Abstract
Gentiana kurroo Royle, a critically endangered medicinal plant species of India is conserved as in vitro slow growing cultures in the In Vitro Genebank (IVGB) at ICAR-National Bureau of Plant Genetic Resources (NBPGR), New Delhi, India. Shoot tips (about 1 mm in length) excised from 8-weeks-old stock cultures subjected to cold hardening for four weeks at 8 °C (dark) were precultured on Murashige and Skoog (MS) medium supplemented with 5% Dimethylsulfoxide (DMSO) at 8 °C (dark) for two days. Thereafter, shoot tips were dehydrated with PVS2 solution (30 min) at 0 °C and cryopreserved using conventional vitrification (V) and droplet-vitrification (DV) techniques. Average post-thaw regeneration after 4 weeks was ~ 35% by V technique and ~ 60% by DV in all the three accessions tested. Genetic stability assessment on the basis of 40 Inter Simple Sequence Repeats and 30 Expressed Sequence Tagged-Simple Sequence Repeats markers revealed no variation between in vitro-cryopreserved plants and controls, in any of the genotypes. Thus, standardized DV method has the potential for long-term conservation of germplasm of G. kurroo and the three accessions are now safely cryobanked at ICAR-NBPGR.
Key message
Gentiana kurroo is a critically endangered medicinal plant endemic to North-western Himalayas, India. A protocol to cryopreserve in vitro shoot-tips was developed using droplet vitrification technique with high recovery rates and genetically stable regnerants. The protocol is being applied for cryobanking of Gentiana germplasm at ICAR-NBPGR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gentiana kurroo Royle, is a critically endangered medicinal plant species endemic to North-western Himalayas (Ved et al. 2015). In fact, in the region of Kashmir Himalayas (Dachigam National Park, Kashmir, India), a wild population was discovered growing after about six decades of reported local extinction (Khuroo et al. 2005). Since ancient times the roots and rhizomes of the plant have been used in indigenous system of medicine in India. Medicinal value of the plant has been scientifically validated and attributed to presence of glycosides and alkaloids in the roots and rhizomes (Skinder et al. 2017). Though the plant produces a large number of seeds (if allowed to produce), there is no report on its domestication/cultivation owing to poor germination and seedling establishment (Tomar et al. 2012). Further, the roots and rhizomes at specific age are collected for trade due to which seed formation and vegetative propagation gets adversely affected. Hence, in vitro clonal conservation was prioritized for this species (Pence, 1999). So far, out of ~ 400 species of gentian, protocols for cryopreservation have been developed for seven species using (1) cell suspensions in G. tibetica (Mikula 2006; Mikula et al. 2008), G. cruciata (Mikula et al. 2005, 2008, 2011a), G. kurroo (Mikula et al. 2006, 2011b); (2) axillary buds of G. scabra (Suzuki et al. 1998); (3) shoot meristems of G. scabra, G. trifora, G. trifora × G. scabra and G. pneumonanthe (Tanaka et al. 2004); (4) hairy root tips of G. macrophylla (Xue et al. 2008) and (5) somatic embryos of G. tibetica and G. cruciata (Mikula et al. 2015). In spite of these reports, Mikula et al. (2015) observed that practical application for conservation of diverse germplasm still requires improvement in members of family Gentianaceae. Hence, in this study, cryopreservation of an organized tissue (shoot tips) in three accessions of G. kurroo was attempted using two different techniques namely, conventional vitrification (V) and droplet-vitrifcation (DV). We also assessed genetic stability of cryopreserved shoot tips using Inter Simple Sequence Repeats (ISSR) and Simple Sequence Repeats (SSR) markers.
