Abstract
Hydrogen peroxide (H2O2) is an important signaling molecule that involved in multiple physiological metabolic processes in plants. Excess H2O2 can destroy biological macromolecules to poison the cell. Thioredoxin peroxidase (Tpx) plays an important role in protecting plants from oxidative damage by clearing H2O2. In this study, tomato Tpx (SlTpx) gene was cloned and bioinformatic analysis was done. The mRNA transcript level of SlTpx in tomato root and leaf was increased significantly after NaCl stress treatment for 12 h. SlTpx overexpression transgenic tobacco plants were obtained to study its function under NaCl stress. The seed germination rate of SlTpx overexpression plants was higher than that in wild type (WT) plants under NaCl treatment. The malondialdehyde (MDA) content and reactive oxygen species (ROS) accumulation in transgenic tobacco were less than in WT under NaCl stress. Transgenic plants had significantly higher antioxidant enzyme activities, proline and total soluble sugar contents, and expression of Na+ metabolism genes in transgenic plants than the WT. Moreover, The SlTpx transgenic seeds showed higher tolerance to H2O2 and methyl viologen (MV) treatment, compared with the WT. Besides, the growth of prokaryotic strain of pET-28a-SlTpx was better than the pET-28a strain with H2O2 treatment. The above results indicate that the SlTpx gene improves the plant salt tolerance by scavenging H2O2.
Key message
Overexpression of tomato SlTpx gene in tobacco enhances the salt stress tolerance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salt stress is one of the most severe environmental challenges, which damages crop production and quality (Munns and Gilliham 2015). Plant cells and tissues were rapidly damaged within few minutes of exposure to salt stress. The harm of salt stress consists of two aspects. The first is a high osmotic potential, leading to root water absorption disorders and osmotic stress (Miller et al. 2010). At this stage, stress signals are rapidly transmitted from the root to the ground, leading to the initiation of salt resistance mechanisms, such as reducing turgor pressure, impaired cell ductility, and inducing abscisic acid biosynthesis, which in turn promotes stomatal closure to reduce transpiration (Cuadros-Rodriguez et al. 2002). The second effect occurs in the long term, salt-induced ion imbalance due to high concentrations of sodium (Na+) and chloride (Cl−), ultimately resulting in ion toxicity and nutritional imbalance (Rana et al. 2008). Furthermore, both osmotic stress and ion toxicity can lead to the accumulation of reactive oxygen species (ROS), thus causing oxidative damage to cellular macromolecules (Miller et al. 2010). In response to salt stress, plants are equipped with effective adaptation strategies such as morphological changes, osmotic substances biosynthesis, antioxidant activation, ion homeostasis, plant hormone response.
While plants suffer from external stress, with the accumulation of ionic toxicity and the photosynthetic rate decreasing, ROS in plants also begin to accumulate. ROS are highly active and toxic and can disrupt biomolecular substances in vivo such as proteins, nucleic acids and lipid membranes (Nathan and Cunningham-Bussel 2013). Hydrogen peroxide (H2O2) is one of the components of intracellular ROS (Kimoto et al. 2012). To remove excess ROS, organisms have developed an extremely effective set of antioxidant mechanisms involving multiple enzymes, such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), thioredoxin peroxidase (Tpx) (Corona and Robinson 2010; Gui-Qin et al. 2012). These enzymes act together to maintain the balance of the redox environment in vivo (Zhang et al. 2019).
Thioredoxin peroxidase (Tpx) is a member of the peroxidase family, which lacks the metal ion auxiliary group required for the catalytic reaction (Corona and Robinson 2010). Tpx has 1–2 conserved cysteine (Cys) residues to replace the function of the metal ion auxiliary and remove various peroxides (Circu and Aw 2010). There are two ways of Tpx reducing the substrate to H2O, one directly catalyzing H2O2 to H2O and another catalyzing the reduction of alkyl H2O2 to the corresponding alcohol and H2O by thioredoxin as the electron donor (Barranco-Medina et al. 2007; Woo et al. 2010). Several studies have explored the role of Tpx genes in peroxide clearance, and the redox regulation of different species under stress conditions (Kim et al. 2018). For example, BvM14-Tpx genes can ease the inhibition of H2O2 on the growth of E.coli. BvM14-Tpx can also improve the salt tolerance ability of transgenic yeast (Zhao et al. 2015). In the cyanobacteria PCC 6803, Tpx acts as a clearance system for H2O2 and alkyl hydroperoxide (Gaber et al. 2004). Human Tpx gene protects cells from H2O2 induced damage (Berggren et al. 2001). In tomato, the role of the Tpx gene has not been extensively studied under salt stress.
To further investigate the potential roles of Tpx of tomato (SlTpx), we amplified the SlTpx gene and found that SlTpx was induced by NaCl treatment. Functional analysis in transgenic tobacco plants revealed that overexpression of SlTpx in tobacco enhanced the salt stress tolerance by scavenging H2O2. The growth of prokaryotic recombinant strain of pET-28a-SlTpx was better than pET-28a strain in medium with H2O2.
