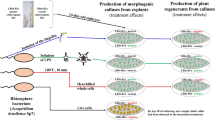
Abstract
Callus tissue is a popular tool in modern plant breeding and biotechnology. Macromolecules of plant-growth-promoting rhizobacteria can be beneficial for callus morphogenesis. We compared the effects of the lipopolysaccharides (LPSs) of three Azospirillum strains (A. brasilense SR55, A. brasilense SR75, and A. lipoferum SR65) on the calluses of two wheat (Triticum aestivum L. cv. Saratovskaya 29) lines (LRht-B1c and LRht-B1a) that differ in their morphogenic activity. The LPSs differed in the chemical structure of their O polysaccharides and in their physicochemical and serological properties. The LPS of A. lipoferum SR65 significantly promoted callus morphogenesis and regenerant development in both wheat lines. The yield of regenerated plants in terms of the total number of explants was significantly increased—2.15-fold in the highly morphogenic line LRht-B1c and 3.75-fold in the weakly morphogenic line LRht-B1a. In both lines, the LPSs of A. brasilense SR55 and SR75 increased either only the yield of morphogenic calluses or only the yield of regenerated plants, respectively. Overall, the Azospirillum LPSs affected the weakly morphogenic line LRht-B1a stronger than they did the highly morphogenic line LRht-B1c, and this resulted in a leveling of differences between the activities of the LRht-B1c and LRht-B1a morphogenic calluses. The LPSs of some Azospirillum strains are promising promoters of plant morphogenesis and may, in the future, find frequent use in plant breeding and genetic engineering experiments with callus tissue.
Graphic abstract

Key message
Lipopolysaccharides isolated from the outer membranes of various strains of plant-growth-promoting rhizobacteria of the genus Azospirillum increase the morphogenic activity of soft wheat calluses with different efficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biotechnologies based on in vitro cell and tissue culture are used widely in modern basic and applied research (Bednarek and Orłowska 2020). Embryogenesis and organogenesis in a somatic cell culture, which are based on the property of totipotency, are induced by various growth stress factors, among which a large part is played by endogenous and exogenous hormonal signals and by nonhormonal inducers (Fehér 2003; Kim and Moon 2007; Lee and Huang 2013; Miroshnichenko et al. 2016; Zang et al. 2001). The influence of bacteria and their metabolites on plant cells has been understudied. There have been sporadic reports describing positive effects of cell suspensions of plant-associated Bacillus spp. on the morphogenic activity of geranium calluses (Visser-Tenyenhuis et al. 1994), positive effects of the diazotrophic bacterium Herbaspirillum seropedicae Z78 on the cells of oil palm (Lim et al. 2016), and positive effects of methylotrophic bacteria on the cells of barley (Shirokikh et al. 2010) and wheat (Kalyaeva et al. 2003). However, in vitro bacterization of plant somatic cells is not always successful, because it may cause culture contamination and/or toxicity (Ilchukov 2012).
Lipopolysaccharide (LPS) is the principal component of the outer layer of the outer membrane of gram-negative bacteria (Serrato 2014). It forms the surface layer of the cell wall and is involved in the interaction of bacteria with biotic and abiotic environmental constituents (Makin and Beveridge 1996). LPS plays a part in bacterial attachment to host plant cells (Lee et al. 2014) and in induction of plant responses to the presence of symbiotic bacteria (Leeman et al. 1995; Sumayo et al. 2013). The LPSs of some rhizosphere strains promote the growth of plant seedlings (Evseeva et al. 2011; Sigida et al. 2020) and improve yields in wheat (Chávez-Herrera et al. 2018). Previously, we have found that by contrast to the LPS of Escherichia coli К-12, the LPS of the plant-growth-promoting bacterium Azospirillum brasilense Sp245 increases the morphogenic activity of wheat calluses, improving the efficacy of culturing of genotypes with low morphogenic potential (Evseeva et al. 2018).
In recent years, much information has been accumulated to show that the O-polysaccharide (OPS) structure and the LPS physicochemical characteristics can be very different in different Azospirillum strains (Burygin et al. 2016; Fedonenko et al. 2015). However, no comparative studies have been made of LPS effects on plant calluses. We examined the effects of Azospirillum LPSs with different chemical structures and properties on the morphogenic activity of soft wheat calluses.
