro de luz tiene un fuerte efecto en el desarrollo del tubérculo de papa. Para investigar el efecto de variación del espectro en el crecimiento y rendimiento del tubérculo en papa, se trasplantaron plántulas de papa bajo luz roja/blanca (RW; luz roja: 200 μmol m-2 s-1; luz blanca: 100 μmol m-2 s-1), luz azul/blanca (BW; luz azul: 200 μmol m-2 s-1; luz blanca: 100 μmol m-2 s-1), y luz blanca/roja/azul (WRB; luz blanca: 100 μmol m-2 s-1; luz roja: 100 μmol m-2 s-1; luz azul: 100 μmol m-2 s-1) con un fotoperíodo de 11/13-h (luz/oscuridad). Las plantas de papa que crecieron bajo luz RW tuvieron la media mas alta de peso fresco de tubérculo y de rendimiento total entre todos los tratamientos. Las plantas bajo RW resultaron en el mayor peso seco de tallo a los 30 y 45 d entre todos los tratamientos, suministrando una fuente fotosintética para los tubérculos. Aun más, las hojas fotosintéticas bajo RW tuvieron significativamente mas alto contenido total de clorofila que los otros tratamientos a los 90 d. Las plantas bajo RW incrementaron significativamente el peso fresco de tubérculo por planta en 57.7% de 60 a 90 d. El espectro de BW benefició el llenado del tubérculo. Las plantas bajo BW a los 40 d resultaron en significativos cambios en sacarosa y almidón entre el día y la noche en comparación con las de 20 d. BW tuvo un efecto positivo en la actividad de ADP-glucosa pirofosforilasa a los 40 d y de sacarosa sintetasa a los 60 d en comparación con RW y WRB durante el día. Entonces, la relación de tubérculo (peso fresco de tubérculo ≥2.0 g) bajo BW fue significativamente mayor que bajo RW y WRB. El rendimiento total de tubérculos bajo WRB fue el menor entre todos los tratamientos.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Potato (Solanum tuberosum L.) is widely cultivated worldwide. Potatoes are propagated asexually by tubers. However, long-term asexual reproduction can cause deterioration of growth potential, dwarfing of plants, shrinking of potato tubers, and deformity. Therefore, potato seeds are cultivated using virus-free potato plantlets in the greenhouse. Traditional greenhouses can be difficult environments to cultivate potatoes because of natural climate constraints, severe continuous cropping obstacles, and a decrease in the breeding base. With the development of plant factories, plants can grow in an ideal environment in artificial systems. Crops can keep phenotypic stability and had great potential to increase the yield in plant factories (Kyoko and Hiroshi 2016; Folta 2019). Thus, potato seeds are cultivated in plant factories for better yields.
An artificial light environment system is an essential part of plant factories. Light systems are used to provide the spectrum, light intensity, and photoperiod needed to meet the plant’s needs. For instance, red light affects the morphology of potato plantlets e.g., plant height and leaf area (Miyashita et al. 1997; Lee et al. 2011), whereas blue light increases the cell surface area and number of chloroplasts and mitochondria per cell (Gukasyan et al. 1994). Furthermore, red light and blue light are the main energy sources for carbon dioxide assimilation and have primary impacts on plant growth and carbohydrate biosynthesis (Lin et al. 2013). In addition, simultaneous provision of blue and red light is essential for optimum potato plantlet growth (Jao and Fang 2004). Blue and red light are necessary for healthy transplants and tuber yield at the nursery stage (Kim and Lee 2004). The enlargement of the storage root in radishes requires red and blue light (Zha and Liu 2018). However, few studies have been performed on the effects of red and blue light on the entire growth cycle of potatoes. In nature, plants always grow in broad-spectrum sunlight, which controls photo-morphogenesis (Briggs 1993). The spectral components are interdependent (Chen et al. 2016), which allows for the acclimatization of plants to the natural environment. Lin et al. (2013) reported that canopy transmission capacity is limited to red and blue light, whereas other spectra in white light are beneficial for photosynthesis of the lower leaves. Therefore, supplementing with white light is advantageous for the production cycle of crops in plant factories compared with a spectrum comprising only red and blue light.