Materials and methods
Plant materials and stress treatment
Tomato (Solamum lycopersicum L.) seeds were germinated in vermiculite and then the young seedlings were hydroponically grown in a greenhouse under normal growth conditions of about a 16 h/8 h (light/dark) photoperiod at 28 ℃/20 ℃ (day/night) (Siddiqi et al. 2002). The 6-week-old tomato seedlings were treated with 100 mM NaCl for 0, 3, 6, 12 and 24 h. The collected samples were immediately placed in liquid nitrogen and stored at − 80 ℃ until use.
Bioinformatics analysis of the SlTpx
The cDNA sequence of the SlTpx was retrieved from the NCBI (https://www.ncbi.nlm.nih.gov/) (GenBank No. NM_001247242.1), with a sequence size of 489 bp. To further understand the relationship between SlTpx and other Tpxs, the deduced amino acid sequence of SlTpx was compared with other protein sequences of Genbank. The phylogenetic tree was constructed using MEGA 7.0 software (Kumar et al. 2016).
Construction of SlTpx overexpression vector and plant transformation
The full-length sequence of SlTpx was amplified with specific primers (SlTpx-BamHI-F: cgggggtaccggatccATGGCTCCAATCGCCG; SlTpx-BamHI-R: cgatgaattc ggatccAAGAGCATTGACGATTTC) (The lower case letters with underline were the homologous recombinant splice sequence). The 489 bp open reading fragment was cloned into pRI101-6flag (Takara, China) using the ClonExpress II one-step cloning kit (Vazyme, China). The recombinant plasmid of pRI101-SlTpx was transformed into the Agrobacterium tumefaciens LBA4404 strain. Transgenic tobacco plants were obtained using the leaf plate method (Sankara and Rohini 1999). The transgenic plants were identified by genomic PCR, qPCR and Western blot analysis. T2-generation transgenic plants were used for further stress treatment.
Western blot analysis
Western blot analysis was performed according to the procedure of Bai et al. (2016). Proteins were subjected to SDS-PAGE and then transferred to a PVDF membrane. Membranes were blocked with 5% skim milk (PBS dilution containing 0.1% Tween-20) and incubated for 2 h at room temperature, then incubated with anti-flag or anti-Beta actin antibody (5% 1:5000 dilution in skim milk) at 4 ℃ for 8 h and washed with PBST for 5 min each. Horseradish peroxidase-labeled sheep anti-mouse IGG (H + L) was incubated with membrane room temperature for 1 h and gently shaken. Finally, the membrane was washed three times with 0.1 M PBST for 10 min each, and colored with ECL (Beijing Kangwei Century Biotechnology Co. Ltd.).
RNA extraction and qRT-PCR analysis
Total plant RNA was extracted with TRIzol reagent (Takara, China) and reverse transcribed into cDNA using the Hieff Clone Plus One Step (YEASEN, China) kit. The mRNA transcript level of SlTpx and several antioxidant and defense-related genes were detected by real-time quantitative PCR (qPCR) using gene-specific primers. qPCR was performed in a 96-well white board each containing 20 µL reaction mixture in triplicate for gene expression level analysis using a Hieff qPCR SYBR Green Master Mix kit (YEASEN, China) using a BioRad CFX 96™ real-time quantification system. The relative transcript level was calculated for each gene according to the method reported by Livak and Schmittgen (2013). In addition, each qPCR experiment was performed in three biological replicates. The primer sequences used for the qRT-PCR analysis are listed in Table S1.
Analysis of transgenic plants under salt stress
Seeds from transgenic and WT tobacco were soaked in 55 ℃ sterile water for 30 min, sterilized with 4% NaClO for 20 min, and rinsed three times in sterile water. The seeds were then seeded on MS agar plates containing 0 mM and 100 mM NaCl, and their daily germination rate was recorded. Seedlings were grown vertically for 12 d on MS solid medium containing 0 mM or 100 mM NaCl.
To determine the salt tolerance of tobacco seedlings, transgenic and WT tobacco seedlings were placed in the soil and grown for 6 weeks to the appropriate size for stress treatment. The plants were watered with 50 mL water per basin (control group) or 50 mL 150 mM NaCl solution (NaCl treatment) every 2 d for 2 weeks (Qi et al. 2020).
Malondialdehyde (MDA) contents and endogenous ROS accumulation analysis
Samples were collected and ground to a fine powder in liquid nitrogen, followed by the addition of 3 ml of 5% TCA to 0.2 g of ground tissue. After centrifugation, 2 ml of the supernatant was transferred into a 10 mL tube, after which 2 mL of 0.67% TBA solution was added. The concentration of MDA was calculated using the formula: 6.45 × (OD532 – OD600) – 0.56 × OD450.
To observe ROS in the tips, the washed tips were placed into EP tubes containing 2 μM H2DCF-DA dye, stained for 30 min, then washed three times with 20 mM HEPES–KOH (pH 7.8) buffer for a total of 45 min and photographed with a fluorescence microscope (Mazel et al. 2004).