Materials and methods
Plant material
Two near-isogenic lines of soft spring wheat (Triticum aestivum L. cv. Saratovskaya 29) were used—LRht-B1c and LRht-B1a. These have been described in detail elsewhere (Evseeva et al. 2018; Lobachev 2000). The LRht-B1c line has greater morphogenic potential in a somatic tissue culture than does its sister line LRht-B1a.
LPS characteristics
We used LPSs from Azospirillum spp. of three serogroups: A. brasilense SR75, A. brasilense SR55, and A. lipoferum SR65 (Fedonenko et al. 2015). The LPSs were isolated by the method of Westphal and Jann (1965). Proteins and nucleic acids were precipitated with CCl3CO2H (Kul’shin et al. 1987) and were centrifuged away. The LPSs were dialyzed and freeze dried (Boyko et al. 2011; Fedonenko et al. 2005, 2008).
The LPS carbohydrate content was measured as recommended by Dubois et al. (1956). The measurements were made on a Specord 250 spectrophotometer (Analytik, Jena, Germany) at 490 nm, by using glucose solutions for calibration. The LPS fatty acids were analyzed as methyl esters by gas–liquid chromatography (GLC) on a GC-2010 chromatograph (Shimadzu, Japan). The acids had been methylated as described by Mayer et al. (1985), with a bacterial acid methyl ester mix (Sigma–Aldrich, USA) as the standard. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) of the LPSs and gel staining were done as recommended by others (Hitchcock and Brown 1983; Tsai and Frasch 1982).
The dynamic light scattering of aqueous LPS solutions (LPS concentration, 2 mg ml−1) was measured with a Malvern Nano-ZS system (Malvern, UK; Burygin et al. 2016). We determined the intensity of light scattering at 173 ° and the correction function of scattering intensity fluctuations in time. Using these data, we estimated the number distribution over the particle size and the most probable modal hydrodynamic diameter (dm). Measurements were made at 37°C. The laser was focused on the cuvette center (4.65 mm), and the diaphragm diameter was constant. Four-sided plastic cuvettes (10 mm; SARSTED, Germany) were used, and the solutions were incubated for 3 min before use. The zeta-potential of micelles in the aqueous LPS solutions (LPS concentration, 2 mg ml−1) was measured with a Malvern Nano-ZS system at 25 °C by using DTS 1060 folded capillary cells (Malvern) and standard settings for zeta-potential measurements.
The serological reactions of antibodies against the O-antigens of Azospirillum serogroups I, II, and III (Fedonenko et al. 2015) with the LPSs were detected by enzyme-linked immunosorbent assay (ELISA) in 96-well polystyrene plates (Yegorenkova et al. 2010). The absorbance at 492 nm was read on a Multiskan Ascent analyzer (Thermo, Finland).
Determination of the morphogenic activity of wheat calluses
This was done as described earlier (Evseeva et al. 2018). Donor plants were grown under field conditions, and immature (14-day-old) embryos were used as explants to produce somatic calluses. For callus formation, sterile embryos were removed and placed scutellum up in petri dishes containing Linsmaier and Skoog’s nutrient medium (Linsmaier and Skoog 1965) with the inclusion of 2 mg l−1 of 2,4-dichlorophenoxyacetic acid and 10 µg ml−1 of the LPS of A. brasilense SR75, A. brasilense SR55, or A. lipoferum SR65. The 10 µg ml−1 concentration was chosen as the most effective on the basis of the earlier work with the LPS of A. brasilense Sp245 (Tkachenko et al. 2010). Because bacterial LPSs are heat stable (Ramos-Sanchez et al. 1991), they were added to the growth medium before autoclaving (pressure, 0.8 atm; time, 20 min). The nativity of the structure and the concentration of each LPS in the autoclaved medium and during the experiment were confirmed by ELISA. All experiments were done in triplicate. In the control treatment, calluses were initiated on an LPS-free medium. No fewer than 50 embryos were used in each treatment. On day 30, the total number of formed calluses and the number of calluses with meristematic centers were counted by eye (He et al. 1986). Morphogenic calluses were transferred to test tubes containing a regeneration medium of the same composition that lacked 2,4-dichlorophenoxyacetic acid but included 0.5 mg l−1 of indole-3-acetic acid and 0.5 mg l−1 of kinetin. Regenerated plants were counted 30 days later.
