Abstract
CRISPR/Cas9 has emerged as a simple, yet efficient gene editing tool to generate targeted mutations in desired genes in crops plants. Agrobacterium tumefaciens, a reliable and inexpensive DNA-delivery mechanism into plant cells, has been used for the generation of CRISPR/Cas9-mediated mutations in crop plants, including potato. However, little information is available as to the progression of gene knockout during various stages of culture following the introduction of CRISPR components in this species. In the current study, the green fluorescent protein (gfp) transgene was first introduced in the genome of a potato variety, Yukon Gold. Two GFP-expressing lines, one with a single gfp copy integrated and another with four gfp copies integrated, were subjected to CRISPR/Cas9-mediated mutations in the transgene(s) using three different gRNAs. Disappearance of GFP fluorescence was monitored during the entire culture/regeneration process. Although all three gRNAs successfully knocked out the transgene(s), their efficiencies differed greatly and did not completely match the predicted scores by some guide RNA prediction tools. The nature of mutations in various knockout events was analyzed. Several lines containing four gfp-copies showed four different types of mutations. These findings suggest that it is possible to target all four alleles of a desired native gene in the tetraploid potato.
Key message
Potato lines, with a single or four copies of the gfp transgene integrated, were subjected to CRISPR/Cas9-mediated mutations. The results indicated that it should be possible to target and knock out multiple copies of a native gene in the tetraploid genotypes of this crop.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potato (Solanum tuberosum L.) is an important and the largest vegetable crop. It ranks fourth worldwide among all the food crops, only behind maize, rice, and wheat in terms of global production tonnage (FAOSTAT 2019). Potato is a global crop with most diverse distribution pattern that is grown in over 150 countries on ~ 16 million hectare of land and serves as a staple food for around 1.3 billion people (Camire et al. 2009; FAOSTAT 2019; Devaux et al. 2020). With approx. 160 cal from a medium size potato, mostly derived from its starch content, it constitutes an important source of energy for a large segment of impoverished people in many parts of the world and also provides other necessary nutrients, including vitamins and minerals. Even in the developed countries, potatoes are widely consumed in many forms, both as freshly-cooked meals and as processed food such as French fries and chips. Thus, given the wide utilization of potato as food among all socioeconomic groups and its contribution to the basic nutrition requirements of a large segment of human population, it offers an attractive target for genetic improvement. Potato is a cool season crop that is relatively sensitive to heat and drought stress. It also suffers from pests such as Colorado beetle, aphids, and nematodes, as well as diseases, early blight, zebra chip, Fusarium dry rot, and especially viral diseases (PVY, PVX, PVA, etc.) and late blight caused by Phytophthora infestans (Haverkort et al. 2013). In the face of a growing global population, environmental degradation and climate change, there is a critical need to improve the productivity of this important crop in an environmentally sustainable manner. Traditional breeding methods to improve potato are slow and cumbersome. This is because the majority of commercially-grown potatoes are autotetraploid with tetrasomic inheritance, highly heterozygous, have large complex genomes, suffer from inbreeding depression and often have self- and cross-compatibility issues (Campos and Ortiz 2020). In addition to these limitations, the vegetative manner of propagation for this crop also slows down introduction and multiplication of new varieties (Stokstad 2019). Thus, considering the vulnerability to many biotic and abiotic stresses and limitations of the traditional breeding methods, potato is a good target for improvement via modern biotechnology tools.
Technologies capable of introducing new traits in a rapid and more efficient manner are valuable and desirable for crops such as potato. If the sequence and the function of a desirable gene in a wild relative of potato are known, the recently developed CRISPR/Cas9 technology offers a more direct and faster means to incorporate the desired traits into the popular commercial varieties (Doudna and Charpentier 2014; Gilbert et al. 2013). The CRISPR/Cas9 system relies on two components: a guide RNA and a nuclease. These two components act together to introduce a targeted double-stranded break (DSB) in the DNA (Jiang and Doudna 2017). A DSB in the cellular DNA is repaired either by the more predominant, non-homologous end-joining (NHEJ) mechanism or, less frequently, by the homologous recombination (HR). The CRISPR/Cas9 system, by generating a DSB at a precise DNA locus, enables these repair pathways to introduce mutations (indels or replacements) at the target site resulting in the loss of function of a desired gene (Puchta 2005; Jiang and Doudna 2017; Qi 2019; Schaart et al. 2021). CRISPR/Cas9 system has been used previously to introduce modifications in the genome of potato. Some examples include: targeting genes such as: S-RNase (S-locus RNase) gene, which is involved in the gametophytic self-incompatibility of the plant (Enciso-Rodriguez et al. 2019), StPPO2 gene which is part of the family of polyphenol oxidases (PPO) genes present in potato that are responsible of the enzymatic browning of the tuber (González et al. 2020), and starch-branching enzyme genes leading to reduction in starch-amylopectin (Tuncel et al. 2019). However, commercial potato being a polyploid, does add to the challenges as compared to diploid crops in terms of its modification by the CRISPR/Cas9 system. (Schaart et al. 2021).