Determination of the soluble sugar and proline contents
The soluble sugar content was determined based on the Yemm method (Yemm and Willis 1954). 0.3 g fresh leaves were put into the tube, then 5 mL of distilled water was added, sealed the tube and boiled for 30 min, and the extract was collected. The extract was added 1.5 mL of water, 0.5 mL anthractrone ethyl acetate and 5 mL concentrated sulfuric acid, boiled for 1 min, and the absorbance was measured at 630 nm.
The free proline content was measured according to the method described by Gay and Gebicki (2003). Weigh 0.5 g of leaves of samples of different treatments, add 5 mL of 3% sulfosalicylic acid for boiling water extraction for 10 min, filter, add 2 mL of glacial acetic acid and acidic ninhydrin respectively, heat for 30 min, add 4 mL of toluene for cooling, and centrifuge to get the supernatant, the absorbance was measured at 520 nm.
Determination of antioxidant enzyme activity
Leaf samples were ground and homogenized in the extraction buffer, then the homogenates were centrifuged. The resulting supernatant was finally collected for enzymatic activity analysis. The SOD enzyme activity was analyzed according to the method described by Madhawa Rao and Sresty (2000). The CAT activity was determined at 240 nm according to the procedure described by Cakmak (Cakmak and Marschner 1992). The APX activity was determined at 290 nm according to the Kang method (Kang et al. 2016). POD activity was determined spectrophotometrically at 470 nm using guaiacol as substrate and was reported as Ug−1 min−1FW, which corresponded to a change in absorbance in 1 min−1 g−1 of FW (Cui et al. 1999).
Histochemical staining of O2 − and H2O2
SlTpx transgenic and WT seeds were treated with 200 mM NaCl for 12 d. The H2O2 accumulation in plants was observed with 3,3′-diaminobenzidine (DAB) staining. The transgenic plants were treated with 24 mg/mL DAB in the dark at 22 ℃, followed by fixation, staining and removal (Qi et al. 2020). To test the O2− content in the plant, transgenic plants were stained with 0.1 mg/mL nitroblue tetrazole (NBT) and treated in darkness for 8 h at room temperature and decolorized with 80% ethanol (He et al. 2016).
MV and H2O2 stress analysis of the transgenic tobacco seeds
SlTpx transgenic and WT tobacco seeds were sterilized and placed on MS medium containing 15 μM MV or 100 μM H2O2 with MS medium as a control. Their daily germination rate was recorded for 12 d. Then the phenotype of the germination seeds was photographed.
Analysis of oxidative stress tolerance of recombinant pET-28a-SlTpx bacteria
The SlTpx gene was amplified and inserted into the pET-28a vector. Recombinant plasmid of pET-28a-SlTpx was transformed into BL21 strain. The pET-28a-SlTpx and pET-28a BL21 strain were inoculated in 5 mL liquid kanamycin-containing LB and incubated at 37℃, 200 rpm/min rocking, overnight. The next day it was transferred to new kanamycin liquid LB medium at 1:100, induced expression with IPTG of 0.5 mM at 37 ℃, while adding 100 and 200 μM H2O2 for oxidative stress tolerance analysis. The LB medium without H2O2 was used as a control. The absorbance value was measured at 650 nm. The experiment was repeated 3 times (Guo et al. 2015).
Statistical analysis
Three replicates of each sample were used for the statistical analysis. Mean comparison was performed by Student's t-test, and the significance level was *P < 0.1 and **P < 0.05, respectively. Data was expressed as mean ± standard deviation (SE) of three independent experiments.
Results
Isolation and bioinformatics analysis of SlTpx
The identified full-length cDNA of SlTpx was 489 bp. The SlTpx encodes a 162-amino-acid protein with a molecular mass of 17.4 kDa. A phylogenetic tree was constructed using SlTpx protein and other Tpx proteins of tobacco, Arabidopsis, potato and pepper (Fig. 1A). SlTpx is closely related to Tpx in potato. SlTpx protein also showed a high sequence identity with Tpx proteins in Arabidopsis thaliana (NM_105270.3, 82%), Nicotiana tabacum (KJ874387.1, 90.12%), Capsicum annuum (AF442385.2, 90.74%), Solanum tuberosum (NM_001288326.1, 98.77%) (Fig. 1B). The SlTpx sequence contains a cysteine-dependent peroxidase (PRX5) domain (Fig. 1C).
Bioinformatics analysis of SlTpx. A Phylogenetic tree analysis of SlTpx with Tpx of other organisms. B Multiple sequence alignment analysis of Tpx. SlTpx: Solanum lycopersicum (NM_001247242.1); StTpx: Solanum tuberosum (NM_001288326.1); NtTpx: Nicotiana tabacum (KJ874387.1); CaTpx: Capsicum annuum (XM_016684704.1); AtTpx: Arabidopsis thaliana (NM_105270.3); LcTpx: Leymus chinensis (GQ397275). C Analysis of the active domains in the SlTpx amino acid sequence
Expression profiles of SlTpx in response to NaCl stress
qPCR analysis of SlTpx expression showed that SlTpx in the leaf and root was increased gradually from 0 to 12 h and then decreased after 24 h NaCl treatment (Fig. 2). The expression of SlTpx significantly increased by 12.9 and 5.5 times after 12 h treatment in leaf and root under NaCl treatment, respectively.