Statistics
Data on the physicochemical and antigenic properties of the LPSs (quantitative indicators) came from at least three independent experiments done in three replicates. The measured data were processed by one-way ANOVA (p ≤ 0.05) and then by Duncan’s multiple range tests for independent samples. The yields of calluses, morphogenic calluses, and regenerated plants (qualitative indicators) were evaluated with the alternative variability formulas given in Evseeva et al. (2018). The significances of differences between control and experimental treatments were tested by Student’s t test for P = 0.10, 0.05, 0.01, and 0.001.
Results
LPS isolation and characterization
Wheat calluses were treated with the LPSs of A. brasilense SR75, A. brasilense SR55, and A. lipoferum SR65, which differ in OPS structure (Table 1). Earlier, these strains had been assigned to three Azospirillum serogroups owing to the substantial differences in OPS structure (Fedonenko et al. 2015). These differences include a linear d-rhamnan in A. brasilense SR75; a branched acidic heteropolysaccharide formed from residues of l-rhamnose, d-galactose, and 3-O-methyl-l-rhamnose in its main chain and a residue of d-glucuronic acid in its side chain in A. brasilense SR55; and a branched heteropolysaccharide formed from l-rhamnose and d-glucose residues in A. lipoferum SR65 (Boyko et al. 2011; Fedonenko et al. 2005, 2008).
LPSs were isolated from dry bacterial mass with hot phenol–water. Proteins were precipitated with trichloroacetic acid, and the extracts were dialyzed and freeze dried. The LPS yields were about 5–7% of the bacterial mass. Fatty acid composition analysis by GLC showed the predominance of 3-hydroxytetradecanoic, 3-hydroxyhexadecanoic, octadecenoic, and hexadecenoic acids. As is known and was observed in this study, the fatty acid profile and ratio are often similar in the LPSs from bacteria of the same genus. SDS–PAGE followed by silver nitrate staining showed the predominance of S-form molecules containing OPSs (Fig. 1).
Silver-stained 12.5% Tris–glycine SDS–PAGE. Lane A, A. brasilense SR75 LPS; lane B, A. brasilense SR55 LPS; lane C, A. lipoferum SR65 LPS; lane M, protein molecular weight marker (β-galactosidase, 116 kDa; bovine serum albumin, 66.2 kDa; ovalbumin, 45 kDa; lactate dehydrogenase, 35 kDa; REase Bsp98I, 25 kDa; β-lactoglobulin, 18.4 kDa; lysozyme, 14.4 kDa)
Determination of the LPS carbohydrate content and measurement of the size and zeta-potential of the micelles formed from LPS molecules in aqueous media revealed the individual characteristics of each of the three LPSs examined (Table 2). The LPS of A. brasilense SR75 had a relatively high carbohydrate content and formed micelles with a diameter of 31 nm and an average zeta-potential. The carbohydrate mass fractions and the micelle sizes for the LPSs of A. brasilense SR55 and A. lipoferum SR65 were small, but the zeta-potentials of the micelles differed greatly. The LPS of A. brasilense SR55 had the most negative zeta-potential, which is due to the presence of glucuronic acid residues in the OPS. By contrast, the zeta-potential of the LPS micelles for A. lipoferum SR65 was the lowest in modulus.
ELISA with rabbit polyclonal monospecific antibodies against the O-antigens of A. brasilense Sp245 (serogroup I), A. brasilense Sp7 (serogroup II), and A. lipoferum Sp59b (serogroup III) showed that the LPSs of A. brasilense SR75, A. brasilense SR55, and A. lipoferum SR65 belonged to serogroups I, II, and III, respectively (Fig. 2). Thus, of the variety of described Azospirillum LPSs, we had chosen those that differed in OPS structure, physicochemical characteristics, and serological properties.
