Abstract
Intact and wounded shoots of in vitro cultured potato were investigated in situ to determine how their phototropic (PT) bending capacity was affected by water availability and exogenous auxin supplementation. Wounding strongly decreased PT bending but it recovered upon supplementation of water, auxin or both. Intact shoots required neither water nor auxin, while shoot segments required both. Shoot cuttings required only water, while, like shoot segments, decapitated shoots had a strong requirement for auxin. Water supplementation was beneficial in all treatments and PT bending was not affected in cultures that were submerged in water for a short period. Sucrose and inorganic salts present in the medium strongly affected PT bending capacity of cultures, favouring combinations with lower concentrations of both. Sucrose alone strongly promoted PT bending up to a concentration of 5%. Osmotic shock induced by the addition of small volumes of highly concentrated carbohydrate solutions (sucrose, glucose or sorbitol) induced a rapid but transient decline in PT bending capacity. These results indicate that water availability is a major factor that affects PT bending in potato plantlets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In vitro culture techniques offer many advantages when applied to tropic studies. Agar solidified media is commonly used in tropic studies of Arabidopsis as a support for aseptic seed germination and for plantlet spatial positioning. In the case of potato, shoot culture techniques help to overcome problems with the seed availability of commercial cultivars. Single node shoot explants of potato rapidly develop into plantlets that exhibit little variability and are suitable for tropic studies. In general, in vitro culture conditions enable strict control over internal (media composition) and external (light parameters and temperature) factors, with minimal need to physically interact with the explants and plantlets situated in their culture vessels.
The potato is one of the five most important cultivated species and is frequently used in biotechnological studies. However, the tropic responses of potato are still largely unknown. Recently we adopted a simple in vitro culture protocol and developed technical procedures to enable potato shoot cultures to be used as a novel model system for tropic response studies of dicotyledonous plants (Vinterhalter et al. 2012, 2015). Studies performed on light-grown potato plantlets revealed some previously unknown features, such as the circadian regulation of tropic movements (Vinterhalter et al. 2015) and multiple phototropic responses including light tracking (Vinterhalter and Vinterhalter 2015). Potato shoots could perform serial phototropic responses with the ability to reverse bending direction or perform lateral phototropic movements (Vinterhalter et al. 2016). Furthermore, potato shoots were shown to exhibit strong competitive interactions between their phototropic (PT) and gravitropic (GT) bending movements (Vinterhalter et al. 2016). Indeed, light grown plants must somehow integrate light and gravity signals to guide their growth (Goyal et al. 2013; Vandenbrink et al. 2014).
Water, needed both for shoot elongation and tropic bending in the shoot elongation zone may be a limiting resource for tropic responses of potato shoots (McIntyre 1980, 2001). Thus, water and inorganic salts (nitrogen) as limiting factors in the regulation of plant development offer alternatives to plant growth regulators as forces mediating the elongation/tropic bending mechanism. In this study we therefore examine hormonal and nutritional effects on the tropic bending of potato, with a focus on IAA (indole-3-acetic acid) supplementation and water availability.
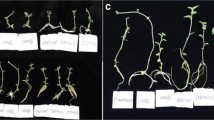
The basic approach was to evaluate phototropic bending in wounded shoot explants that are known to respond to exogenous IAA supplementation (Kutschera 1994; Hager 2003). Phototropic bending capacity of intact shoots was compared with the capacity of different categories of wounded shoots (decapitated, stem cuttings and shoot segments; Fig. 1) exposed to variable levels of water availability and in the presence or absence of exogenously supplied IAA.