Three genotypes of G. kurroo (IC266697, IC554589 and IC612563; referred as GK1, GK2, and GK3, respectively) maintained in the In Vitro Genebank at ICAR-National Bureau of Plant Genetic Resources (NBPGR), New Delhi, India, were used as source material. Stock cultures were multiplied on MS medium (Murashige and Skoog 1962) supplemented with 8.9 µM 6-benzyladenine (BA) (Sigma-Aldrich®, St. Louis, MO) and 1.1 µM 1-naphthalene acetic acid (NAA) (Sigma-Aldrich®), 3% (w/v, 0.08 M) sucrose (Hi-Media®, Mumbai, India), 0.8% (w/v) bacteriological agar (Hi-Media®) at pH 5.8, henceforth, referred as shoot multiplication (SM) medium (Sharma et al. 1993). Cultures were maintained at 25 ± 2 °C under standard culture room conditions (SCC), a 16 h photoperiod with light intensity of 40 μEm−2 s−1, provided by 40 W cool-white fluorescent tubes (Philips, Mumbai, India). Detailed experiments on standardization of V and DV techniques were carried out on genotype GK1. Preliminary trials were conducted to standardize preconditioning, pregrowth medium and Plant Vitrification Solution 2 [PVS2 − 0.4 M sucrose, 30% (w/v) glycerol, 15% (w/v) ethylene glycol and 15% (w/v)] dehydration using V technique. Preconditioning was done by cold hardening the 4-weeks-old stock cultures at 8 °C in dark for four weeks using following media – (1) SM medium, (2) SM medium supplemented with high sucrose (6% or 9%) and (3) SM medium with modified nitrate concentrations (1/2, 1/4, 3/4 and 1.5 times). Cultures grown on SM medium and incubated under SCC conditions for 8 weeks (non cold-hardening) served as control. For all the experiments (both V and DV), excised shoot tips were precultured on MS + 5% DMSO (Sigma-Aldrich®) at 8˚C (dark) for 2 days.
For V technique, precultured shoot tips were dehydrated in 2.0 ml cryovials (Thermo Fisher Scientific®, Roskilde, Denmark) containing pre-chilled 1.0 ml PVS2 at 0 °C for 0, 10, 20, 30 and 40 min. After the desired period in PVS2, cryovials were quickly plunged into liquid nitrogen (LN) in a Dewar flask and retained for at least 1 h. For thawing, cryovials were plunged in warm water at 45 °C for 2 min with constant stirring, followed by 1 min at 25 °C. After thawing, PVS2 solution was quickly removed and shoot tips were rinsed five times with unloading solution (US; 1.2 M sucrose in liquid MS medium; pH 5.8) over 20 min period. Thereafter, shoot tips were placed on sterile dry filter papers over SM medium held in Petri dishes. For DV technique, precultured shoot tips were dehydrated for 30 min in pre-chilled PVS2 at 0 °C in cryovials. About 5 µL droplet of PVS2 solution was placed on a strip of sterile aluminium foil (20 × 5 mm) held in a sterile Petri plate and 10 explants were placed in each drop (2 min before the end of the PVS2 exposure time). Following PVS2 dehydration, aluminium foil strips with shoot tips were directly plunged into LN and immediately placed into sterile cryovials (2 ml) filled with LN in a Dewar flask for at least 1 h. For thawing, the aluminium foils were rinsed in 10 ml US for 20 min at 25 °C (Sharma et al. 2017). In both V and DV, shoot tips treated with PVS2 (cryoprotected but non-frozen) and rinsed with US for 20 min at 25 °C served as controls. After incubation in dark for a week, cultures were transferred to SCC, on fresh SM medium. The experiments were repeated at least three times. Survival was assessed by visual examination as the ability of shoot tips to turn green and initiate sprouting within 10 days and regrowth by scoring the number of shoot tips that had formed normal shoots 4 weeks after plating. After 8 weeks, growing shoot tips were transferred to SM medium in culture tubes (Qualigens Fine Chemicals Pvt., Ltd., Mumbai, India) (150 mm × 25 mm). Results are presented as means (%) ± standard error of means. Statistical difference between mean values was assessed using Analysis of Variance (ANOVA) and Duncan’s multiple range test (p ≤ 0.05). Prior to analysis, the original percentage data were arcsin transformed to satisfy the prerequisites of ANOVA.