ELISA of solutions of the LPSs (10 µg ml−1) from A. brasilense SR75, A. brasilense SR55, and A. lipoferum SR65 by using antibodies against the O-antigens of A. brasilense Sp245 (serogroup I; 1), A. brasilense Sp7 (serogroup II; 2), and A. lipoferum Sp59b (serogroup III; 3). Bars indicate standard deviation of the mean. Different letters (a, b, c) show that values differ significantly at p ≤ 0.05, according to Duncan’s multiple range test
LPS effects on callus formation and on yield of morphogenic calluses
Explants of the LRht-B1c and LRht-B1a lines were cultured on LPS-containing media to form calluses. On day 30 of culturing, the callus yield from explants (Table 3) of both lines treated with the LPSs from A. brasilense SR55 and A. lipoferum SR65 increased significantly (at P = 0.001), as compared to the controls (calluses obtained on LPS-free media). No significant differences between the genotypes were detected in any experimental treatment. For LRht-B1c, the yield of morphogenic calluses increased 1.7- and 1.6-fold with the LPSs of A. brasilense SR55 (P = 0.05) and A. lipoferum SR65 (P = 0.01), respectively; for LRht-B1a, it increased 2.4- and 2.0-fold (P = 0.001), respectively (Fig. 3). When the explants were cultured with the LPS of A. brasilense SR75, the formation of calluses and the yield of morphogenic calluses did not differ from the control in either line.
Yield of morphogenic calluses on LPS-containing media (sample proportion q, %). Bars indicate confidence intervals at P = 0.05 (significance level, 95%). Differences between controls (no LPS) for the two callus genotypes are significant at P = 0.05; t = 1.98 > t0.05 = 1.97. The asterisks show the significance of the differences between the LPS-treated calluses and the respective controls: one asterisk, at P = 0.05; two asterisks, at P = 0.01; and three asterisks, at P = 0.001
Yield of regenerated plants
After the morphogenic calluses were transferred to Linsmaier and Skoog’s regeneration medium (no LPS), the yield of LRht-B1a regenerants increased significantly—2.6-fold (P = 0.05) with the LPS of A. brasilense SR75 in the initial medium and 1.8-fold (P = 0.05) with the LPS of A. lipoferum SR65 (Table S1). The LPS of A. brasilense SR55 did not significantly affect regeneration from LRht-B1a morphogenic calluses. The yield of LRht-B1c regenerants did not significantly change relative to the control in any of the LPS treatments.
Thus, the use of the LPS from A. lipoferum SR65 significantly increased the yield of regenerants from the LRht-B1c and LRht-B1a calluses obtained at the first stage. The respective increases were 2.15- and 3.75-fold (P = 0.01), as compared to the controls (Table 4). A positive effect of the A. brasilense SR55 and SR75 LPSs on the final yield of regenerants was detected only for the weakly morphogenic LRht-B1a line (P = 0.1). However, the final yields of LRht-B1a regenerants in all LPS treatments did not differ significantly from the corresponding treatments or from the controls for the LRht-B1c line. This is in contrast to the control for LRht-B1a, for which the yield of regenerants was 2.4-fold lower (P = 0.01) than it was for the LRht-B1c control.
Discussion
LPS is one of the most conserved structures within all gram-negative bacteria. This property makes LPS an important pathogen-associated molecular pattern (PAMP) to be recognized by mammals and plants (Kutschera and Ranf 2019). In plants, LPSs activate the genes of the salicylate pathway of plant immunity (pr genes) (Iizasa et al. 2016) and initiate systemic resistance to plant pathogens (Leeman et al. 1995; Sumayo et al. 2013). The great progress in the study of Azospirillum OPSs that has been made in the past two decades (Fedonenko et al. 2005) has made it possible here to choose LPSs that differ in their physicochemical and serological properties. The OPSs of many Azospirillum strains contain two or more types of repeating oligosaccharide units (Fedonenko et al. 2015; Matora et al. 2008). The OPS of each LPS used in this study contained only one type of repeating unit with a unique chemical structure.