In this investigation, we targeted the genome-integrated gfp transgene whose disruption results in a phenotype that is easily and rapidly detected in a non-destructive manner. Its use allowed us to monitor the progression of gene knockout over time and provided some clues as to the timing to initiate the regeneration process in cases where native genes are the targets for mutations, but do not result in easily visible phenotypes. The same three DNA sequences within the gfp were targeted as the ones in a study previously conducted on cotton in our laboratory (Janga et al. 2017). This deliberate choice of the targets was made in order to examine the relative efficacies of the three gRNAs and also to compare the nature of mutations generated in the same gene residing in the genomes of two unrelated species.
Since potato is a tetraploid, it will be necessary to disrupt the function of all four alleles of a given native gene to engineer certain traits. Therefore, GFP-expressing lines with a single transgene copy and also four transgene copies integrated were targeted with the CRISPR/Cas9 to examine the efficacy of the system in knocking out multiple copies of a desired gene.
Of the three popular methods, including protoplast, gene gun and Agrobacterium, used for genetic manipulation of plants, the Agrobacterium-mediated transformation has proven to be more reliable, efficient and least expensive. Agrobacterium tumefaciens-mediated transformation methods for potato are well established and successful reports of transgene introduction into tuber and leaf explants were published as early as 1988 (De Block 1988; Sheerman and Bevan 1988; Stiekema et al. 1988). Therefore, the Agrobacterium system was used first to introduce the gfp gene(s) into potato genome and then to create CRISPR/Cas9-mediated mutations in the transgene. The specific objectives were: (i) to establish a workable transformation protocol using the gfp transgene, (ii) to evaluate transformation/regeneration efficiencies in three different potato varieties using a construct designed to express the gfp and nptII genes, (iii) to create mutations in the gfp transgene using the CRISPR/Cas9-mediated DSBs, and iv) to evaluate the efficiency and nature of mutations generated.
Materials and methods
Transformation with gfp vector
The three potato varieties used were Russet Norkotah (fresh market russet), White LaSoda (fresh market, white flesh, white-skin clonal variant of the red skin potato clone Red LaSoda) and Texas Yukon Gold (TXYG79; a clonal variant of Yukon Gold with fewer eyes), obtained from the Texas A&M Potato Breeding Program. The transformation protocol utilized was essentially that described by Chetty et al. (2015). A. tumefaciens (LBA4404) carrying the binary vector, pBINmGFP5-ER (Sunilkumar et al. 2002), was used to transform leaf and internodes explants obtained from in vitro cultured plantlets. The T-DNA within pBINmGFP5-ER harbors a kanamycin-resistance gene (nptII) cassette and also a modified green fluorescence protein (mgfp) expression cassette (Siemering et al. 1996; Haseloff et al. 1997). Following Agrobacterium infection, the reporter gene allowed us to evaluate transformation/regeneration ability of the three potato varieties. Using the protocol described by Chetty et al. (2015), we were able to obtain transgenic plants only of the variety Yukon Gold. Briefly, the growing callus at the cut end of the explant were transferred to shoot induction medium under light after four weeks of culture. Following regeneration of shoots, these were excised and individually transferred to root induction media. The regenerated plants expressing the gfp gene were grown to tuberization stage, both in vitro and in soil. Plantlets were cultured in dark on Murashige and Skoog (MS) medium containing 90 g/L sucrose to induce microtuber formation. Selected lines were also grown in soil (Pro-Mix BX Mycorrhizae) in a growth chamber under fluorescent lights, 20 °C temperature and the photoperiod being: 16 h light/8 h dark for plant development and 12 h light/12 h dark until these reached the tuberization stage.
Fluorescence observations and scoring
Stable, kanamycin-resistant transgenic lines were observed under a Zeiss M2 BIO Fluorescence Combination Zoom Stereo/Compound microscope during all stages of culture until plant regeneration. Fluorescence was visualized using either a 500 nm Long Pass Filter or a 525 nm Narrow Band Pass Filter that blocks red-colored fluorescence from plastids and the images were captured with a Zeiss AxioCam color digital camera coupled to the microscope. The shoots that showed green fluorescence resulting from gfp expression were selected and transferred to root induction media and further cultured to develop plants capable of growing in soil. Leaves and stem from the regenerated lines were scored microscopically for the intensity of GFP fluorescence.
Southern blot analysis
Southern blot analysis was performed to confirm integration and to ascertain transgene copy number in the regenerated plants. Genomic DNA was obtained from leaves using the Plant Isolate DNA Extraction Kit (Alfa Aesar; Catlog no. J67554) and Southern blot analysis was performed following restriction enzyme digestion of the genomic DNA with EcoRI and using standard protocols. Radio-labeled [α-32P] deoxycytidine triphosphate (dCTP; PerkinElmer, Waltham, MA) probes were used to detect the presence and integration of gfp and nptII genes in the genomes of transgenic lines. Note that different GFP-expressing plants are referred to as ‘lines’ in this paper.
CRISPR/Cas9-mediated knock out of gfp transgene
Two transgenic lines were selected for targeted mutagenesis, one with a single copy gfp transgene integrated in its genome and the second one with four integrated copies of the gfp transgene. Note that since the nptII gene and kanamycin were used to select the gfp expressing lines, hygromycin-resistance gene (hpt) and hygromycin selection were used for introducing the Cas9-sgRNA construct to knock out the gfp transgene.