The process of tuberization starts with the production of photosynthates via photosynthesis, which are distributed to the tubers. Sucrose is the primary photosynthate, as a carbon source for plant energy carriers. The large amount of carbohydrates that accumulates during the day is deposited transiently in chloroplasts in the form of starch and is degraded at night into sugars for growth. Sucrose-phosphate synthase (SPS) plays a major role in sucrose biosynthesis (Huber and Huber 1996). Studies show that sucrose synthase (SS) is necessary for starch synthesis (Stein and Granot 2019; Baroja-Fernández et al. 2012), and ADP-glucose pyrophosphorylase (AGPase) is important for starch biosynthesis. Reduced AGPase activity leads to low-starch, high-sucrose tubers. SPS and AGPase activities in leaves affect sucrose and transient starch biosynthesis (Müller-Röber et al. 1992). The activities of SS and AGPase mainly reflect starch metabolism in potato tubers. Therefore, this study used red and blue light as the basal light and white light as supplemental light to research their effects on potato plants according to plant growth, carbohydrate content, and the activities of critical enzymes during potato development.
Materials and Methods
Light-Emitting Diode (LED) Settings
All LED devices used in this study were designed at the College of Agriculture, Nanjing Agricultural University, Nanjing, China. The three spectral treatments were red/white light (RW; red light: 200 μmol m−2 s−1, white light: 100 μmol m−2 s−1; red light peak wavelength: 660 nm), blue/white light (BW; blue light: 200 μmol m−2 s−1, white light: 100 μmol m−2 s−1; blue light peak wavelength: 450 nm), and white/red/blue light (WRB; white light: 100 μmol m−2 s−1, red light: 100 μmol m−2 s−1, blue light: 100 μmol m−2 s−1). The photoperiod was 11/13 h (light/dark). The distance between LED panels and the plant canopy was 15 cm. Light intensity was measured using a spectroradiometer (OPT-2000; ABDPE Co., Beijing, China), and is shown in Table 1. The combination of the LED lighting and ventilation systems maintained the environmental temperature.
Plant Materials and Growth
Solanum tuberosum cv. Favorita was selected for the experiments, and was obtained from Nanjing Agricultural University. Stem segments (1–2 cm in length with one leaf) were dissected vertically from virus-free potato plantlets in vitro. Then, they were grown in vitro using Murashige and Skoog medium supplemented with 40 g L−1 sucrose and 8 g L−1 agar under white light. The photoperiod was 16/8 h (light/dark), and light intensity was 100 μmol m−2 s−1. Four weeks after the in vitro subculture, the plantlets were transferred to 600-mL pots containing compost and grown under conditions of 11 h white light (21 °C) and 13 h dark (16 °C). The lights came on at 08:00 and turned off at 19:00. Light intensity ranged from 150 to 300 μmol m−2 s−1 and relative humidity (RH) was kept at 70%. After 5 d, the plants began to grow under the different light treatments and followed the same patterns of temperature and RH. The nutrient solution used was MX (Xu et al. 2018), which was maintained at a standard concentration. The plants were irrigated once every 5 d. All plants were rotated once every 5 d.
Measurements of Plant Growth, Morphology, and Yield
Growth characteristics, such as shoot dry weight (leaves and stems per plant), leaf number, and leaf area, were assessed with three replications per treatment and measured once every 15 d during the 90-d experiment. Plant samples were oven-dried at 80 °C until a constant weight was reached. Growth analysis of shoots was carried out using the relative growth rate (RGR; g g−1 d−1) and the net assimilation rate (NAR; g m−2 d−1) (Matsuda et al. 2016). Tuber yield was measured according to the yield of 30 plants. Tuber fresh weight was ≥2.0 g in tuber yield.
Photosynthetic Measurement
Photosynthetic efficiency rates (α) were measured according to the initial slope of the rapid light curves. Rapid light response curves were measured with a portable fluorometer (PAM-2500; Walz, Effeltrich, Germany) operated with PAMWIN-3 software. The transpiration rate (Tr) was measured using a portable photosynthesis instrument (LI-6400; LI-COR, Lincoln, NE, USA) at 30 and 60 d. Light intensity was the same as the growth irradiance of 300 μmol m−2 s−1. The experimental conditions (i.e., leaf temperature, carbon dioxide concentration, and RH) were set at 21 °C, 400 μmol m−2 s−1, and 65%, respectively.
Photosynthetic Pigment Measurement
Chlorophyll (Chl) was extracted from the leaves of three seedlings located at similar positions within each treatment at 30, 60, and 90 d. The Chl a + b contents were measured by spectrophotometry following the method of Arnon (1949).