Genetic stability of cryopreserved plants of the three genotypes was assessed by Inter Simple Sequence Repeats (ISSR) and Expressed Sequence Tagged-Simple Sequence Repeats (EST-SSR) marker systems. Three randomly selected plants each of the in vitro multiplied plants (tissue culture controls), cryopreservation control plants, plants obtained after post-thaw regrowth after V and plants obtained after post-thaw regrowth after DV were compared in each of the three accessions. Total genomic DNA was isolated from fresh, young leaf samples using the CTAB method of DNA isolation (Doyle and Doyle 1987). Isolated DNA was quantified using NanoDrop 1000 (Thermo Fisher Scientific™, USA) and diluted to 40 ng/μL for further analysis. A total of 40 ISSR primers (UBC Series) were tested for PCR amplification in a thermal cycler (Gene Pro, Hangzhou Bioer Technology Co., China). A 10 μL reaction mixture was set up for each primer using 1U Taq DNA Polymerase (Vivantis Technologies, Malaysia). The thermal profile was programmed as: initial denaturation at 94 °C for 2 min, followed by 37 cycles of denaturation at 94 °C (10 s), primer annealing Tm varying as per primer (30 s) and primer extension at 72 °C (65 s) and a final extension at 72 °C for 10 min. The EST-SSR based analysis was carried out using 30 in silico derived EST-SSR primers (unpublished data). PCR reactions were set up similar to the ISSR reactions and amplification profile was programmed as follows: initial denaturation at 94 °C for 5 min, followed by 40 cycles of denaturation at 94 °C (1 min), primer annealing Tm varying as per primer (30 s) and primer extension at 72 °C (1 min) and a final extension at 72 °C for 10 min. The amplification products were resolved on ethidium bromide stained 2% and 3% agarose gel in 1 × TAE buffer for ISSR and EST-SSR markers, respectively, and visualized under UV light. Pairwise similarity among the analyzed samples was estimated based on Jaccard’s similarity coefficient (Jaccard 1908) and cluster analyses were performed based on the generated similarity matrices by the unweighted pair group method with arithmetic mean (UPGMA) using the NTSYS-PC version 2.0 (Rohlf 1998) software package.
In the present study using V technique, irrespective of cold hardening and PVS2 dehydration duration, the non-frozen controls (but PVS2 treated) exhibited 100% survival and regrowth (data not presented), indicating ability of shoot tips to tolerate PVS2 dehydration. As shown in Fig. 1a, shoot tips excised from non-cold hardened cultures either did not survive (in 0 or 40 min PVS2 treatment) or showed very low survival (~ 10%) when subjected to 10–30 min PVS2 dehydration, and failed to form shoots after LN freezing. Shoot tips excised from cold-hardened cultures led to significant increase in post-thaw regrowth (8–40%). Cold hardening of in vitro grown cultures (often called preconditioning or pregrowth) or shoot tips (often called preculture) has been reported to be an important factor for successful cryopreservation in several cold hardy plants such as apple, birch, pear, kiwifruit, mulberry, Prunus and Rubus (Suzuki et al. 2005). Cold hardening has a tendency to stimulate an intrinsic tolerance to low temperature and desiccation by activating genes responsible for cold stress (Takagi 2000). In various Gentiana spp. cold hardening has significantly increased survival and regrowth, with optimal duration (10–50 days) and temperature (5–15 °C) varying with species and technique of cryopreservation employed (Kikuchi and Yashiro 1995; Tanaka et al. 2004). Our results also confirm the importance of cold hardening to enhance post-thaw survival and regrowth, as non-cold hardened cultures failed to tolerate LN freezing. It is also important to study the effect of PVS2 dehydration period, as it determines the level of cell dehydration and the amount of PVS2 permeating the cells. In the present study, survival and regrowth increased with increase in PVS2 dehydration time (Fig. 1a) and was highest when shoot tips were dehydrated for 30 min (c. 43% survival and c. 40% regrowth), then slightly decreasing with further exposure to 40 min (c. 38% survival and regrowth). No shoot tips survived cryopreservation without PVS2 treatment. Thus, the experiment clearly indicated that PVS2 dehydration for 30 min resulted in optimum recovery after 4-weeks cold acclimation (at 8 °C in dark) of cultures pregrown on SM medium. The next set of experiments was aimed at optimizing pregrowth medium for best post-thaw recovery. In almost all treatments (6% and 9% sucrose), the shoot tips survived (80–100%) and exhibited normal growth in non-frozen controls. In contrast, only 40% post-thaw survival and ~ 18% regrowth using 6% sucrose, ~ 20% survival without recovery growth using 9% sucrose, whereas ~ 40% survival and regrowth was observed using 3% sucrose. The effect of modifying (lowering or increasing) nitrate in MS medium (by using 1/4, 1/2, 3/4 and 1 × 1/2 nitrate) resulted in reduction in post-thaw recovery compared to pregrowth on SM medium with 3% sucrose (data not given). It is, therefore, concluded that inclusion of higher concentration of sucrose (6% and 9%) and modification in nitrate concentration in the pregrowth medium was not beneficial in improving post-thaw survival and regrowth.