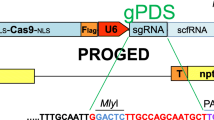
The binary vector needed for this part of the project was assembled using plasmids pTC212, pTC241 and pCGS751, kindly provided by Dr. Daniel Voytas, University of Minnesota. The same three sequences within the gfp gene (Target 1: GGGCACAAATTTTCTGTCAGTGG, Target 2: CTTGTCACTACTTTCTCTTATGG, and Target 3: GATACCCAGATCATATGAAGCGG) were targeted as those done by Janga et al. (2017). Using the Golden Gate cloning method and Esp31 as the restriction enzyme, each pair of oligonucleotides were annealed, phosphorylated and introduced into pTC241 (Thermo Fisher Scientific, USA) following the method described in Cermak et al. (2015); Cong et al. (2013) with slight modifications. The plasmid vector pTC212 contains the Cas9 gene that was codon optimized for Arabidopsis. (Baltes et al. 2014). The two DNA sequences (regulating the expression of sgRNA and Cas9), contained within pTC241 and pTC212, were cloned into the binary vector pCGS751 by the Golden Gate cloning method using AarI restriction enzyme (Thermo Fisher Scientific, USA) (Cermak et al. 2015; Cong et al. 2013). The final order of the selectable marker gene and CRISPR components in the T-DNA is shown in Fig. 1. Three such plasmids were prepared, one for each gRNA and transformed independently into either single copy or four copy gfp-expressing line.
T-DNA region of the binary vector. It harbors hygromycin resistance gene (hpt) under the control of the CaMV 35S promoter, CRISPR associated protein 9 (CAS9) under the control of the CaMV 35S promoter and the single guide RNA (sgRNA) expression sequence under the control of the Arabidopsis U6 promoter (AtU6 promoter)
Qualitative analysis of knockout events
Following transformation with the Cas9-sgRNA constructs, the culture/regeneration procedure described above was used to obtain gfp knockout events. The explants and growing cultures were observed via fluorescence microscopy every week to examine the knockout phenotype, counting the sectors that had lost their GFP fluorescence and showed red fluorescence under UV light. At the time of transferring regenerated shoots from Petri dishes to the root induction media in glass jars, only the shoots that showed the gfp-knockout phenotype were transferred. From this group of regenerated, gfp-knockout plantlets, ten events were selected randomly to move forward for molecular characterization. Note that following the transfer of selected events to jars, it was not possible to use the microscope for observations. Instead, a handheld UV light source NIGHTSEA BlueStar™ flashlight in combination with VG2 Filter Glasses (CNTech Lab Supplies, Wisbech, Cambridgeshire, UK) were used to observe the fluorescence status of the plantlets and also to choose 1–2 leaves for DNA isolation for sequencing purposes. Note that different gfp-knockout plants are referred to as ‘events’ in this paper.
Molecular characterization of mutations
To ascertain the nature of mutations present in the knockout events, PCR primers were designed from the flanking regions of the target site as specified by Janga et al. (2017). The amplicons (650 bp) were sequenced and aligned to the original sequence. This step allowed us to determine the nature of mutations present in each event. Sanger sequencing was used for the single-gfp-copy knockout events. While for the four-gfp-copies knockout events, next generation sequencing was performed (Novogene Corporations Inc., Sacramento, CA, USA). Primers with tags were designed and used to amplify the sequences around the target region. First, Trimmomatic software was used on the sequences obtained to trim and filter out low-quality reads (Bolger et al. 2014) and then FLASH2 was used to merge paired sequences (Magoč and Salzberg 2011). Finally, CRISPResso was used to assess the nature of mutations present in each event (Pinello et al. 2016). The results obtained were further confirmed using CLC Genomics Workbench 8.0.1 software (QIAGEN).
Results
Transformation and regeneration
Initially, we utilized both leaf segments and internode explants, however, only the internode explants produced callus following transformation with Agrobacterium. All three potato varieties used produced callus. However, following the excision of such callus growth from the internode and its transfer to fresh selection medium, not all calli survived. Only the calli that continued to grow on fresh medium supplemented with kanamycin and those showing GFP fluorescence were considered successfully transformed. Out of the three varieties evaluated, only one, Yukon Gold, was able to regenerate into plantlets (Fig. 2a). Russet Norkotah and White LaSoda did not progress beyond the callus stage (Fig. 2a). Transformation to microtuber formation stages for Yukon Gold are shown in Fig. 2b.
Recovery of transformants following Agrobacterium-mediated transformation of potato. a Comparison among three varieties (Russet Norkotah, White LaSoda and Texas Yukon Gold) related to their ability to form callus and regenerate following transformation. b Stages depicting potato transformation/regeneration/microtuber formation. I: Internode explants used for transformation; II: Callus formation; III: Shoot formation; IV: Root formation; V: Maintenance/plantlet development in a culture jar; VI: In vitro tuberization in a culture jar
Transgene expression analysis
Ten randomly selected lines were evaluated visually for GFP expression. These were scored on an arbitrary scale as follows: ( +) Low GFP expression, (+ +) Medium GFP expression, and (+ + +) High GFP expression. Examples of various GFP expression levels are shown in Fig. 3. Lines 3, 4 and 10 being the ones with the lowest score, Lines 1, 5, 8 and 9 having a medium score, and Lines 2, 6 and 7 being the lines with the highest score.