Schematic presentation of explant types employed in the study. Intact and decapitated plantlets were used in their original culture vessels. In decapitated explants the whole apical leaf whorl containing the apical bud was removed. For the preparation of stem cutting and shoot segment explants, entire plantlets were removed from their culture vessels. They were trimmed, defoliated and then inserted by their lower cut end into fresh medium in an upright position
In decapitated shoots, elongation rate quickly deteriorates (Christian and Lüthen 2000), reaching only 30% of the elongation rate of intact shoots (Kutschera 1994). Decapitation also decreases the PT bending rate of shoots. For instance, in decapitated Pisum shoots, the magnitude of PT bending falls from 60° to 8° (Britz and Galston 1983). In coleoptile tips, excision also decreases PT and GT bending, as shown by Iino (1995) in maize. Recovery of the PT response in decapitated shoots/coleoptiles due to IAA supplementation has been well documented (Britz and Galston 1983; Kaldenhoff and Iino 1997).
Auxins are plant growth regulators with multiple roles including strong growth-promoting activity that induces shoot elongation (Christian and Lüthen 2000). Experimental evidence indicates a direct connection between the presence of IAA (natural auxin) and the shoot elongation or tropic bending responses. IAA is considered the main mediating factor for both gravitropic (Evans 1991; Chen et al. 1999) and phototropic (Christie and Murphy 2013) bending. The pattern of IAA distribution in tissues provides ample support for the core of the well-known Cholodny–Went theory (CWT). In short, CWT assumes that IAA is synthesised in the apical shoot bud and then is basipetally translocated, providing gradients along the shoot plus lateral redistribution and asymmetry in the case of tropic responses. In phototropic responses, IAA is laterally redistributed from the illuminated to the shaded side of the plant (Whippo and Hangarter 2006).
In the closed circulatory system of intact plants there is a well organized flow of endogenous auxin regulated by a complex system of auxin transporters. Their activity can be modified by tropic stimuli resulting in the redirection of polar auxin transport and then tropic bending (Spalding 2013). If the shoot integrity is compromised by wounding recovery of PT bending capacity in most cases will positively respond to addition of exogenous auxin. However, some plant species, such as the sunflower, do not fit well into the CWT as their tropic bending develops without the previous formation of any IAA gradients (Bruinsma et al. 1975).
Shoot segments were the material of choice for studies on the Acid Growth Theory (AGT), which was started by studies of Rayle and Cleland (1970) and Hager et al. (1971). The main objective of AGT studies was to examine tissue acidification as the motivating force for shoot elongation; therefore, segment preparation for these studies was particularly well elaborated. Unfortunately, segments were always layered in water prior to treatments with the intention of removing excess and residual IAA; therefore, the effects of water supplementation alone could not be studied in this system.
Fukaki et al. (1996a) showed that shoot segments and stem cuttings prepared from Arabidopsis flower stalks and mounted in gellan gum were highly responsive to gravistimulation. The phototropic responses of the same explants were less pronounced (Fukaki et al. 1996b). Both studies showed that stem cuttings and shoot segments can be used to study tropic responses and that the presence of apical shoot buds is not of crucial importance for segments to develop the proper tropic response following adequate stimulation. The redundancy of the apical shoot tip in the PT bending of plants has been further confirmed in studies by Preuten et al. (2013) and Yamamoto et al. (2014).
The development of tropic responses in explants missing the apical shoot tip does not necessarily negate the CWT as some of its modifications consider that polar IAA translocation is redundant for PT responses triggered locally by signals generated in narrow tissue zones at the same level as the bending response (Preuten et al. 2013). It is, however, possible that CWT, with its hormonal approach to shoot elongation and tropic bending, is still missing a vital factor. We believe this factor could be water availability, as intensive shoot elongation and tropic bending require that their target cells and tissues be provided with a high and constant supply of water inducing the cell volume increase (Kutschera and Niklas 2013). A stimulatory effect of distilled water on initial elongation of shoot segments has been observed and reported by Rayle and Cleland (1992).
We therefore studied the effects of water availability on the phototropic bending of potato shoots by creating experimental conditions in which water was present in surplus (submersion) or in abrupt shortage (osmotic stress). Fine tuning of water availability was tested in treatments with plantlets growing on media with various sucrose concentrations and high or low content of macro mineral salts.
The role of auxin in tropic responses remains unchanged and unchallenged by results presented here, except for the finding that in potato its promotion of the tropic response was limited only to explants with an excised apical bud. Water availability was beneficial for restoring PT bending capacity in all types of wounded explants.