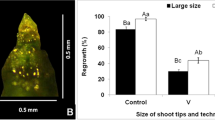
a Effect of cold hardening and PVS2 dehydration duration on post-thaw survival and regrowth of shoot tips of Gentiana kurroo (genotype GK1); NC not cold hardened and C cold hardened cultures. b Comparison of efficacy of conventional vitrification (V) and droplet-vitrification (DV) techniques in three genotypes (GK1, GK2 and GK3). Error bars represent standard errors. Bars marked by the same letter are not significantly different for survival (upper case) and regrowth (lower case) according to Duncan's Multiple Range Test
Since first reported for sweet potato shoot tips (Pennycooke and Towill 2000), the DV technique has been successfully applied to a large number of plant species owing to high post-thaw recovery and being adopted for long-term conservation of many crops in the cryobanks across the world (Panis 2019; Wang et al. 2020). However, there are no publications regarding its implementation in Gentiana spp. Hence, an attempt was made to compare shoot tips cryopreservation of G. kurroo using V and DV techniques, in three genotypes. Using DV technique, significant improvement in post-thaw survival (c. 65%) as well as regrowth (c. 60%), as compared to V was observed (~ 35% regrowth) in all the three genotypes (Fig. 1b). Secondly, there was comparatively less variation between replicates in terms of regrowth within a genotype and among the genotypes using DV. The major difference between these two techniques is freezing rate/cooling rate. The cooling rate in DV is about 130 °C s−1 is faster than that for V of about 6 °C s−1, therefore facilitating rapid transition of intracellular water from a liquid state to a glassy state while decreasing water crystallization (Towill and Bonnart 2003). These results are in conformity with publications in many plant species including those from our laboratory (Agrawal et al. 2004). Genotype-to-genotype variation with respect to success of any protocol and post-thaw recovery are well documented in Gentiana species (Tanaka et al. 2004) and other species (Agrawal et al. 2008; Sharma et al. 2017). In contrast, similar regrowth of cryopreserved shoot tips across genotypes in the present study was observed. Regrowth of cryopreserved shoot tips without transitory callus (Fig. 2a–c) is significant as it is indicative of low risk of genetic instability. After 2–3 weeks, multiple shoots were formed which could be transferred to fresh SM medium (Fig. 2d). Profuse rooting was also observed.