Examples of each type of GFP expression score: ( +) Low GFP expression, (+ +) Medium GFP expression, and (+ + +) High GFP expression. Untransformed control images are also shown for comparison. The tissues examined were leaf and stem, and were observed either with 500 nm long pass filter or 525 nm narrow band-pass filter
Southern blot analysis
EcoRI-digested genomic DNA from ten regenerated lines was used for southern blot analysis. Two different probes were used separately to detect either the nptII gene or the gfp gene. Using the nptII probe, we found six lines with two copies and four lines with a single-copy integration. Lines 2, 6, 7 and 8 showed two bands each with similar banding pattern indicating that these lines may possibly be siblings. Four lines were probed with the gfp probe. Line 6 showed integration of four copies of the gfp gene, while lines 5, 9 and 10 showed single-copy integration of the gfp gene (Fig. 4). Although line 6 contained four copies of the gfp gene, the possibility exists that not all copies are functional, which depends on the site of integration in the genome and the integrity of the entire GFP expression cassette. We have observed such differences between integrated copy numbers for two different transgenes, residing on the same T-DNA, in some of our previous studies on cotton. Integration of intact and partial copies of the T-DNAs at different sites in the genome was found to be the basis for such results (unpublished).
Southern blot analysis of GFP expressing lines. a Partial map of the transformation construct harboring gfp and nptII genes. The positions of EcoRI restriction site and the two probes are also shown. b Blots obtained using the nptII probe (left) and the gfp probe (right). Lanes labelled (P) and (C) are lanes with and without the binary vector
Qualitative analysis of knockout events
Two lines, showing gfp integration and expression, were selected to generate CRISPR/Cas9-mediated mutations. These include Line 5 and Line 6, with a single copy and four copies of the gfp transgene integrated, respectively. Three different gRNAs designed previously by Janga et al. (2017) were used.
Transformed explants were monitored via fluorescence microscopy every week for the knockout phenotype. At week three, calli originating from the use of gRNA2 and gRNA3 started to show gfp-knockout phenotype. At this stage, few, if any, gRNA1-targeted calli showed gfp-knockout phenotype. It should be noted that the half-life of mGFP5-ER is not known, however, wild-type GFP has a half-life of ~ 26 h in mammalian cells (Corish and Tyler-Smith 1999). Thus, it is possible that observations at this three-week stage do not reflect the true extent of all the knockout events. At week four and five, several calli for gRNA1 began to show sectors that lacked GFP fluorescence. Progression of knockout phenotype is indicated by the diminution of GFP fluorescence and appearance of plastid-based, red fluorescence as shown for time points 3-, 4- and 5-weeks with all three gRNAs (Fig. 5a). Thirty days after infection, the highest knockout efficiencies were observed with gRNA3 at 59.7% (single gfp copy line) and 53.2% (four gfp copies line) based on visual observations, followed by 23% and 26.3% for gRNA2 (single and four gfp-copy, respectively) and 11.7% and 23.1% for gRNA1 (single and four gfp-copy, respectively). Thus, gRNA3 proved to be the most efficient while gRNA1 being the least effective in knocking out gfp gene function (Fig. 5b). Nine weeks after infection, only the emerging shoots showing gfp-knockout phenotype were transferred to the root induction media to regenerate complete plantlets from which events were selected randomly to perform molecular characterization.
Progression of CRISPR/Cas9-mediated mutations in the gfp gene in callus tissue growing at the cut surface of internode explants obtained from a four-copy line. a At 3 weeks, 4 weeks and 5 weeks following transformation. Red arrows show parts of callus where the gfp gene has been successfully mutated and became non-functional. b GFP-fluorescence knockout phenotype (percentage) observed in cultures at 20- and 30 days post infection in response to three different gRNA. Calli were observed under a fluorescence microscope. Dpi: days post-infection
Molecular characterization of mutations
Sanger sequencing results for the knockout events resulting from the targeting of single-copy gfp line showed a variety of mutations, including SNPs, small (1–4 bp) indels and large deletions (10–73 bp). Various types of mutations observed are shown in Fig. 6. Interestingly, gRNA1 and gRNA2 resulted in a wider variety of mutations compared to gRNA3, including some large deletions. Whereas gRNA3 generated relatively smaller deletions (1–4 bp) that were similar in nature among different knockout events.