As shown by Tkachenko et al. (2010), the LPS of A. brasilense Sp245 promotes morphogenesis in wheat. The 10 µg ml−1 LPS concentration was found optimal for the promotion of morphogenesis in a wheat somatic callus culture. Here, however, we have found that when used at 10 µg ml−1, the LPSs differed considerably in the magnitude of their effect. The most active LPS was that isolated from A. lipoferum SR65 (serogroup III). Its micelles in water had a diameter of 21 nm and a weak zeta-potential. This LPS, like that from A. brasilense Sp245 (serotype I), promoted morphogenesis at all stages of culturing in vitro. By contrast, the LPS of A. brasilense SR75, in which the OPS repeating unit is structurally identical to that in A. brasilense Sp245 (Fedonenko et al. 2005), increased only the yield of regenerants from morphogenic calluses in the weakly embryogenic line LRht-1Ba, but it did not affect the yield of morphogenic calluses in either line, as compared to the control.
The considerable differences in the effect of the LPSs of A. brasilense SR75 and Sp245 on wheat calluses are difficult to explain. These LPSs contain structurally identical OPSs (Fedonenko et al. 2005) but differ slightly in the mass ratio between carbohydrates and in the physicochemical characteristics of their micelles (Table 2). The structural identity of the OPS units of A. brasilense SR75 and Sp245 suggests that neither OPSs nor their fragments are essential for the enhancement of the morphogenetic activity in wheat calluses. The LPS of A. brasilense Sp245 greatly promoted morphogenesis in both lines (Evseeva et al. 2018), as distinct from the LPS of A. brasilense SR75 (this study). This difference may be due to the multifactorial nature of the interaction between LPS molecules and callus cells. The combined effect of the small differences between the sizes of the LPS molecules and between the zeta-potentials of their micelles, together with the influence of other parameters, may be important for the formation of meristematic centers in dedifferentiated plant tissue. Also, one should not overlook the specificity of the action of lipids A and core oligosaccharides. These LPS portions are highly conserved (Caroff and Novikov 2019; Casillo et al. 2017); therefore, we did not account for their effects in this work. Further, one cannot rule out the differences in the structure of lipid A or the core between bacterial strains of the same species. These differences are important for interaction with plant cells (Caroff and Novikov 2019; Raetz and Whitfield 2002). Desaki et al. (2018) showed that the lipid A of the plant pathogen Xanthomonas oryzae pv. oryzae is implicated in the activation of plant responses through the specific interaction with the multifunctional co-receptor kinase OsCERK1 in suspension-cultured rice cells. It should be assumed that there are other plant receptors that trigger different biochemical and physiological responses of plants to LPS.
The LPS of A. brasilense SR55 (d = 23 nm, high negative zeta-potential, serogroup II) significantly promoted the yield of morphogenic calluses in both lines but not the yield of regenerants from morphogenic calluses. Nonetheless, for calluses of the weakly embryogenic line LRht-1Ba grown with A. brasilense SR55 LPS, we experimentally observed a 2.9-fold (P = 0.1) increase in regenerant yield (from explants to regenerants), as compared to the control group. This increase was due to the substantial activation of morphogenesis during the formation of morphogenic calluses.
In summary, the observed effects of the three Azospirillum LPSs tested, together with their physicochemical and serological properties, have enabled us to establish a (weak) relationship between the ability to increase morphogenic activity in wheat calluses and the low value of the zeta-potential of micelles. Yet, it may be that the LPS promotion of callus growth is not related to the chemical structure of the OPS repeating unit or to the size of the micelles formed. The entire mechanism of LPS action on plants is still poorly understood. Of note also is that the LPSs of different Azospirillum strains may differ in the concentration at which they are most effective as activators of wheat callus morphogenesis. The data generated in this study could be a step to elucidating the mechanisms by which the LPSs of plant-growth-promoting rhizobacteria affect plant cell differentiation.
Conclusion
The production of regenerants and the regulation of culture development in biotechnological approaches that use plant tissue culture (calluses) should be improved. The use of substances elaborated by bacteria may assist in this process. The study of plant tissue culture responses to bacterial components may both contribute to the understanding of basic issues in plant–microbe interactions and help researchers choose bacterial molecules able to regulate plant development.