a–c Comparison of efficacy of conventional vitrification (V) and droplet-vitrification (DV) techniques in genotype GK1 of Gentiana kurroo (3 weeks after plating). a Control (PVS2 dehydration and non-frozen). b Post-thaw regrowth of cryopreserved shoot tips using V technique. c Post-thaw regrowth of cryopreserved shoot tips using DV technique. d Applicability of DV protocol for cryopreservation of shoot tips in three genotypes of G. kurroo. e, f Representative gel image of amplification of three genotypes with e EST–SSR primer G14. f ISSR primer IS-10, Lane L: 100 bp DNA marker (L). TCC tissue culture control,—represents control (PVS2 dehydration and non-frozen), + V conventional vitrification technique, + DV droplet-vitrification technique
Genetic stability of plants regenerated from cryopreserved shoot tips using both V and DV techniques was confirmed by molecular analysis using 40 ISSR and 30 EST-SSR markers in all the three genotypes. Representative banding profiles of plantlets derived from cryopreserved shoot tips using ISSR and EST-SSR primers are depicted in Fig. 2e, f, respectively. Out of the 40 ISSR markers tested, 18 primers (45%) amplified in all the three genotypes, while 19 out of the 30 EST-SSR markers gave scorable amplification (63.33%). A UPGMA based dendrogram based on Jaccard’s similarity co-efficient generated for each genotype and marker system separately, depicted high levels of genetic similarity between plants of the same accession. Further, no significant variation was observed among the controls and plants regenerated from cryopreserved shoot tips at any of the tested marker loci, in all the three genotypes. Hence, it can be concluded that all the in vitro propagated plantlets were genetically similar to their respective mother plants. Flow cytometry showed that cryopreservation did not change the genome size of the proembryogenic mass or regenerants in Gentiana cruciata, G. tibetica (Mikula et al. 2008) and in G. cruciata using AFLP (Mikula et al. 2011a). The results of present study regarding confirmation of genetic fidelity in cryopreserved plants using ISSR and/or SSR markers are in agreement with reports in other plant species such as Bacopa monnieri (Sharma et al. 2017), banana (Agrawal et al. 2014), Rubus (Reed et al. 2010), wild pear (Condello et al. 2009) and wild Solanum lycopersicum (Zevallos et al. 2014).
Until now, cryopreservation has been attempted in seven species of Gentiana and in most of the cases unorganized tissues (cell suspensions) were used as explants. In general, use of these tissues as explants has few practical difficulties viz., difficult to develop, maintain and handle, require longer period like 2–3.5 years in G. cruciata (Mikula et al. 2011a), 10 years in G. tibetica (Mikula 2006) and 11 years in G. cruciata and G. tibetica (Mikula et al. 2008). Suzuki et al. (1998) attempted cryopreservation in G. scabra using axillary buds, obtained 78–90% of post-thaw survival following air desiccation (exact regrowth percentage is not mentioned). Tanaka et al. (2004) reported post-thaw survival of 60–76.7% (V) to 80–93.3% (encapsulation-vitrification, EV) in G. scabra, 16.7–46.7% (V) to 43.3–83.3% (EV) in G. triflora and 40% (V) to 80% (EV) in G. pneumonanthe. In both the cases, ~ 70–72 days are required for obtaining shoot tips for cryopreservation. In contrast, our protocol requires only 58 days, thus shortening the total period by 12–14 days with post-thaw regrowth of ~ 60% using DV and ~ 35% using V. Thus, the DV protocol standardized here has the potential for long-term conservation of germplasm of the critically endangered plant, G. kurroo. All the three genotypes of G. kurroo (IC266697, IC554589 and IC612563) have been safely cryobanked at ICAR-NBPGR, New Delhi, India, using the DV technique.