Effect of salt stress on SlTpx expression in tomato leaf and root. A Gene expression of SlTpx in tomato leaf under 100 mM NaCl stress treatment for 0, 3, 6, 12, 24 h. B Gene expression of SlTpx in tomato root under 100 mM NaCl stress treatment for 0, 3, 6, 12, 24 h. All the results represented mean ± standard deviation (SD) of three biological replicates. Data were compared using Student's t-test with gene expression of 0 h indicated by *P < 0.1; **P < 0.05
Characterization of SlTpx overexpression transgenic tobacco
To investigate the function of SlTpx, putative transgenic tobacco plants were obtained by A. tumefaciens-mediated transformation. Molecular characterization by PCR showed that the expected SlTpx fragment was detected in the transgenic plants, while this gene was not detected in the WT plants (Fig. 3A). qPCR showed that the SlTpx gene expression was significantly higher than WT (Fig. 3B). Western blot analysis showed that expression protein was found in transgenic plants with anti-flag antibody, while there was no protein found in the WT (Fig. 3C). These results demonstrated that the three lines were transgenic lines and were selected for further analysis.
Identification of SlTpx overexpression transgenic tobacco plants. WT: wild type plants. OE-1, 2, 3: three different transgenic lines. A Genomic PCR analysis. B The relative expression level of SlTpx in transgenic plants by qRT-PCR. C Western blot analysis of the SlTpx transgenic plants. All the results represented mean ± standard deviation (SD) of three biological replicates. Data was analysed with Student's t-test compared with WT plants under similar conditions and indicated by *P < 0.1; **P < 0.05
The seed germination of SlTpx overexpression tobacco plants under salt stress
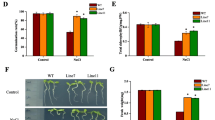
Seeds of transgenic lines (OE-1, OE-2, OE-3) and WT were grown on MS medium containing 0 or 100 mM NaCl for 12 d, respectively. There was no difference in the germination rate of transgenic and WT plants on MS medium while the germination rate in SlTpx overexpressing plants was significantly higher than WT plants under 100 mM NaCl stress (Fig. 4A, B). The three transgenic lines were also subjected to vertical plate growth experiments on MS medium in absence or presence (100 mM) of NaCl (Fig. 4C). Under salt stress, the root length of OE-1, OE-2 and OE-3 was 1.7, 1.4 and 1.9 times of WT plants, respectively, significantly longer in transgenic tobacco (Fig. 4D). DAB and NBT staining result showed that H2O2 and O2− content in WT leaves was obviously higher than the three transgenic plants (Fig. 4E, F). These results indicated that SlTpx overexpression transgenic tobacco plants had improved salt stress tolerance.
Effect of NaCl stress on the seed germination, H2O2 and O2– contents in SlTpx overexpression transgenic plants seeds. A Phenotypes of tobacco seeds grown on MS medium in absence or presence (100 mM) of NaCl for 12 d. B Seed germination rate. C Growth phenotype and root length of tobacco seeds observed by vertical plate. D Root length of tobacco in the vertical plate. E Leaf H2O2 content observed by DAB staining. F The O2− content in the leaves visualized by NBT staining
Effect of salt stress on phenotype, MDA and ROS contents in SlTpx transgenic tobacco seedlings
To investigate the effect of salt tolerance on SlTpx overexpression transgenic plants, 6-week-old WT and transgenic tobacco seedlings were treated with 150 mM NaCl for 14 d. In the un-treated control, there is no phenotype and visible differences in the growth between WT and transgenic plants. After NaCl treatment, the growth of WT and transgenic plants seedlings were all inhibited and the inhibition of WT plants was more than the transgenic plants (Fig. 5A). The membrane lipid peroxidation between WT and transgenic plants were then analyzed. After salt stress, MDA of WT was significantly higher than the three transgenic lines (Fig. 5B), indicating less membrane lipid peroxidation in transgenic plants than WT. Then the ROS contents in roots were determined. As shown in Fig. 5C, the ROS contents in roots of WT plants were higher than in transgenic plants under NaCl stress, indicating that transgenic tobacco have less oxidative damage caused by NaCl treatment.