Determination of Root Activity
Lateral root tips (0.5 g fresh weight) were collected from three seedlings randomly selected from each treatment at 30 d. Root activity was determined according to Li et al. (2010).
Sucrose and Starch Measurements
Leaves and tubers from similar positions within each treatment were sampled at 08:00, 19:00, and 06:00 the following day at four stages (20, 40, 60, and 80 d). The samples were chopped and dried at 80 °C until reaching a constant weight, and were ground into a fine powder. Sucrose and starch contents were measured using the modified anthrone method of Fairbairn (1953).
Enzyme Extraction and Assay
Leaves and tubers from similar positions within each treatment were sampled at 12:00 in the daytime at four stages (20, 40, 60, and 80 d). SPS (EC2.4.1.14) activity in potato leaves was determined following the method of Zhu et al. (2017) in a 50-μL reaction solution and 50 μL of the crude enzyme extract sample. The procedure for the SS (EC2.4.1.13) assay in tubers was the same as that of SPS except for the replacement of fructose-6-phosphate with 0.084 M fructose in the reaction mixture. Enzyme activity is described in micrograms of sucrose generated per minute per gram fresh weight of samples. The AGPase (EC2.7.7.27) activity assay was performed using the AGPase test kit (F7895-B, ELISA; Jiangsu Feiya Technology Co., Ltd., Jiangsu, China). The experimental procedures were carried out in strict accordance with the kit instructions.
Statistical Analysis
The mean values of three replicate samples were recorded, except tuber yield was the mean value of 30 replicates, and each sample was measured three times. Statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Data were analyzed using analysis of variance; differences among means were tested using the LSD multiple range test (P < 0.05).
Results
Effects of Different Spectra on Tuber Yield
Tuber yield differed markedly under the three light treatments. The mean fresh weight of tubers under RW was 27.72% more than that under BW and 35.99% more than that under WRB (Table 2). The ratio of tubers (> 20 g) under RW in total tuber yield was higher than those under BW and WRB. Tubers (2–10 g) grown under RW had the lowest ratio among all treatments. In contrast, the tuber ratios (number of tubers with ≥2.0 g/number of all tubers) under RW and WRB were significantly lower than that under BW. BW with fewer than two tubers per plant and with more three tubers per plant were compared with other treatments, although there was no significant difference in the number of plants having three or more tubers among the three treatments.
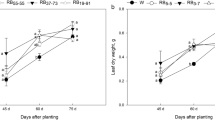
Although the tuber fresh weight per plant of the three treatments was not different at 60 d, RW, BW and WRB significantly increased tuber fresh weight per plant by 57.7%, 21.2% and 16.7% at 60–90 d, respectively (Fig. 1). Tuber yield per plant under WRB was significantly lower than that under RW at 90 d.
Plants Growth Characteristics under the Light Treatments
The different ratios of red and blue light affected shoot growth. The changes in shoot dry weight and leaf number all tended to increase first and then decreased during the 90 d (Fig. 2). RW increased shoot dry weight from 15 to 45 d, and then significantly decreased shoot dry weight compared with WRB at 60 d. In contrast, shoot dry weight under BW and WRB reached a peak at 60 d and then began to decline. BW had the lowest shoot dry weight among the three treatments.
Effects of different light treatments on shoot dry weight, leaf number, relative growth rate and net assimilation rate during 90 days. Note: Different letters for the same parameter indicate significant differences at the 5% level, according to the LSD’s test (n = 3). The bars represent the standard error. The same as below
The RGR and NAR were high during the first 15 d, and then decreased gradually. RW resulted in a lower RGR than BW and WRB during 45–60 d, while RW did not decrease again during 60–90 d. For example, plants grown under RW had a significantly higher RGR during 75–90 d and a higher NAR during 60–75 d than those under BW and WRB.
Effects of Different Spectra on Photosynthetic Parameters and Root Activities
The photosynthetic characteristics of the leaves and root activities showed the effects of the different light treatments on growth. The Tr and root activity of potato seedlings at 30 d grown under WRB were significantly higher than those under RW and BW, although no significant differences were observed in the photosynthetic efficiency rate or Chl a + b content among the three treatments at 30 d (Fig. 3). At 60 d, the Tr, photosynthetic efficiency rate, and Chl (a + b) under BW and WRB were not significantly different, but those under BW and WRB were higher than RW. At harvest time (90 d), the Chl a + b of plants grown under BW decreased to the lowest value among all treatments, whereas the Chl a + b content under RW decreased significantly less than that under BW and WRB.