Specifically, soft wheat calluses have weak morphogenic activity, which makes the production of regenerated plants less effective. The study of the effects of the LPSs from three Azospirillum strains has found significant promotion by A. lipoferum SR65 LPS of the morphogenic activity of wheat calluses—an effect comparable to that of A. brasilense Sp245 LPS (Evseeva et al. 2018). Not all Azospirillum strains are effective in activating plant tissue culture morphogenesis through their LPSs. Presumably, neither the structural differences between OPS repeating units nor the physicochemical characteristics of micelles determine the nature of LPS action on callus cells. Although LPS effects on plant objects (cells, tissues, organs) is a very promising area of plant biotechnology, the nature of the differences in efficacy and the mechanisms of LPS action on plant calluses remain unknown and call for further research.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bednarek PT, Orłowska R (2020) Plant tissue culture environment as a switch-key of (epi)genetic changes. Plant Cell Tissue Organ Cult 140:245–257. https://doi.org/10.1007/s11240-019-01724-1
Boyko AS, Konnova SA, Fedonenko YP, Zdorovenko EL et al (2011) Structural and functional peculiarities of the lipopolysaccharide of Azospirillum brasilense SR55, isolated from the roots of Triticum durum. Microbiol Res 166:585–593. https://doi.org/10.1016/j.micres.2011.01.002
Burygin GL, Sigida EN, Fedonenko YP, Khlebtsov BN, Shchyogolev SY (2016) The use and development of the dynamic light-scattering method to investigate supramolecular structures in aqueous solutions of bacterial lipopolysaccharides. Biophysics 61:547–557. https://doi.org/10.1134/S0006350916040059
Caroff M, Novikov A (2019) LPS structure, function, and heterogeneity. In: Williams K (ed) Endotoxin detection and control in pharma, limulus, and mammalian systems. Springer, Cham, pp 53–93
Casillo A, Ziaco M, Lindner B, Parrilli E, Schwudke D, Holgado A, Verstrepen L, Sannino F, Beyaert R, Lanzetta R, Tutino ML, Corsaro MM (2017) Unusual lipid A from a cold adapted bacterium: detailed structural characterization. ChemBioChem 18:1845–1854. https://doi.org/10.1002/cbic.201700287
Chávez-Herrera E, Hernández-Esquivel AA, Castro-Mercado E, García-Pineda E (2018) Effect of Azospirillum brasilense Sp245 lipopolysaccharides on wheat plant development. J Plant Growth Regul 37:859–866. https://doi.org/10.1007/s00344-018-9782-2
Desaki Y, Kouzai Y, Ninomiya Y, Iwase R, Shimizu Y, Seko K, Nishizawa Y (2018) OsCERK 1 plays a crucial role in the lipopolysaccharide-induced immune response of rice. New Phytol 217:1042–1049. https://doi.org/10.1111/nph.14941
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Evseeva NV, Matora LY, Burygin GL, Dmitrienko VV, Shchyogolev SY (2011) Effect of Azospirillum brasilense Sp245 lipopolysaccharide on the functional activity of wheat root meristematic cells. Plant Soil 346:181–188. https://doi.org/10.1007/s11104-011-0808-9
Evseeva NV, Tkachenko OV, Burygin GL, Matora LY, Lobachev YV, Shchyogolev SY (2018) Effect of bacterial lipopolysaccharides on morphogenetic activity in wheat somatic calluses. World J Microbiol Biotechnol 34:3. https://doi.org/10.1007/s11274-017-2386-3
Fedonenko YP, Borisov IV, Konnova ON, Zdorovenko EL, Katsy EI, Konnova SA, Ignatov VV (2005) Determination of the structure of the repeated unit of the Azospirillum brasilense SR75 O-specific polysaccharide and homology of the lps loci in the plasmids of Azospirillum brasilense strains SR75 and Sp245. Microbiology 74:542–548. https://doi.org/10.1007/s11021-005-0101-0
Fedonenko YP, Zdorovenko EL, Konnova SA, Kachala VV, Ignatov VV (2008) Structural analysis of the O-antigen of the lipopolysaccharide from Azospirillum lipoferum SR65. Carbohydr Res 343:2841–2844. https://doi.org/10.1016/j.carres.2008.05.022