Materials and methods
All experiments were performed using potato cv. Désireé maintained under conditions of in vitro culture previously described by Vinterhalter et al. (2016). In short, shoot cultures were maintained on plant growth regulator-free medium according to a continual propagation procedure recommended by Hussey and Stacey (1981) using MS (Murashige and Skoog 1962) ingredients with 3% (w/v) sucrose and 0.7% (w/v) agar. Percentage concentrations were always prepared as w/v. Sub-culturing was done at 3–4 weeks intervals and treatments were set with single node explants in a hexagonal arrangement. Glass vessels (Boneco 250 ml, Vetropack) equipped with translucent 57 mm twist-off polypropylene closures contained 40–50 ml medium. Cultures were grown in growth chambers under long day (16/8 h light to darkness photoperiod), with 70 µM m−2s−1 light irradiance provided by cool white Philips TLD fluorescent lamps and measured with Li-Cor 1400 spectrophotometer with a quantum sensor. Temperature in the growth chambers and dark chambers where PT stimulation treatments were performed was maintained at 24 ± 1 °C. Submersion treatments were conducted with deionised distilled water adjusted to the same temperature. The water used for media preparation and submersion treatments was freshly processed from distilled water by a Milli Q-UF (Millipore) water deionizer.
Cultures required 9–12 days for single node explants to reach 40–55 mm height considered here as optimal for phototropic studies of intact shoots. At this stage, explants had well-developed adventitious roots and they were referred to as plantlets. Plantlets used in wounding treatments were cultured somewhat longer enabling explants to reach height of 60 mm or more. Decapitated (DEC) cultures were left in their original culture vessels; only the apical shoot whorl was removed. For preparation of cuttings (CUT) and shoot segments (SEG), we aseptically removed plantlets from their culture vessels, aligned and trimmed them on cardboard plates. Leaves were removed, shoots were detached from roots and in SEG explants apical shoot whorl was also removed as in preparation of DEC explants. CUT and SEG explants were immediately inserted in appropriate medium in vertical position and mounted for phototropic stimulation.
Studies were conducted 1–2 h after the beginning of the day in the growth chambers. Phototropic stimulation was performed by unilateral irradiation of flasks using a single Phillips blue 1 W GU10 accent LED lamp producing 24 μmol m−2 s−1 irradiance per flask with plantlet inside. The peak emission of blue LED lamps was at 470 nm (Vinterhalter et al. 2012).
Tandem treatments comprising two culture vessels each irradiated with a separate LED lamp were replicated 3–4 times providing no less than 30 plantlets per treatment. The bending process was documented by Nikon Coolpix P510 and P520 cameras under a 5 min time-lapse regime. Curvatures were measured from stored digital images using Gimp 2.8 (http://www.gimp.org). Graphic presentations of PT bending comprising mean values and their standard errors were drawn with OriginPro 8 (http://www.originlab.com).
Results
Phototropic bending in submersed shoots
Conditions of high water availability were maintained by submersion of the culture vessel in distilled water. As field-grown potato is susceptible to excess water in the soil, we first had to demonstrate that water submersion for 2–3 h was not detrimental to cultures or their PT bending capacity. PT bending in this study was usually monitored for 3 h after the beginning of PT stimulation. However, in an assessment of the durability of cultures to extended submersion treatment, it was found that little deterioration of the PT bending capacity occurred after 18 h of submersion including an 8 h-long period during the night (Fig. 2).
Over the long term, water submersion is detrimental for most plants except for a small number of highly specialised, tolerant species. However, short submersion exposures of 2–3 h were not harmful, enabling high PT bending responses. Water submersion solved the problem of direct IAA supplementation to plants, offering an alternative to IAA application at the cut ends or to supportive media. Plants cultured in vitro on agar solidified media are considered to be well supplied with water. There are, however, species in which in vitro culture conditions may induce hyper hydric responses (vitrification) characterised by high water content of tissues (Debergh et al. 1992). The decrease in PT bending at the end of treatments with submersed shoots is due to the presence of air in leaf aerenchyma, making shoots buoyant. In treatments where shoots were removed from culture vessels (stem cuttings and shoot segments). The problem of excess buoyancy was efficiently solved by leaf removal.