Nature of mutations ascertained by sequencing the PCR products that were amplified from the genomic DNA obtained from the leaves of various knockout events. A single, integrated gfp copy line was subjected to mutations with three different gRNAs. The target sequence in the unedited, wild-type (WT) gfp is shown with an overline, PAM nucleotides are indicated with darker overline, and the Cas9 cleavage site is denoted with “-”. T#-# indicates the target and the knockout event number for each target
Although it is not possible to know whether all four gfp copies of the gene are functional in the 4-gfp-copies line subjected to CRISPR/Cas9 treatment, if at least four different mutations are seen in an event that has lost GFP fluorescence, it would indicate that all four copies were targeted and mutated. The sequencing results for mutations in the three target regions in this 4-gfp-copies line are shown in Figs. 7, 8. In the case of target 1 and 2, sequences showing 1–3 types of mutations and wild-type sequences were observed. For Target 3, most of the events showed 1–4 different types of mutations, with the exception of event T3-4 that had one wild-type sequence. For this Target 3, three events showed only one type of mutation (same 1 bp deletion in T3-1, T3-2 and T3-3). Another interesting result for all three targets was that multiple events showed exactly the same type of large deletion. For example, for Target 1, events T1-3, T1-5 and T1-6 show the same 43 bp deletion, for Target 2, events T2-2, T2-3, T2-4 and T2-5 show the same 94 bp deletion, and for Target 3, events T3-5, T3-6, T3-7 and T3-9 show the same 78 bp deletion. In the case of target 3, with the exception of event T3-4, none of the events showed an unmutated wild-type copy indicating that all copies of the gfp gene were mutated. The fact that it is possible to achieve mutations in all four copies of the transgene present in the potato genome suggests that when targeting a native gene in the tetraploid potato, it should be possible to knock out all four alleles of a native gene using the CRISPR system.
Nature of mutations ascertained by the next generation sequencing of PCR products that were amplified from the genomic DNA of leaves from different events that showed complete GFP-knockout phenotype. A line with four integrated gfp copies was subjected to mutations at Target 1 and Target 2. The target sequence in the unedited, wild-type (WT) gfp is shown with an overline, PAM nucleotides are indicated with darker overline, and the Cas9 cleavage site is denoted with “-”. T#-#-alphabet indicates the target, the knockout event number for each target and the type of sequence obtained
Nature of mutations ascertained by the next generation sequencing of PCR products that were amplified from the genomic DNA of leaves from different events that showed complete GFP-knockout phenotype. A line with four integrated gfp copies was subjected to mutations at Target 3. The target sequence in the unedited, wild-type (WT) gfp is shown with an overline, PAM nucleotides are indicated with darker overline, and the Cas9 cleavage site is denoted with “-”. T#-#-alphabet indicates the target, the knockout event number for each target and the type of sequence obtained
Discussion
Potato, being an important food crop, makes a good target for introducing new, desirable traits. As stated earlier, the development of new commercial varieties of potatoes by plant breeding is difficult and infrequent. In general, the plant breeding process is slow for most crops; it is even slower for potato. Breeders have to be mindful that when desired traits are brought into commercial varieties from the wild genotypes, there is a high possibility that the next generation might lose the established traits and express unwanted traits co-introduced from the wild relatives (Stokstad 2019).
Potato is a relatively easy species to transform, being one of the first crops to be successfully transformed using the Agrobacterium method as demonstrated by a study that utilized A. rhizogenes to determine the transformation efficiency (Ooms et al. 1986). As is the case with most plant species, the transformation efficiency of potato depends on the variety and the type of explant used. In the current study, the explants used for transformation were internodes. As shown by Bruce and Shoup Rupp (2019), this explant has shown the greatest transformation efficiency. During the establishment of potato transformation protocol in the current study, leaves were also evaluated in addition to the internodes. However, following co-cultivation of leaf segments with Agrobacterium, the tissue showed severe damage and no callus growth. All three varieties that were examined using the protocol described in materials and methods proved amenable to Agrobacterium-mediated transformation. However, only one variety (Yukon Gold) was able to go through regeneration stage to yield transgenic plants. Our results support general observations by others in that regeneration and also the Agrobacterium-mediated transformation depend on the plant genotype used. By optimizing the media and culture conditions, it may be possible to regenerate the two potato varieties that failed to do so in the current investigation.
In this study, Agrobacterium-mediated transformation was used to introduce the CRISPR reagents that created mutations in a non-native, integrated gfp transgene in the potato genome. The objective was to target the gfp gene in two different GFP expressing lines: one with a single copy of the gfp integrated and another one with four copies of the gfp gene integrated. In order to compare the mutability of various sequences within the gfp gene targeted by the CRISPR/Cas9 system in potato, the same three gRNAs used by Janga et al. (2017) in cotton were deployed in this study. The knockout efficiency of the system depends on multiple factors related to the gRNA sequence, for example, percentage of GC content and the secondary structures. Expression levels of Cas9 and gRNA also have an effect on the efficiency of the system (Baysal et al. 2016; Liang et al. 2016; Ma et al. 2015).