Daily Changes in Sucrose and Starch Contents of Potato Leaves and Tubers
The carbohydrate products in potato mainly consist of sucrose and starch. Changes in sucrose and starch contents in the potato leaves and tubers were observed according to the light treatments from 08:00 to 19:00 and during darkness from 19:00 to 06:00 the next day. Leaves under RW and WRB had significantly enhanced sucrose content during the day, whereas only WRB significantly reduced sucrose content during the dark at 20 d (Fig. 4). The starch content of leaves under RW also increased significantly during the day, but no obvious decrease was detected in the dark at 20 d. Carbohydrate metabolism occurred under RW, BW, and WRB leaves until 40 d. Plants grown under all light conditions showed significantly increased sucrose and starch contents during the day, and deceased contents during the dark at 40 d. The RW and BW plants showed apparent increases in sucrose contents during the day and clear decreases in starch contents among the RW, BW, and WRB conditions at 60 d. The carbohydrate content of leaves at 80 d was lower than that at 40 and 60 d. However, significant differences were observed between sucrose and starch contents for all treatments, especially BW. Leaf starch accumulated 37.0% of the biomass during the light and consumed 46.3% during the night under BW.
The sucrose contents of tubers under RW decreased by 21.9% and 22.2% from 19:00 to 06:00 (in the dark) at 20 and 40 d, respectively (Fig. 5). The starch content of the tubers in all treatments increased during the night at 20 d. The BW-treated tubers had significantly increased sucrose contents from 08:00 to 19:00 at 40 d (during the day) and reduced sucrose contents in the dark at 60 d. RW, BW, and WRB at 80 d all resulted in increases during the day and decreases at night. For example, BW and WRB rapidly decreased by 76.6% and 64.3% in the dark. There were no distinct changes in starch content of the tubers from 40 to 80 d.
Enzyme Activities Related to Starch and Sucrose Metabolism in Potato Leaves and Tubers
SPS and AGPase activities revealed sucrose and starch metabolism in leaves. No differences in SPS and AGPase activities were observed in potato leaves among any of the treatments (Fig. 6). In tubers, the SS activities under RW, BW, and WRB increased continually from 20 to 60 d, and then decreased to the 20-d level. BW-treated tubers had higher SS activity than RW and BW-treated tubers at 60 d. The AGPase of BW-treated tubers reached its highest activity at 40 d during the entire growth cycle, while RW-treated tubers had the highest AGPase activity at 80 d.
Discussion
RW Increases Tuber Yield
The effects of light wavelength are not only limited to plant growth. Many studies have confirmed that the spectra affect carbohydrate metabolism (Chen et al. 2018). Bantis et al. (2020) reported that watermelon seedlings under red light promoted dry weight. Lettuce and peanut plants treated with high ratio of red light enhanced biomass accumulation (Poulet et al. 2014; Li et al. 2018). Potato plants enhance tuber yield by stimulating carbohydrate metabolism. In this study, tuber fresh weight per plant under RW increased during the last 30 d compared with BW and WRB (Fig. 2). The AGPase activity of RW-treated tubers was significantly higher than that of BW-treated tubers at 80 d. The ratio of RW-treated tubers (> 20 g) in the total tuber yield was higher than other treatments, whereas RW-treated tubers (2–10 g) had the lowest ratio in total tuber yield among all treatments. These results were attributed to higher total Chl content under RW at 90 d than those under BW and WRB. Thiele et al. (1999) reported that the beginning of senescence is not delayed for transgenic potato (Solanum tuberosum) plants expressing Arabidopsis phytochrome B, but rather deceleration of Chl degradation extends the lifetime of photosynthetically active plants. Transgenic potato plants extend underground organs with increased tuber yields. Drozdova et al. (2001) showed that radish accumulates high dry storage root biomass at later stages of growth under 170 μmol m−2 s−1 red light alone compared with blue light.