Effect of NaCl stress on the phenotype, MDA and ROS contents in SlTpx overexpression plants. A Tobacco phenotype after two weeks of 150 mM NaCl treatment. B MDA contents after two weeks of 150 mM NaCl treatment. C ROS accumulation in tobacco roots after two weeks of 150 mM NaCl treatment. All the results represented mean ± standard deviation (SD) of three biological replicates. Data was analysed with Student's t-test compared with WT plants under similar conditions and indicated by *P < 0.1; **P < 0.05
Effect of salt stress on the antioxidant enzyme activities and osmotic substance contents in SlTpx transgenic tobacco seedlings
Under normal conditions, the SOD, POD, APX activities of the transgenic plants were similar to the WT, while the CAT activity of transgenic plants was significantly higher than that of WT. After salt stress treatment, the SOD, POD, CAT, and APX activities were significantly higher in the transgenic plants, compared with the WT (Fig. 6). Soluble sugar and proline play important roles when plants facing salt stress. The soluble sugar and proline contents in the transgenic leaves were significantly higher than the WT after NaCl stress treatment (Fig. 6E, F). We also analyzed the gene expression of some genes related to the synthesis of osmotic substances, including LEA5, P5CS and Osmotin. Under normal treatment, the gene expression in transgenic plants was similar to that in WT plants. After NaCl treatment, the gene expression of transgenic plants was dramatically higher than the WT plants (Fig. 6G–I). These results suggest that the transgenic plants under NaCl stress may regulate the osmotic pressure by increasing the soluble sugar and proline contents under salt stress.
Effect of NaCl stress on plant antioxidant enzyme activity, osmotic substance contents in SlTpx overexpression plants. A–D SOD, POD, CAT and APX activities. E, F soluble sugar and proline contents. G–I Expression of NtP5CS, NtLEA5, NtOsmotin by qRT-PCR. The transcript levels were normalized to NtACTIN. All the results represented mean ± standard deviation (SD) of three biological replicates. Data was analysed with Student's t-test compared with WT plants under similar conditions and indicated by *P < 0.1; **P < 0.05
Effect of salt stress on Na+ transport related gene expression in SlTpx transgenic tobacco seedlings
The expression levels of several Na+ transport-related genes were analyzed by qPCR. These genes include the tonoplast Na+/H+ antiporters gene NtNHX1, NtSOS1 and high affinity potassium transporter (HKT) gene of NtHKT555 and NtHKT586. The results showed that the transgenic plants had significantly higher expression of NtNHX1, NtHKT555 and NtHKT586, NtSOS1 compared with WT plants after salt stress treatment (Fig. 7).
Effect of NaCl stress on transcript levels of Na+ transport related gene in SlTpx overexpression plants. The expression of NtHKT555 (A), NtHKT586 (B), NtNHX1 (C), NtSOS1 (D) was done with qPCR analysis. The transcript levels were normalized to NtACTIN. All the results represented mean ± standard deviation (SD) of three biological replicates. Data was analysed with Student's t-test compared with WT plants under similar conditions and indicated by *P < 0.1;**P < 0.05
Effect of MV and H2O2 stress on the seed germination rate of SlTpx overexpression transgenic tobacco
To investigate the oxidative stress tolerance of SlTpx overexpression transgenic tobacco, seeds were germinated on MS medium containing 15 μM MV and 100 μM H2O2. As shown in Fig. 8A, C, the transgenic tobacco seed germinate earlier and have higher germination rate than WT under MV and H2O2 treatment. Under MV treatment, the germination rate of WT seeds was 76.1%, while the germination rate of OE-1, OE-2 and OE-3 were 92.2%, 96.0% and 100.0% respectively (Fig. 8B). Under H2O2 treatment, the germination rate of WT seeds was 80.1%, and that of OE-1, OE-2 and OE-3 were 92.2%, 96.1% and 96.2% respectively (Fig. 8D). These results suggested that transgenic tobacco seeds have improved tolerance to oxidative stress caused by MV and H2O2.
Effect of MV and H2O2 stress on the germination rate of SlTpx overexpression plants. A Phenotypes of tobacco seeds grown on MS medium containing 0 or 15 μM of MV for 12 d. B Germination rate of tobacco seeds under MV stress. C Phenotypes of tobacco seeds grown on MS medium containing 0 or 100 μM H2O2 for 12 d. D Germination rate of tobacco seeds under H2O2 stress
Analysis of the oxidative stress tolerance of the SlTpx recombinant strain
Oxidative stress analysis of the SlTpx recombinant bacteria with 100 and 200 μM H2O2 was applied at 37 ℃. In normal LB medium, the growth rate of pET-28a-SlTpx, and pET-28a empty vector was similar. After exogenous application of 100 and 200 μM H2O2, the pET-28a-SlTpx recombinant strain consistently grew faster than the pET-28a empty vector. At 540 min, the absorbance values of the recombinant pET-28a-SlTpx at 100 μM and 200 μM were 1.0 and 0.7, respectively, and the absorbance values of the empty vector at 100 μM and 200 μM were 0.7 and 0.5, respectively (Fig. 9). The results showed that the tolerance to oxidative stress was enhanced in the pET-28a-SlTpx recombinant bacteria.