Fedonenko YP, Sigida EN, Konnova SA, Ignatov VV (2015) Structure and serology of O-antigens of nitrogen-fixing rhizobacteria of the genus Azospirillum. Russ Chem Bull 64:1024–1031. https://doi.org/10.1007/s11172-015-0971-x
Fehér A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult 74:201–228. https://doi.org/10.1023/A:1024033216561
He DG, Tanner G, Scott KJ (1986) Somatic embryogenesis and morphogenesis in callus derived from the epiblast of immature embryos of wheat (Triticum aestivum). Plant Sci 45:119–124. https://doi.org/10.1016/0168-9452(86)90047-6
Hitchcock PJ, Brown TM (1983) Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stain polyacrylamide gels. J Bacteriol 154:269–277
Iizasa S, Iizasa EI, Matsuzaki S, Tanaka H, Kodama Y, Watanabe K, Nagano Y (2016) Arabidopsis LBP/BPI related-1 and-2 bind to LPS directly and regulate PR1 expression. Sci Rep 6:27527. https://doi.org/10.1038/srep27527
Ilchukov VV (2012) Cultivation of the callus tissue of wheat with the nitrogen-fixing bacteria of the genus Azospirillum. Vestnik Saratovskogo gosagrouniversiteta im. N.I. Vavilova 6:28–29 (in Russian)
Kalyaeva MA, Ivanova EG, Doronina NV, Zakharchenko NS, Trotsenko YuA, Buryanov YI (2003) The effect of aerobic methylotrophic bacteria on the in vitro morphogenesis of soft wheat (Triticum aestivum). Russ J Plant Physiol 50:313–317. https://doi.org/10.1023/A:1023861918193
Kim YW, Moon HK (2007) Enhancement of somatic embryogenesis and plant regeneration of Japanese larch (Larix leptolepis). Plant Cell Tissue Organ Cult 88:241–245. https://doi.org/10.1007/s11240-007-9202-y
Kul’shin VA, Yakovlev AP, Avaeva SN, Dmitriev BA, (1987) Simplified method for lipopolysaccharide isolation from gram-negative bacteria. Mol Genet Mikrobiol Virusol 5:44–46 (in Russian)
Kutschera A, Ranf S (2019) The multifaceted functions of lipopolysaccharide in plant-bacteria interactions. Biochimie 159:93–98. https://doi.org/10.1016/j.biochi.2018.07.028
Lee ST, Huang WL (2013) Cytokinin, auxin, and abscisic acid affects sucrose metabolism conduce to de novo shoot organogenesis in rice (Oryza sativa L.) callus. Bot Stud 54:5. https://doi.org/10.1186/1999-3110-54-5
Lee HI, In YH, Jeong SY, Jeon JM, Noh JG, So JS, Chang WS (2014) Inactivation of the lpcC gene alters surface-related properties and symbiotic capability of Bradyrhizobium japonicum. Lett Appl Microbiol 59:9–16. https://doi.org/10.1111/lam.12232
Leeman M, Van Pelt JA, Den Ouden FM, Heinsbroek M, Bakker PAHM, Schippers B (1995) Induction of systemic resistance against Fusarium wilt of radish by lipopolysaccharides of Pseudomonas fluorescens. Phytopathology 85:1021–1027
Lim S, Subramaniam S, Zamzuri I, Amir HG (2016) Biotization of in vitro calli and embryogenic calli of oil palm (Elaeis guineensis Jacq.) with diazotrophic bacteria Herbaspirillum seropedicae (Z78). Plant Cell Tissue Organ Cult 127:251–262. https://doi.org/10.1007/s11240-016-1048-8
Linsmaier E, Skoog F (1965) Organic growth factor requirements of tobacco tissue culture. Physiol Plant 18:100–127
Lobachev YuV (2000) Effects of the dwarfing genes in spring wheats in the Lower Volga region. Saratov State Agrarian University named after NI Vavilov, Saratov (in Russian)
Makin SA, Beveridge TJ (1996) The influence of A-band and B-band lipopolysaccharide on the surface characteristics and adhesion of Pseudomonas aeruginosa to surfaces. Microbiology 142:299–307. https://doi.org/10.1099/13500872-142-2-299
Matora LY, Burygin GL, Shchyogolev SY (2008) Study of immunochemical heterogeneity of Azospirillum brasilense lipopolysaccharides. Microbiology 77:166–170. https://doi.org/10.1134/S0026261708020070