The lowest shoot biomass and total Chl content under BW were observed compared with RW and WRB at 90 d. The RGR of BW was below zero during 45–90 d, indicating that potato plant aging was severe under BW. In addition, BW showed lower AGPase activity than RW in tubers at 80 d. These results indicate that BW-treated tubers slowly developed until 80 d. Additionally, WRB resulted in similar leaf senescence to that of BW, according to RGR and Chl content. The results suggested that mature potato leaves under high ratio of red light kept chlorophyll content and reduced leaf senescence. However, this phenomenon may vary depending on leaf age and species. Antioxidant capacities are important for leaf senescence in plants. Lettuce and water spinach under blue light increased chlorophyll content and antioxidant capacity in leaves (Son and Oh 2013; Kitayama et al. n.d.). Camptotheca acuminata seedlings and jute under red light increased antioxidant capacity to avoid leaf senescence (Yu et al. 2017; Saleem et al. 2019).
WRB did not have a higher tuber ratio or carbohydrate metabolic enzyme activities at 40–60 d than BW, although WRB had active carbohydrate metabolism in leaves at 20 and 80 d (Fig. 4). For comparison, radish requires a supplement of 10% blue light to the red-light environment (PPF = 200 μmol m−2 s−1) to increase storage root dry weight (Yorio et al. 2001; Samuolienė et al. 2011). Cope et al. (2014) reported the importance of the interaction between blue light and light intensity for radish roots. These findings suggest that the ratio of blue for WRB (37.2%) with 300 μmol m−2 s−1 of PPF was not sufficient to significantly stimulate the bulking stage of the tubers.
Positive Effect of BW on Tuber Bulking
BW leaves showed active sucrose and starch metabolism at 40 d (Fig. 4). Struik et al. (1991) showed that the tuber growth is closely related to the ability of photosynthates to be converted to starch. Tuncel and Okita (2013) reported that potatoes could increase yield by increasing SS activity in tubers. Tubers under BW had significantly higher AGPase activity at 40 d and SS activity at 60 d compared with RW and WRB at 40 and 60 d. As a result, BW plants had a high carbohydrate metabolism during the bulking stage compared with RW and WRB plants. This led to a greater distribution of carbohydrates and a significantly higher tuber ratio under BW. Additionally, BW resulted in 10% more plants with three or more tubers than RW and WRB. These results indicate that the high proportion of blue light had a positive influence on the bulking stage. The reason might be that blue light causes an energy shift to protein synthesis from carbohydrate accumulation during the tuber bulking stage. The tuber information includes starch synthesis (Tauberger et al. 2000) and accumulation of storage protein (Taylor et al. 1992). Jungandreas et al. (2014) also revealed that the photosynthates of Phaeodactylum tricornutum under red light are mainly used to accumulate carbohydrates, whereas blue light causes an energy shift from carbohydrate accumulation to protein synthesis. Amino acids (primary amides: asparagine/aspartate and glutamine) are used to synthesize storage proteins in the tuber (Tiwari et al. 2018).
The sucrose content of leaves under RW significantly increased during the day compared with those of BW and WRB, while SS activity in leaves did not increase at 40 d under RW. The starch content of RW also changed little during the day. These results suggest that starch degradation was stronger than starch synthesis in leaves treated with RW, which led to higher sucrose content in RW plants. Consequently, RW produced a higher sucrose content than the other treatments in tubers at 40 d. The AGPase activity in tubers under RW might not match the high sucrose content; thus, it did not stimulate starch synthesis. As Mugford et al. (2014) found that there was a balanced relationship between sucrose and starch in plant organs. Some newly formed tubers under RW might lose starch at the initial bulking stage. This also explained why the tuber ratio of RW plants was apparently lower than that of BW plants.
Beneficial Effect of WRB on Root Development in Potato Seedlings
Light plays important roles for plant growth, which provides energy for carbohydrate synthesis and drives a number of light-dependent biochemical reactions (Berkovich et al. 2017; Zheng et al. 2019). Red and blue light are primary spectra for plants. RW clearly resulted in more accumulated sucrose and starch contents during the day than BW in potato leaves at 20 d, while carbohydrates in leaves under RW did not degrade significantly at night. At 30 d, RW plants also had a higher shoot dry weight than BW and WRB plants (Fig. 2). The reason may be that the high ratio of red light (71.5%) under RW benefited shoot growth; thus, the leaves were an RW sink at 20 d. A similar phenomenon was reported for leaf dry weight of Wasabia japonica under a red-light treatment alone (Kim and You 2013). Lettuce leaf area and dry weight increased significantly under a red + blue mixed light treatment with an increasing red LED light ratio (Wang et al. 2016). WRB changed the sucrose content as well. The leaves of WRB degraded more starch content than those of RW at night after 20 d. The starch in WRB plants may have been transported underground as a sink. WRB with the proper blue light ratio resulted in the highest root activities and Tr among the three treatments at 30 d (Fig. 3) by providing energy from starch consumption. Johkan et al. (2012) indicated that raising lettuce seedlings treated with blue light promotes growth after transplant. Lim and Eom (2013) also showed that blue light was the most effective light source for acclimating basil cuttings compared with red, fluorescent light, and natural sunlight.