In the investigation to examine the efficiency of three gRNAs, gRNA3 proved to be the most efficient in knocking out GFP expression (59.69%–single gfp copy line and 53.23%–four gfp copies line), followed by gRNA 2 (22.96%–single gfp copy line and 26.25%–four gfp copies line) while gRNA1 was the least efficient (11.73%–single gfp copy line and 23.13%–four gfp copies line). These results for gRNA1 and gRNA2 efficiencies, while not consistent with the predicted scores, are in line with those observed in cotton by Janga et al. (2017). In the cotton study, gRNA1, gRNA2 and gRNA3 sequences were based on scores obtained from gRNA Scorer 1.0. The observed mutation efficiency for gRNA 3 (predicted score: 91.2) was highest in the cotton study and thus in compliance with the predicted score. However, the expected and observed knockout efficiencies showed discrepancies for the other two gRNAs. gRNA 1, with medium score (predicted score: 51.2), proved to be the least efficient while the gRNA2 with the lowest score (predicted score: 2.2) showed medium efficiency (Janga et al. 2017). Such discrepancy prompted these investigators to examine the prediction score for gfp using another web tool, WU-CRISPR (Wong et al. 2015). This program considers various characteristics of gRNA such as the GC content, secondary structure, contiguous stretch of the same nucleotides, and free accessibility of the seed region for target recognition (i.e., avoiding the use of U at position 19 and use of C or U at position 20 in the guide sequence). Thirteen different possible targets were predicted by this tool. Only the gRNA3 target sequence, but not gRNA1- and gRNA2-target sequences, was predicted to be among these 13 targets (Janga et al. 2017). The discrepancies between the predicted and observed gRNA scores that were observed in these two investigations on potato and cotton have also been reported by Baysal et al. (2016) in rice and Wang et al. (2016) in wheat. Our results, taken together with those from these previous studies suggest that the efficiency of the CRISPR/Cas9 system depends on sequence of the gRNA and not on the plant species in which it is being used. Taken together, the results from various studies suggest that it is advisable to use more than one prediction program to design the gRNAs and then select the one with high efficiency score which is also predicted by multiple guide RNA design tools.
In both cases, CRISPR-targeting of single copy- and four copies gfp-integrated lines, it is interesting that gRNA1 and gRNA2 were less efficient (Fig. 5a, b). However, in the case of single gfp copy line, both gRNA1 and gRNA2 resulted in a wider variety of mutations compared to gRNA3, including some large deletions. Whereas gRNA3 generated relatively smaller deletions (1–4 bp) that were similar in nature among different knockout events. These results with regards to large (gRNA1 and gRNA2) or small deletions (gRNA3) are generally in line with those observed by Janga et al. (2017) in cotton. Such differences in the nature of mutations among the three gRNAs were not observed when the line with four gfp copies was subjected to CRISPR treatment.
In the gfp knockout experiment, wherein the single-gfp copy line was targeted by the CRISPR, sequencing of PCR amplicons showed mutations (indels and substitutions) in all the regenerated events. Interpretation of the results shown in Fig. 7, 8 for the knockout experiments on the four-gfp copies line is complicated by two factors. Firstly, the mode of regeneration in potato is via organogenesis resulting in chimeric shoots, secondly, the genome-integrated Cas9-sgRNA cassettes continue to be active throughout the growth of cultures and plantlets. However, some conclusions can be drawn safely. In the case of CRISPR-targeting of four-gfp copies line, several knockout events showed 1–3 types of mutations along with unmutated (wild-type) copy. Great care was taken to select shoots that showed complete knockout of the gfp gene. This fact taken together with the presence of wild-type sequences suggests that not all four copies of the gfp gene were functional. The differences in transgene copy numbers for the gfp and nptII genes also suggest that some T-DNAs were partially integrated in line 6 (Fig. 4). However, there were some events that showed four different types of mutations with gRNA3 (T3-6, T3-7, T3-8 and T3-9) indicating that it was possible to target four different copies of a gene in the potato genome with the CRISPR system. Some events had 2–3 mutated copies but no wild-type copy (T2-7, T3-5 and T3-10) suggesting the possibility of homozygous mutations in these events as previously observed in cotton by Janga et al. (2017), in rice by Ma et al. (2015), and in tomato by Pan et al. (2016).
The two alternative methods, protoplast and gene gun, have the advantage in terms of direct delivery of the required gene editing reagents: Cas9 enzyme and sgRNA (ribonucleoproteins, RNP), thus avoiding transgene integration in the cellular genome. Crops edited in this manner lessen the regulatory burden in some countries and allow the edited plants to be labeled as “GMO-free”. However, the edited plants obtained from the protoplast method are more likely to contain somaclonal variations due to much longer culture period. Shepard et al. (1980) showed that genetic variations are common when using protoplasts for potato transformation. Gene gun-mediated delivery method has been shown to cause unintended damage to the genome of the recipient cell (Liu et al. 2019). While the Agrobacterium-mediated delivery, followed by integration of Cas9 and sgRNA cassettes whose continued activities through callus growth, plant regeneration and development, offers a more reliable and less disruptive system to edit all intended targets. The desired mutations can then be segregated away from the CRISPR-encoding reagents by traditional breeding methods.
Agrobacterium proved to be an efficient system to introduce CRISPR/Cas9-mediated mutations in a targeted manner in the potato genome. The results pertaining to the progressive knockout of gfp transgene indicate that it is important to wait for at least 30 days following transformation before transfer of the cultured tissue to shoot regeneration medium is attempted. As expected, the efficiencies of various gRNAs varied and the results suggest the need for careful choice of the target sequence within a gene selected for mutations using multiple gRNA prediction tools. However, if an efficient gRNA is used, it should be possible to knock out all four alleles of a native gene in the tetraploid potato. In conclusion, the results described in this paper will be helpful in future studies involving CRISPR/Cas9-mediated mutations in the native genes of potato. The Agrobacterium-based, CRISPR/Cas9 delivery method described in this paper has been used to knock out all four alleles of the gbssI gene in tetraploid potato, variety Yukon Gold to obtain amylose-free starch in the tubers (Toinga-Villafuerte et al. 2022).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on a reasonable request.