Growth of pET-28a-SlTpx recombinant strain under H2O2 stress. Oxidative stress analysis of pET-28a-SlTpx recombinant strain was applied with 0, 100 and 200 μM H2O2 at 37 ℃. The absorbance of OD 650 nm was done to compare the growth rate of different strain. All the results represented mean ± standard deviation (SD) of three biological replicates
Discussion
Abiotic stresses from the outside world can produce large amounts of ROS in the plants, destroy the macromolecular material in the organism, and thus affect the plant growth and development. Tpx is involved in the antioxidant system by clearing H2O2 and is an important enzyme for maintaining redox homeostasis in plants (Koh et al. 2007). The specific function of SlTpx under salt stress is still unclear. In this study, we cloned the SlTpx gene from tomato. The results of multiple sequence alignment showed that tomato SlTpx had high sequence similarity with other plants Tpx (Fig. 1).
To further investigate the SlTpx function under salt stress, we transformed tobacco with a SlTpx gene through Agrobacterium tumefaciens-mediated transformation, and we have regenerated three different transgenic plant lines which were characterized by PCR, western blot and qPCR analyses (Fig. 3). Our results showed that the germination rate of the transgenic seeds was higher than that of the WT under salt stress conditions (Fig. 4A–D). A reduced H2O2 and superoxide anion content in transgenic plants by DAB and NBT staining indicate that transgenic plants have a stronger ability to remove ROS than WT plants and can effectively alleviate oxidative damage in tobacco plants upon stress (Fig. 4E, F). Heterologous expressed Tpx also increase salt and low temperature tolerance in Arabidopsis (Jing et al. 2006). Increasing evidence suggests that overexpression of Tpx enhances plant tolerance to MV-induced oxidative stress and salt-induced osmotic stress (Dietz et al. 2002). In our study, the germination rate of the transgenic plants under H2O2 and MV stress conditions was higher than that of the WT plants (Fig. 8), indicating that transgenic plants have improved tolerance to oxidative stress. Besides, we constructed a Tpx prokaryotic expression vector (Fig. 9), and found that recombinant strain has enhanced the oxidative stress to further verify the function of the Tpx protein to remove H2O2.
In an organism, free radicals act on lipid peroxidation reaction. The oxidation end product is MDA, which will cause crosslinking polymerization of vital macromolecules such as proteins and nucleic acids (Hongbo et al. 2005). MDA content is an important parameter to reflect the body's potential ability to resist oxidation, which can reflect the body's lipid peroxidation rate and intensity, and can also indirectly reflect the degree of tissue peroxidation damage (Alessio et al. 1988; Huang et al. 2009). In our study, there was significantly lower MDA contents in transgenic plant than WT plants after salt stress (Fig. 5B). Tpx increases the clear efficiency of ROS by regulating antioxidant enzyme activity, and subsequently plays a key role in stress regulation (Kowaltowski et al. 2000). In this study, after NaCl stress the SlTpx transgenic tobacco lines showed increased activities of SOD, POD, CAT, and APX as compared to the WT plants (Fig. 6A–D).
As osmoprotective substances, proline and soluble sugars are important components of increasing permeability solutes and play an important role in the resistance physiology of plants (Xiao et al. 2005). Sugars are small molecules that regulate osmotic stress as an important member of increased permeability solutes when plants subjected to stress (Berkowitz and Masmoudi 2007). Proline, however, is involved in the synthesis by sugar and phosphorylation under stress (Roger 2001), and the glutamate pathway is the main pathway of proline synthesis under osmotic stress (Delauney and Verma 1993). Our study evaluated the soluble sugar and proline content of transgenic tobacco under salt stress (Fig. 6E, F), which resulted significantly higher than WT plants. These results showed that the overexpression of Tpx gene improved the synthesis of soluble sugar and proline, and then stabilized the osmotic pressure of plants, and parodied the growth of transgenic plants under salt stress. At the same time, we also measured the gene expression levels of P5CS, LEA5 and Osmotin (Fig. 6G–I), three proteins related to osmoregulation, and found that the gene expression levels in transgenic plants were significantly higher than those in WT. This indicated that the Tpx transgenic plants had improved salt tolerance by regulating the osmotic substance synthesis.