Mayer H, Tharanathan RN, Weckesser J (1985) Analysis of lipopolysaccharides of gram-negative bacteria. Meth Microbiol 18:157–207. https://doi.org/10.1016/S0580-9517(08)70475-6
Miroshnichenko D, Chernobrovkina M, Dolgov S (2016) Somatic embryogenesis and plant regeneration from immature embryos of Triticum timopheevii Zhuk. and Triticum kiharae Dorof. et Migusch, wheat species with G genome. Plant Cell Tissue Organ Cult 125:495–508. https://doi.org/10.1007/s11240-016-0965-x
Raetz CR, Whitfield C (2002) Lipopolysaccharide endotoxins. Annu Rev Biochem 71:635–700. https://doi.org/10.1146/annurev.biochem.71.110601.135414
Ramos-Sanchez MC, Rodriguez-Torres A, Leal JA, Martin-Gil FJ, Martin-Gil J (1991) Thermolytical techniques to characterize fungal polysaccharides and bacterial lipopolysaccharides. Biotechnol Prog 7:526–533. https://doi.org/10.1021/bp00012a007
Serrato RV (2014) Lipopolysaccharides in diazotrophic bacteria. Front Cell Infect Microbiol 4:119. https://doi.org/10.3389/fcimb.2014.00119
Shirokikh IG, Shupletsova ON, Ogorodnikova SYu, Shirokikh AA (2010) Reaction of the callus culture and regenerative plants of barley on bacterization with Methylobacterium mesophylicum. Theor Appl Ecol 54–62 (in Russian)
Sigida EN, Kargapolova KY, Shashkov AS, Zdorovenko EL, Ponomaryova TS, Meshcheryakova AA, Tkachenko OV, Burygin GL, Knirel YA (2020) Structure, gene cluster of the O antigen and biological activity of the lipopolysaccharide from the rhizospheric bacterium Ochrobactrum cytisi IPA7.2. Int J Biol Macromol 154:1375–1381. https://doi.org/10.1016/j.ijbiomac.2019.10.092
Sumayo M, Hahm MS, Ghim SY (2013) Determinants of plant growth promoting Ochrobactrum lupini KUDC1013 involved in induction of systemic resistance against Pectobacterium carotovorum subsp. carotovorum in tobacco leaves. Plant Pathol J 29:174–181. https://doi.org/10.5423/PPJ.SI.09.2012.0143
Tkachenko OV, Lobachev YuV, Matora LYu, Evseeva NV, Burygin GL, Shchyogolev SYu (2010) Effect of bacterial lipopolysaccharide on the morphogenetic potential of wheat callus cells in vitro. Annual Wheat Newsletter 56:216–217
Tsai CM, Frasch CE (1982) A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem 119:115–119. https://doi.org/10.1016/0003-2697(82)90673-x
Visser-Tenyenhuis C, Murthy BNS, Odumeru J, Saxena PK (1994) Modulation of somatic embryogenesis in hypocotyl-derived cultures of geranium (Pelargonium x hortorum Bailey) cv Ringo Rose by a bacterium. Vitro Cell Dev Biol Plant 30:140–143. https://doi.org/10.1007/BF02632203
Yegorenkova IV, Tregubova KV, Matora LY, Burygin GL, Ignatov VV (2010) Use of ELISA with antiexopolysaccharide antibodies to evaluate wheat-root colonization by the rhizobacterium Paenibacillus polymyxa. Curr Microbiol 61:376–380. https://doi.org/10.1007/s00284-010-9622-5
Westphal O, Jann K (1965) Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem 5:83–91
Zang P, Phansiri S, Puonti-Kaerlas J (2001) Improvement of cassava shoot organogenesis by the use of silver nitrate in vitro. Plant Cell Tissue Organ Cult 67:47–54. https://doi.org/10.1023/A:1011654128198
Acknowledgements
Thanks are due to Mr. Dmitry N. Tychinin (IBPPM RAS) for translating the original manuscript into English.
Funding
This work was funded from the state budget (Project No. 121031100266-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
This article does not contain any human or animal studies performed by any of the authors.
Additional information
Communicated by Manoj Prasad.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tkachenko, O.V., Burygin, G.L., Evseeva, N.V. et al. Morphogenesis of wheat calluses treated with Azospirillum lipopolysaccharides. Plant Cell Tiss Organ Cult 147, 147–155 (2021). https://doi.org/10.1007/s11240-021-02114-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02114-2