References
Agrawal A, Sanayaima R, Singh R, Tandon R, Verma S, Tyagi RK (2014) Phenotypic and molecular studies for genetic stability assessment of cryopreserved banana meristems derived from field and in vitro explant sources. Vitro Cell Dev Biol Plant 50:345–356. https://doi.org/10.1007/s11627-014-9606-4
Agrawal A, Swennen R, Panis B (2004) A comparison of four methods for cryopreservation of meristems in banana (Musa spp.). CryoLetters 25:101–110
Agrawal A, Tyagi RK, Goswami R (2008) Cryopreservation of subgroup Monthan (ABB) of Indian cooking bananas (Musa spp.). Curr Sci 94:1125–1128
Condello E, Palombi MA, Tonelli MG (2009) Genetic stability of wild pear (Pyrus pyraster Burgsd) after cryopreservation by encapsulation dehydration. Agric Food Sci 18:136–143. https://doi.org/10.2137/145960609789267533
Doyle JJ, Doyle JJ (1987) A rapid DNA isolation procedure from small quantities of fresh leaf tissues. Phytochem Bull 19:11–15
Jaccard P (1908) Nouvelles Recherches sur la distribution florale. Bull de la Soc Vaudoise des Sci Nat 44:223–270
Khuroo AA, Dar GH, Khan ZS, Reshi ZA (2005) Observations on Gentiana kurroo Royle, a critically endangered medicinal plant from the Kashmir Himalaya, India. Endanger Species Update 22:137–146
Kikuchi M, Yashiro N (1995) Cryopreservation of lateral buds of gentian by the encapsulation–dehydration technique. Japanese Society for Plant Tissue Culture, Program and Abstracts of the 14th Meeting
Mikuła A (2006) Comparison of three techniques for cryopreservation and re-establishment of long-term Gentiana tibetica suspension culture. CryoLetters 27:268–282
Mikuła A, Fiuk A, Rybczyński JJ (2005) Induction, maintenance and preservation of embryogenic competence of Gentiana cruciata L. cultures. Acta Biol Crac Ser Bot 47:227–236
Mikuła A, Niedzielski M, Rybczyński JJ (2006) The use of TTC reduction assay for assessment of Gentiana spp. cell suspension viability after cryopreservation. Acta Physiol Plant 28:315–324. https://doi.org/10.1007/s11738-006-0027-9
Mikuła A, Olas M, Śliwińska E, Rybczyński JJ (2008) Cryopreservation by encapsulation of Gentiana spp. cell suspensions maintains re-growth, embryogenic competence and DNA content. CryoLetters 29:409–418
Mikuła A, Tomiczak K, Rybczyński JJ (2011a) Cryopreservation enhances embryogenic capacity of Gentiana cruciata (L.) suspension culture and maintains (epi)genetic uniformity of regenerants. Plant Cell Rep 30:565–574. https://doi.org/10.1007/s00299-010-0970-1
Mikuła A, Tomiczak K, Wójcik A, Rybczyński JJ (2011b) Encapsulation-dehydration method of cryopreservation elevates embryogenesity of Gentiana kurroo cell suspension and carrying on genetic stability of its regenerants after cryopreservation. Acta Hortic 908:143–154. https://doi.org/10.17660/ActaHortic.2011.908.16
Mikuła A, Tomiczak K, Domżalska L, Rybczyński JJ (2015) Cryopreservation of gentianaceae: trends and applications. In: Rybczyński J, Davey M, Mikuła A (eds) The gentianaceae—volume 2: biotechnology and applications. Springer, Berlin, Heidelberg, pp 267–286. https://doi.org/10.1007/978-3-642-54102-5_11
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Panis B (2019) Sixty years of plant cryopreservation: from freezing hardy mulberry twigs to establishing reference crop collections for future generations. Acta Hortic 1234:1–8. https://doi.org/10.17660/ActaHortic.2019.1234.1
Pence VC (1999) The application of biotechnology for the conservation of endangered plants. Plant Conserv Biotech 15:227–241
Pennycooke JC, Towill LE (2000) Cryopreservation of shoot tips from in vitro plants of sweet potato (Ipomoea batatas (L.) Lam.) by vitrification. Plant Cell Rep 19:733–737. https://doi.org/10.1007/s002999900171
Reed BM, Castillo N, Wada S, Bassil NV (2010) Genetic stability of cryopreserved shoot tips of Rubus Germplasm. Vitro Cell Dev Biol Plant 46:246–256. https://doi.org/10.1007/s11627-009-9265-z