References
Baltes NJ, Gil-Humanes J, Cermak T, Atkins PA, Voytas DF (2014) DNA replicons for plant genome engineering. Plant Cell 26:151–163. https://doi.org/10.1105/tpc.113.119792
Baysal C, Bortesi L, Zhu C, Farré G, Schillberg S, Christou P (2016) CRISPR/Cas9 activity in the rice OsBEIIb gene does not induce off-target effects in the closely related paralog OsBEIIa. Mol Breed 36:108–119. https://doi.org/10.1007/s11032-016-0533-4
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinform (oxford, England) 30:2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Bruce MA, Shoup Rupp JL (2019) Agrobacterium-mediated transformation of Solanum tuberosum L., potato. In: Kumar S, Barone P, Smith M (eds) Transgenic Plants: Methods and Protocols 1864. Springer, New York, pp 203–223
Camire ME, Kubow S, Donnelly DJ (2009) Potatoes and human health. Crit Rev Food Sci Nutr 49:823–840. https://doi.org/10.1080/10408390903041996
Campos H, Ortiz O (2020) The potato crop, its agricultural nutritional and social contribution to humankind. Springer, Lima
Cermak T, Starker CG, Voytas DF (2015) Efficient Design and Assembly of Custom TALENs Using the Golden Gate Platform. Chromosomal Mutagenesis. Springer Science, New York
Chetty VJ, Narváez-Vásquez J, Orozco-Cárdenas ML (2015) Potato (Solanum tuberosum L.). In: Wang K (ed) Agrobacterium Protocols. Springer, New York
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823. https://doi.org/10.1126/science.1231143
Corish P, Tyler-Smith C (1999) Attenuation of greenfluorescent protein half-life in mammalian cells. Protein Eng 12:1035–1040. https://doi.org/10.1093/protein/12.12.1035
De Block M (1988) Genotype-independent leaf disc transformation of potato (Solanum tuberosum) using Agrobacterium tumefaciens. Theor Appl Genet 76:767–774. https://doi.org/10.1007/BF00303524
Devaux A, Goffart J-P, Petsakos A, Kromann P, Gatto M, Okello J, Suarez V, Hareau G (2020) Global Food Security, Contributions from Sustainable Potato Agri-Food Systems. In: Campos H, Ortiz O (eds) The Potato Crop. Springer, Cham
Doudna JA, Charpentier E (2014) The new frontier of genome engineering with CRISPR-Cas9. Science 346:1258096. https://doi.org/10.1126/science.1258096
Enciso-Rodriguez F, Manrique-Carpintero NC, Nadakuduti SS, Buell CR, Zarka D, Douches D (2019) Overcoming self-incompatibility in diploid potato using CRISPR-Cas9. Front Plant Sci 10:376. https://doi.org/10.3389/fpls.2019.00376
FAOSTAT (2019) Crops and livestock products. https://www.fao.org/faostat/en/#data/QCL. Accessed 31 January 2022
Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA, Lim WA, Weissman JS, Qi LS (2013) CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154:442–451. https://doi.org/10.1016/j.cell.2013.06.044
González MN, Massa GA, Andersson M, Turesson H, Olsson N, Fält A-S, Storani L, Décima Oneto CA, Hofvander P, Feingold SE (2020) Reduced enzymatic browning in potato tubers by specific editing of a polyphenol oxidase gene via ribonucleoprotein complexes delivery of the CRISPR/Cas9 system. Front Plant Sci 10:1649. https://doi.org/10.3389/fpls.2019.01649
Haseloff J, Siemering KR, Prasher DC, Hodge S (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. PNAS 94:2122–2127. https://doi.org/10.1073/pnas.94.6.2122
Haverkort AJ, De Ruijter FJ, Van Evert FK, Conijn JG, Rutgers B (2013) Worldwide sustainability hotspots in potato cultivation. 1 identification and mapping. Potato Res 56:343–353. https://doi.org/10.1007/s11540-013-9247-8
Janga MR, Campbell LM, Rathore KS (2017) CRISPR/Cas9-mediated targeted mutagenesis in upland cotton (Gossypium hirsutum L.). Plant Mol Biol 94:349–360. https://doi.org/10.1007/s11103-017-0599-3
Jiang F, Doudna JA (2017) CRISPR–Cas9 structures and mechanisms. Annu Rev Biophys 46:505–529. https://doi.org/10.1146/annurev-biophys-062215-010822
Liang G, Zhang H, Lou D, Yu D (2016) Selection of highly efficient sgRNAs for CRISPR/Cas9-based plant genome editing. Sci Rep 6:21451. https://doi.org/10.1038/srep21451
Liu J, Nannas NJ, Fu FF, Shi J, Aspinwall B, Parrott WA, Dawe RK (2019) Genome-scale sequence disruption following biolistic transformation in rice and maize. Plant Cell 31:368–383. https://doi.org/10.1105/tpc.18.00613
Ma X, Zhang Q, Zhu Q, Liu W, Chen Y, Qiu R, Wang B, Yang Z, Li H, Xie Y, Shen R, Chen S, Wang Z, Chen Y, Guo J, Chen L, Zhao X, Dong Z, Liu Y (2015) A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant 8:1274–1284. https://doi.org/10.1016/j.molp.2015.04.007