Conclusion
In this study, we found that RW accumulated starch in potato plant leaves to enhance shoot growth of potato seedlings, while WRB had the best root activity at 30 d. BW resulted in synchronous circadian rhythm changes in sucrose and starch contents in leaves during tuber development. BW-treated tubers showed significantly more AGPase activity at 40 d and SS activity at 60 d than RW and WRB-treated tubers. Blue light was useful for potato bulking, which made the tuber ratio of BW higher than those of RW and WRB. RW had higher Chl a + b and RGR than BW and WRB during 60–90 d. The better shoot growth supported the increase in tuber fresh weight compared with BW and WRB.
References
Arnon, D.L. 1949. Copper enzymer in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiology 24: 1–15.
Bantis, F., A. Koukounaras, A.S. Siomos, M.N. Fotelli, and D. Kintzonidis. 2020. Bichromatic red and blue LEDs during healing enhance the vegetative growth and quality of grafted watermelon seedlings. Scientia Horticulturae 261.
Baroja-Fernández, E., F.J. Muñoz, J. Li, A. Bahaji, G. Almagro, M. Montero, Ed Etxeberria, T.M. Sesma, and J. Pozueta-Romero. 2012. Sucrose synthase activity in the sus1/sus2/sus3/sus4 Arabidopsis mutant is sufficient to support normal cellulose and starch production. Proceedings of the National Academy of Sciences of the United States of America 109: 321–326.
Berkovich, Y.A., I.O. Konovalova, S.O. Smolyanina, A.N. Erokhin, O.V. Avercheva, E.M. Bassarskaya, G.V. Kochetova, T.v. Zhigalova, O.S. Yakovleva, and I.G. Tarakanov. 2017. LED crop illumination inside space greenhouses. Reach Reviews in Human Space Exploration 6: 11–24.
Briggs, W.R. 1993. New light on stem growth. Nature 366: 110–111.
Chen, X.L., X.Z. Xue, W.Z. Guo, L.C. Wang, and X.J. Qiao. 2016. Growth and nutritional properties of lettuce affected by mixed irradiation of white and supplemental light provided by light-emitting diode. Scientia Horticulturae 200: 111–118.
Chen, Y.Y., B. Zhou, J.L. Li, H. Tang, J.C. Tang, and Z.Y. Yang. 2018. Formation and change of chloroplast-located plant metabolites in response to light conditions. International Journal of Molecular Sciences 19 (3): 654.
Cope, K.R., M.C. Snowden, and B. Bugbee. 2014. Photobiological interactions of blue light and photosynthetic photon flux: Effects of monochromatic and broad-spectrum light sources. Photochemistry and Photobiology 90: 574–584.
Drozdova, I.S., V.V. Bondar, N.G. Bukhov, A.A. Kotov, L.M. Kotova, S.N. Maevskaya, and A.T. Mokronosov. 2001. Effects of light spectral quality on morphogenesis and source–sink relations in radish plants. Russian Journal of Plant Physiology 48: 415–420.
Fairbairn, N.J. 1953. A modified anthrone reagent. Chemistry & Industry 4: 86.
Folta, K.M. 2019. Breeding new varieties for controlled environments. Plant Biology 21 (SI(1)): 6–12.
Gukasyan, I.A., T.N. Konstantinova, L.V. Chistyakova, and N.P. Aksenova. 1994. Effects of day length and light quality on the ultrastructure of the palisade parenchyma cells of potato plants. Russian Journal of Plant Physiology 41 (1): 24–29.
Huber, S.C., and J.L. Huber. 1996. Role and regulation of sucrose-phosphate synthase in higher plants. Annual Review of Plant Physiology and Plant Molecular Biology 47: 431–444.