References
Alessio HM, Goldfarb AH, Cutler RG (1988) MDA content increases in fast- and slow-twitch skeletal muscle with intensity of exercise in a rat. Am J Physiol 255(6):C874. https://doi.org/10.1152/ajpcell.1988.255.6.C874
Bai X, Long J, He X, Yan J, Chen X, Tan Y, Li K, Chen L, Xu H (2016) Overexpression of spinach non-symbiotic hemoglobin in Arabidopsis resulted in decreased NO content and lowered nitrate and other abiotic stresses tolerance. Sci Rep 6(1):26400. https://doi.org/10.1038/srep26400
Barranco-Medina S, Krell T, Finkemeier I, Sevilla F, Lázaro J, Dietz KJ (2007) Biochemical and molecular characterization of the mitochondrial peroxiredoxin PsPrxII F from Pisum sativum. Plant Physiol Biochem 45(10–11):729–739. https://doi.org/10.1016/j.plaphy.2007.07.017
Berggren MI, Husbeck B, Samulitis B, Baker AF, Gallegos A, Powis G (2001) Thioredoxin peroxidase-1 (peroxiredoxin-1) is increased in thioredoxin-1 transfected cells and results in enhanced protection against apoptosis caused by hydrogen peroxide but not by other agents including dexamethasone, etoposide, and doxorubicin. Arch Biochem Biophys 392(1):103–109. https://doi.org/10.1006/abbi.2001.2435
Berkowitz FBMHIMGA, Masmoudi K (2007) Overexpression of wheat Na+/H+ antiporter TNHX1 and H+-pyrophosphatase TVP1 improve salt- and drought-stress tolerance in Arabidopsis thaliana plants. J Exp Bot 58(2):301–308. https://doi.org/10.1093/jxb/erl251
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98(4):1222–1227. https://doi.org/10.1104/pp.98.4.1222
Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48(6):749–762. https://doi.org/10.1016/j.freeradbiomed.2009.12.022
Corona M, Robinson GE (2010) Genes of the antioxidant system of the honey bee: annotation and phylogeny. Insect Mol Biol. https://doi.org/10.1111/j.1365-2583.2006.00695.x
Cuadros-Rodriguez L, Torres MH, Lopez EA, Gonzalez FE, Liebanas FA, Martinez Vidal JL (2002) Assessment of uncertainty in pesticide multiresidue analytical methods: main sources and estimation. Anal Chim Acta 454(2):297–314. https://doi.org/10.1016/S0003-2670(01)01546-X
Cui C, Sheng LI, Fang M (1999) The research situation about effects of nitrogen on certain physiological and biochemical process in plants. J Northwest Agric Univ 27(4):102–108
Delauney AJ, Verma D (1993) Proline biosynthesis and osmoregulation in plants. Plant J. https://doi.org/10.1046/j.1365-313X.1993.04020215.x
Dietz KJ, HorlingBaier FM (2002) The function of the chloroplast 2-cysteine peroxiredoxin in peroxide detoxification and its regulation. J Exp Bot 53(372):1321–1329. https://doi.org/10.1093/jexbot/53.372.1321
Gaber A, Yoshimura K, Tamoi M, Takeda T, Shigeoka NS (2004) Induction and functional analysis of two reduced nicotinamide adenine dinucleotide phosphate-dependent glutathione peroxidase-like proteins in Synechocystis PCC 6803 during the progression of oxidative stress. Plant Physiol 136(1):2855–2861. https://doi.org/10.1104/pp.104.044842
Gay CA, Gebicki JM (2003) Measurement of protein and lipid hydroperoxides in biological systems by the ferric-xylenol orange method. Anal Biochem 315(1):29–35. https://doi.org/10.1016/s0003-2697(02)00606-1
Gui-Qin S, Quan-You Yu, Zhang (2012) Annotation and evolution of the antioxidant genes in the silkworm, Bombyx mori. Arch Insect Biochem Physiol 79(2):87–103. https://doi.org/10.1002/arch.21014
Guo ZL, Bai XG, Yan JP, Chen XQ, Kun-Zhi LI, Hui-Ni XU (2015) Prokaryotic expression and function analysis of So Hb from spinach. China Biotechnol 33(1):80–85
He X, Zhuo R, Han X, Liu M, Jianju (2016) Overexpression of quinone reductase from Salix matsudana Koidz enhances salt tolerance in transgenic Arabidopsis thaliana. Gene Int J Focus Gene Cloning Gene Struct Funct 576(1):520–527. https://doi.org/10.1016/j.gene.2015.10.069
Hongbo S, Zongsuo L, Mingan S (2005) Changes of anti-oxidative enzymes and MDA content under soil water deficits among 10 wheat (Triticum aestivum L.) genotypes at maturation stage. Colloids Surf B 45(1):7–13. https://doi.org/10.1016/j.colsurfb.2005.06.016
Huang Y, Zhang W, Zhao L, Cao H (2009) Effects of Si on the index of root activity, MDA content and nutritional elements uptake of rice under salt stress. Asian Journal of Ecotoxicology 4(6):860–866
Jing LW, Chen SH, Guo XL, Zhang H, Zhao YX (2006) Overexpression of a chloroplast-located Peroxiredoxin Q gene, SsPrxQ, increases the salt and low-temperature tolerance of arabidopsis. J Bot 48(10):6. https://doi.org/10.1111/j.1744-7909.2006.00357.x
Kang H, Zhang M, Zhou S, Guo Q, Chen F, Wu J, Wang W (2016) Overexpression of wheat ubiquitin gene, Ta-Ub2, improves abiotic stress tolerance of Brachypodium distachyon. Plant Sci 248:102–115. https://doi.org/10.1016/j.plantsci.2016.04.015