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinform (oxford, England) 27:2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Ooms G, Bossen ME, Burrell MM, Karp A (1986) Genetic manipulation in potato with Agrobacterium rhizogenes. Potato Res 29:367–379. https://doi.org/10.1007/BF02359965
Pan C, Ye L, Qin L, Liu X, He Y, Wang J, Chen L, Lu G (2016) CRISPR/Cas9-mediated efficient and heritable targeted mutagenesis in tomato plants in the first and later generations. Sci Rep 6:24765. https://doi.org/10.1038/srep24765
Pinello L, Canver MC, Hoban MD, Orkin SH, Kohn DB, Bauer DE, Yuan G (2016) Analyzing CRISPR genome-editing experiments with CRISPResso. Nat Biotechnol 34:695–697. https://doi.org/10.1038/nbt.3583
Puchta H (2005) The repair of double-strand breaks in plants: mechanisms and consequences for genome evolution. J Exp Bot 56:1–14. https://doi.org/10.1093/jxb/eri025
Qi Y (2019) Plant genome editing with CRISPR systems. Humana Press, New York
Schaart JG, Van De Wiel CCM, Smulders MJM (2021) Genome editing of polyploid crops: prospects, achievements and bottlenecks. Transgenic Res 30:337–351. https://doi.org/10.1007/s11248-021-00251-0
Sheerman S, Bevan MW (1988) A rapid transformation method for Solanum tuberosum using binary Agrobacterium tumefaciens vectors. Plant Cell Rep 7:13–16. https://doi.org/10.1007/BF00272967
Shepard JF, Bidney D, Shahin E (1980) Potato protoplasts in crop improvement. Science 208:17–24. https://doi.org/10.1126/science.208.4439.17
Siemering KR, Golbik R, Sever R, Haseloff J (1996) Mutations that suppress the thermosensitivity of green fluorescent protein. Curr Biol 6:1653–1663. https://doi.org/10.1016/S0960-9822(02)70789-6
Stiekema WJ, Heidekamp F, Louwerse JD, Verhoeven HA, Dijkhuis P (1988) Introduction of foreign genes into potato cultivars Bintje and Désirée using an Agrobacterium tumefaciens binary vector. Plant Cell Rep 7:47–50. https://doi.org/10.1007/BF00272976
Stokstad E (2019) The new potato. Science 363:574–577. https://doi.org/10.1126/science.363.6427.574
Sunilkumar G, Mohr L, Lopata-Finch E, Emani C, Rathore KS (2002) Developmental and tissue-specific expression of CaMV 35S promoter in cotton as revealed by GFP. Plant Mol Biol 50:463–479. https://doi.org/10.1023/A:1019832123444
Toinga-Villafuerte S, Vales MI, Awika JM, Rathore KS (2022) CRISPR/Cas9-mediated mutagenesis of the granule-bound starch synthase gene in the potato variety Yukon Gold to obtain amylose-free starch in tubers. Int J Mol Sci 23:4640. https://doi.org/10.3390/ijms23094640
Tuncel A, Corbin KR, Ahn-Jarvis J, Harris S, Hawkins E, Smedley MA, Harwood W, Warren FJ, Patron NJ, Smith AM (2019) Cas9-mediated mutagenesis of potato starch-branching enzymes generates a range of tuber starch phenotypes. Plant Biotechnol J 12:2259–2271. https://doi.org/10.1111/pbi.13137
Wang W, Akhunova A, Chao S, Akhunov E (2016) Optimizing multiplex CRISPR/Cas9-based genome editing for wheat. bioRxiv 051342. https://doi.org/10.1101/051342
Wong N, Liu W, Wang X (2015) WU-CRISPR: characteristics of functional guide RNAs for the CRISPR/Cas9 system. Genome Biol 16:218. https://doi.org/10.1186/s13059-015-0784-0
Acknowledgements
The authors thank Dr. Devendra Pandeya, and Ms. LeAnne Campbell for their assistance at various stages of this project.
Funding
Stephany T. Villafuerte’s Ph.D. research described in this paper was supported in part by the College of Agriculture & Life Sciences—Grand Challenges Program and the Molecular & Environmental Plant Sciences Program, Texas A&M University.
Author information
Authors and Affiliations
Contributions
KSR designed experiments and supervised research; STV performed research with some help from MRJ and analyzed the data; KSR and STV wrote the manuscript with some editing from MIV. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Communicated by Degao Liu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toinga-Villafuerte, S., Janga, M.R., Isabel Vales, M. et al. Green fluorescent protein gene as a tool to examine the efficacy of Agrobacterium-delivered CRISPR/Cas9 reagents to generate targeted mutations in the potato genome. Plant Cell Tiss Organ Cult 150, 587–598 (2022). https://doi.org/10.1007/s11240-022-02310-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-022-02310-8