Jao, R.C., and W. Fang. 2004. Effects of frequency and duty ratio on the growth of potato plantlets invirto using light-emitting diodes. Hortscience 39 (2): 375–379.
Johkan, M., K. Shoji, F. Goto, S. Hahida, and T. Yoshihara. 2012. Effect of green light wavelength and intensity on photomorphogenesis and photosynthesis in Lactuca sativa. Environmental and Experimental Botany 75: 128–133.
Jungandreas, A., B.S. Costa, T. Jakob, M.V. Bergen, S. Baumann, and C. Wilhelm. 2014. The acclimation of phaeodactylum tricornutum to blue and red light does not influence the photosynthetic light reaction but strongly disturbs the carbon allocation pattern. PLoS One 9 (8): e99727.
Kim, Y.H., and M.G. Lee. 2004. Tuber production and growth of potato transplants grown under different light quality. VII International Symposium on Protected Cultivation in Mild Winter Climates: Production. Acta Horticulturae 659: 267–272.
Kim, H.R., and Y.H. You. 2013. Effects of red, blue, white, and far-red led source on growth responses of wasabia japonica seedlings in plant factory. Korean Journal of Horticultural Science & Technology 31 (4): 415–422.
Kitayama, M., D.T.P. Nguyen, N. Lu, and M. Takagaki. 2019. Effect of light quality on physiological disorder, growth, and secondary metabolite content of water spinach (Ipomoea aquatica forsk) cultivated in a closed-type plant production system. Horticultural Science and Technology 37 (2): 206–218.
Kyoko, H.T., and E. Hiroshi. 2016. Molecular breeding to create optimized crops: from genetic manipulation to potential applications in plant factories. Frontiers in Plant Science 7(539): 1–7.
Lee, Y.I., W. Fang, and C.C. Chen. 2011. Histological observation on the growth of potato plantlets in vitro under six different led light qualities. Acta Horticulturae 907: 393–395.
Li, H.M., Z.G. Xu, and C.M. Tang. 2010. Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell, Tissue and Organ Culture 103: 155–163.
Li, C., D. Liu, L.L. Li, S.X. Hu, Z.G. Xu, and C.M. Tang. 2018. Effects of light-emitting diodes on the growth of Peanut plants. Agronomy Journal 110 (6): 2369–2377.
Lim, Y.J., and S.H. Eom. 2013. Effects of different light types on root formation of ocimum basilicum L. cuttings. Scientia Horticulturae 164: 552–555.
Lin, K.H., M.Y. Huang, W.D. Huang, et al. 2013. The effect of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Scientia Horticulturae 150: 86–91.
Matsuda, R., T. Yamano, K. Murakami, and K. Fujiwara. 2016. Effects of spectral distribution and photosynthetic photon flux density for overnight LED light irradiation on tomato seedling growth and leaf injury. Scientia Horticulturae 198: 363–369.
Miyashita, Y., Kimura, T., Kitaya, Y., Kubota, C., Kozai, T., 1997. Effects of red light on the growth and morphology of potato plantlets in vitro: Using light emitting diodes (LEDs) as a light source for micropropagation. Third International Symposium on Artificial Lighting In Horticulture, Acta Hortic. 418: 169–173.
Mugford, S.T., O. Fernandez, J. Brinton, A. Flis, N. Krohn, B. Encke, R. Feil, R. Sulpice, J.E. Lunn, M. Stitt, and A.M. Smith. 2014. Regulatory properties of adp glucose pyrophosphorylase are required for adjustment of leaf starch synthesis in different photoperiods. Plant Physiology 166 (4): 1733–1747.
Müller-Röber, B., U. Sonnewald, and L. Willmitzer. 1992. Inhibition of the ADP-glucose pyrophosphorylase in transgenic potatoes leads to sugar-storing tubers and influences tuber formation and expression of tuber storage protein genes. The EMBO Journal 11: 1229–1238.
Poulet, L., G.D. Massa, R.C. Morrow, C.M. Bourget, R.M. Wheeler, and C.A. Mitchell. 2014. Significant reduction in energy for plant-growth lighting in space using targeted LED lighting and spectral manipulation. Life Sciences in Space Research 2: 43–53.
Saleem, M.H., M. Rehman, M. Zahid, M. Imran, W. Xiang, and L.J. Liu. 2019. Morphological changes and antioxidative capacity of jute (Corchorus capsularis, Malvaceae) under different color light-emitting diodes. The Brazilian Journal of Botany 42 (4): 581–590.