Kim YS, Kim JJ, Park SI, Diamond S, Yoon HS (2018) Expression of OsTPX gene improves cellular redox homeostasis and photosynthesis efficiency in Synechococcus elongatus PCC 7942. Front Plant Sci 9:1848. https://doi.org/10.3389/fpls.2018.01848
Kimoto H, Yoshimune K, Matsuyma H, Yumoto I (2012) Characterization of catalase from psychrotolerant Psychrobacter piscatorii T-3 exhibiting high catalase activity. Int J Mol Sci 13(2):1733–1746. https://doi.org/10.3390/ijms13021733
Koh CS, Didierjean C, Navrot N, Panjikar S, Mulliert G, Rouhier N, Jacquot JP, Aubry A, Shawkataly O, Corbier C (2007) Crystal structures of a poplar thioredoxin peroxidase that exhibits the structure of glutathione peroxidases: insights into redox-driven conformational changes. J Mol Biol 370(3):512–529. https://doi.org/10.1016/j.jmb.2007.04.031
Kowaltowski AJ, Vercesi AE, Rhee SG, Netto L (2000) Catalases and thioredoxin peroxidase protect Saccharomyces cerevisiae against Ca(2+)-induced mitochondrial membrane permeabilization and cell death. FEBS Lett 473(2):177–182. https://doi.org/10.1016/S0014-5793(00)01526-X
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. https://doi.org/10.1093/molbev/msw054
Livak KJ, Schmittgen TD (2013) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (san Diego, CA) 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Madhava Rao K, Sresty T (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157(1):113–128. https://doi.org/10.1016/s0168-9452(00)00273-9
Mazel A, Leshem Y, Tiwar BS, Levine A (2004) Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7 (AtRabG3e). Plant Physiol 134(1):118–128. https://doi.org/10.1104/pp.103.025379
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33(4):453–467. https://doi.org/10.1111/j.1365-3040.2009.02041.x
Munns R, Gilliham M (2015) Salinity tolerance of crops—what is the cost? N Phytologist 208(3).
Nathan C, Cunningham-Bussel A (2013) Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol 13(5):349–361. https://doi.org/10.1038/nri3423
Qi Q, Dong Y, Liang Y, Li K, Sun X (2020) Overexpression of SlMDHAR in transgenic tobacco increased salt stress tolerance involving S-nitrosylation regulation. Plant Sci 299:110609. https://doi.org/10.1016/j.plantsci.2020.110609
Rana M, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Roger MR (2001) Handbook of plant ecophysiology techniques. Handb Plant Ecophysiol Tech 42(4):1387–1388. https://doi.org/10.2135/cropsci2002.1387a
Sankara RK, Rohini VK (1999) Agrobacterium -mediated transformation of sunflower (Helianthus annuus L.): a simple protocol. Ann Bot. https://doi.org/10.1006/anbo.1998.0828
Siddiqi MY, Malhotra B, Min X, Glass A (2002) Effects of ammonium and inorganic carbon enrichment on growth and yield of a hydroponic tomato crop. J Plant Nutr Soil Sci 165(2):191. https://doi.org/10.1002/1522-2624(200204)165:2%3c191::AID-JPLN191%3e3.0.CO;2-D
Woo HA, Sun HY, Dong HS, Kang D, Yu DY, Rhee SG (2010) Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell 140(4):517–528. https://doi.org/10.1016/j.cell.2010.01.009
Xiao Q, Zheng H, Chen Y, Huang W, Zhu Z (2005) Effects of salinity on the growth and proline, soluble sugar and protein contents of Spartina alterniflora. Chin J Ecol 4:373–376
Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57(3):508. https://doi.org/10.1042/bj0570508
Zhang Y, Mi K, Ding X, Wang Y, Dou T, Ding J, Wei W (2019) Characterization of a classical 2-cysteine peroxiredoxin1 gene from Chinese soft-shelled turtle Pelodiscus sinensis with its potent antioxidant properties and putative immune response. Dev Comp Immunol 101:103456–103456. https://doi.org/10.1016/j.dci.2019.103456
Zhao CX, Nan JD, Chun-Quan MA, Bing YU, Chen SX, Hai-Ying LI (2015) Oxidation resistance and salt resistance analysis of Thioredoxin peroxidase(BvM14-Tpx) gene from Sugar Beet M14 line in transgenic prokaryotic and eukaryotic cells. J Eng Heilongjiang Univ 6(3):62–67
Acknowledgements
This research was funded by the National Natural Science Foundation of China (Grant No. 31760582) and the Yunnan Ten Thousand Talents Plan: Young & Elite Talents Project.
Funding
Funding was provided by National Natural Science Foundation of China (Grant No. 31760582).
Author information
Authors and Affiliations
Contributions
HNX designed the project and wrote the article. STQ and YF, conducted the experiments and analyzed the data. JPY and KZL helped in the writing of the article. All authors agreed on the final content of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest related to this study.
Additional information
Communicated by Jose M. Segui-Simarro.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiao, S., Feng, Y., Yan, J. et al. Overexpression of tomato SlTpx improves salt stress tolerance in transgenic tobacco plants by scavenging H2O2. Plant Cell Tiss Organ Cult 151, 321–333 (2022). https://doi.org/10.1007/s11240-022-02354-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-022-02354-w