Rohlf FJ (1998) NTSYS-pc numerical taxonomy and multivariate analysis. Version 2.0. Applied Biostatistics Inc., New York
Sharma N, Chandel KPS, Paul A (1993) In vitro propagation of Gentiana kurroo—an indigenous threatened plant of medicinal importance. Plant Cell Tissue Org Cult 34:3307–3309. https://doi.org/10.1007/BF00029722
Sharma N, Singh R, Pandey R, Kaushik N (2017) Genetic and biochemical stability assessment of plants regenerated from cryopreserved shoot tips of a commercially valuable medicinal herb Bacopa monnieri (L.) Wettst. Vitro Cell Dev Biol Plant 53:346–351. https://doi.org/10.1007/s11627-017-9826-5
Skinder BM, Ganai BA, Wani AH (2017) Scientific study of Gentiana kurroo Royle. Medicines 4:74. https://doi.org/10.3390/medicines4040074
Suzuki M, Akihama T, Ishikawa M (2005) Cryopreservation of encapsulation gentian axillary buds following 2 step-preculture with sucrose and desiccation. Plant Cell Tissue Org Cult 83:115–121. https://doi.org/10.1007/s11240-005-4854-y
Suzuki M, Ishikawa M, Akihama T (1998) A novel preculture method for the induction of desiccation tolerance in gentian axillary buds for cryopreservation. Plant Sci 135:69–76. https://doi.org/10.1016/S0168-9452(98)00054-5
Takagi, H (2000). Recent developments in cryopreservation of shoot apices of tropical species. In: Engelmann F, Takagi H (eds) Cryopreservation of tropical plant germplasm. Current research progress and application. Japan International Research Center for Agricultural Sciences, Tsukuba/International Plant Genetic Resources Institute, Rome, pp 178–193
Tanaka D, Niino T, Isuzugawa K, Hikage T, Uemura M (2004) Cryopreservation of shoot apices of in-vitro grown gentian plants: comparison of vitrification and encapsulation-vitrification protocols. CryoLetters 25:167–176
Tomar A, Manhas RK, Srivastava RK (2012) Seed germination studies in Gentiana kurroo Royle—an endangered medicinal herb. J Med Aromat Plant Sci 34:166–171
Towill LE, Bonnart R (2003) Cracking in a vitrification solution during cooling or warming does not affect growth of cryopreserved mint shoot tips. CryoLetters 24(6):341–346
Ved D, Saha D, Ravikumar K, Haridasan K (2015) Gentiana kurroo. The IUCN Red List of Threatened Species 2015:e.T50126594A50131345. https://doi.org/10.2305/IUCN.UK.2015-2.RLTS.T50126594A50131345.en
Wang M, Lambardi M, Engelmann F, Pathirana R, Panis B, Volk GM, Wang QC (2020) Advances in cryopreservation of in vitro-derived propagules: technologies and explant sources. Plant Cell Tissue Org Cult. https://doi.org/10.1007/s11240-020-01770-0
Xue SH, Luo XJ, Wu ZH, Zhang HL, Wang XY (2008) Cold storage and cryopreservation of hairy root cultures of medicinal plant Eruca sativa Mill., Astragalus membranaceus and Gentiana macrophylla Pall. Plant Cell Tissue Org Cult 92:251–260. https://doi.org/10.1007/s11240-007-9329-x
Zevallos B, Cejas I, Engelmann F, Carputo D, Aversano R, Scarano MT, Yanes E, Martínez-Montero M, Lorenzo JC (2014) Phenotypic and molecular characterization of plants regenerated from non-cryopreserved and cryopreserved wild Solanum lycopersicum Mill. seeds. CryoLetters 35:216–225
Acknowledgements
We thank Director, ICAR-NBPGR, New Delhi, for facilities and encouragement. Financial support provided by the Department of Biotechnology, Ministry of Science and Technology, is gratefully acknowledged. Thanks are due to Mr Ramesh Chandra and Mr Suresh Mali for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Ranjith Pathirana.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, N., Gowthami, R., Devi, S.V. et al. Cryopreservation of shoot tips of Gentiana kurroo Royle – a critically endangered medicinal plant of India. Plant Cell Tiss Organ Cult 144, 67–72 (2021). https://doi.org/10.1007/s11240-020-01879-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01879-2