Samuolienė, G., R. Sirtautas, A. Brazaitytė, J. Sakalauskaitė, S. Sakalauskienė, and P. Duchovskis. 2011. The impact of red and blue light-emitting diode illumination on radish physiological indices. Central European Journal of Biology 6: 821–828.
Son, K.H., and M.M. Oh. 2013. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. Hortiscience 48(8): 988–995.
Stein, O., and D. Granot. 2019. An overview of sucrose synthesis in plants. Frontiers in Plant Science 10 (95): 1–14.
Struik, P.C., D. Vreugdenhil, A.J. Haverkort, C.B. Bus, and R. Dankert. 1991. Possible mechanisms of size hierarchy among tubers on one stem of a potato (Solanum tuberosum, L.) plant. Potato Research 34 (2): 187–203.
Tauberger, E., A.R. Fernie, M. Emmermann, A. Renz, J. Kossmann, L. Willmitzer, and R.N. Trethewey. 2000. Antisense inhibition of plastidial phosphoglucomutase provides compelling evidence that potato tuber amyloplasts import carbon from the cytosol in the form of glucose-6-phosphate. The Plant Journal 23 (1): 43–53.
Taylor, M.A., S.A. Mad Arif, A. Kumar, H.V. Davies, L.A. Scobie, S.R. Pearce, and A.J. Flawell. 1992. Expression and sequence analysis of cDNAs induced during the early stages of tuberization in different organs of the potato plant (Solanum tuberosum, L.). Plant Molecular Biology 20: 641–651.
Thiele, A., M. Herold, I. Lenk, and Q.C. Gatz. 1999. Heterologous expression of arabidopsis phytochrome b in transgenic potato influences photosynthetic performance and tuber development. Plant Physiology 120 (1): 73–81.
Tiwari, J.K., P. Darren, G. Trevor, S.K. Chakrabarti, and R.K. Singh. 2018. Integrated genomics, physiology and breeding approaches for improving nitrogen use efficiency in potato: Translating knowledge from other crops. Functional Plant Biology 45 (6): 587–605.
Tuncel, A., and T.W. Okita. 2013. Improving starch yield in cereals by over-expression of ADPglucose pyrophosphorylase: Expectations and unanticipated outcomes. Plant Science 211: 52–60.
Wang, J., W. Lu, Y.X. Tong, and Q.C. Yang. 2016. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Frontiers in Plant Science 7: 250.
Xu, Z.G., X.X. Wang, S. Chen, and L.J. Gan. 2018. Optimal regulation of nutrient solution types and concentrations for virus-free seed potato breeding in plant factory. Transactions of the CSAE 34 (17): 199–206.
Yorio, N.C., G.D. Goins, H.R. Kagie, R.M. Wheeler, and J.C. Sager. 2001. Improving spinach, radish, and lettuce growth under red light emitting diodes LEDs with blue light supplementation. HortScience 36: 380–383.
Yu, W.W., Y. Liu, and L.L. Song. 2017. Effect of differential light quality on morphology, photosynthesis, and antioxidant enzyme activity in Camptotheca acuminata seedlings. Journal of Plant Growth Regulation 36 (1): 148–160.
Zha, L., and W. Liu. 2018. Effects of light quality, light intensity, and photoperiod on growth and yield of cherry radish grown under red plus blue leds. Horticulture, Environment, and Biotechnology 59 (4): 511–518.
Zheng, L., H.M. He, and W.T. Song. 2019. Application of light-emitting diodes and the effect of light quality on horticultural crops: A review. Hortiscience 54 (10): 1656–1661.
Zhu, X.D., C.B. Zhang, W.M. Wu, X.P. Li, C. Zhang, and J.G. Fang. 2017. Enzyme activities and gene expression of starch metabolism provide insights into grape berry development. Horticulture Research: Nature Pubkishing Group, Macmillan Building, 4 Crinan St, London N1 9XW, England.
Acknowledgements
This research was supported by the National Key R&D Program of China (2017YFB0403903).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
He, W., Miao, C., You, J. et al. Effects of Red and Blue Light with Supplemental White Light on Growth, Carbohydrate Metabolism, and Yield of Virus-Free Potato in Plant Factories. Am. J. Potato Res. 97, 554–564 (2020). https://doi.org/10.1007/s12230-020-09803-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-020-09803-2