Effects of supplementing shoot segments with auxin
The effect of auxin on PT bending was studied in shoot segments submerged in water and supplemented with IAA. Shoot segments were selected as they are known from the literature to respond well to IAA supplementation, demonstrating a restored PT capacity.
The effect of IAA on PT bending of potato shoot segments is presented in Fig. 3. IAA stimulated PT bending over a wide range of IAA concentrations, although differences between them were not great. Therefore, IAA is a factor that strongly supports PT bending of severely wounded potato shoots. The 6 µmol IAA concentration exhibited the greatest stimulatory effect. This concentration was noted by Kutschera (1994) as the most frequently used IAA concentration in AGT studies. It should be noted that water alone (no IAA) supported reasonably high PT bending in potato shoot segments, leading us to further elaborate on its role.
Comparison of intact and wounded shoots
The phototropic bending response of intact and three types of wounded shoots is presented in Fig. 4. Treatments in which explants were not submerged in water are denoted as Air treatments and they are considered as reference treatments for all comparisons. The other two treatments, submerged in water (Sub) and submerged in 6 µM IAA (Sub + IAA) differed from the air treatment as they provided high water availability to explants. The air treatment of intact shoots showed the highest PT response, which was not surpassed by the treatments providing abundant water and auxin supplementation.
Decapitation (DEC) significantly decreased the PT bending ability of shoots in both air and Sub treatments (Fig. 4b). However, the Sub + IAA treatment significantly restored the decapitation damage. The addition of water alone was not sufficient in this treatment to improve the low PT bending response of the standard air treatment.
The results of stem cuttings (CUT) treatments are of interest as these explants contained intact apical buds but were severed from their root system. CUT explants manifested a large decrease in their PT bending capacity when in the Air treatment. Sub and Sub + IAA treatments were much less affected, and there was a significant recovery of the PT bending response when the cuttings were supplemented with water (Fig. 4c). Since there was no great difference between the Sub and Sub + IAA treatments, it is clear that the recovery of PT bending capacity requires only water and not IAA.
In the segment (SEG) treatments, shoots were both decapitated and detached from their root system. PT bending in the Air treatment was low but it significantly improved in the Sub + IAA treatment while the Sub treatment produced intermediate results (Fig. 4d). Therefore, shoot segments require both IAA supplementation and water to restore their PT bending capacity.
Wounding treatments confirm the important role that IAA plays in the restoration of PT bending capacity of wounded shoots but they also demonstrate a unique effect of water. The water promotion effect was best observed in CUT explants in which water supplementation was obligatory for the recovery of PT bending capacity while IAA was redundant.
CUT and SEG explant types, which upon excision were mounted in fresh medium for PT stimulation, could be subjected to some additional treatments by supplementing IAA instead of water into the new medium. When IAA is present in the medium itself and not in the bathing solution, it is expected to enter shoots mainly through the lower cut end of the explant. Thus, for CUT and SEG explants, two more treatments were performed by supplementing IAA at 6 µM concentration into the medium and exposing the explants either to Air or Sub conditions with distilled water (Fig. 5). For comparison, data values of the Air and Submersed treatments from Fig. 4 are also provided as small symbols.
PT bending of stem cuttings (a) and shoot segments (b) surrounded by air or water (submersed) with IAA added to the agar medium at a concentration of 6 µM. As a reference, Submersion and Air treatment values from Fig. 4 are also included (small symbols)
In CUT explants, there was no difference between Submersion and Submersion + IAA in agar. There was also no difference between the treatments Air and Air + IAA in agar. Therefore, in CUT explant types, IAA supplemented to the agar medium had no apparent effect on PT bending capacity, which was enhanced by water irrespective of the presence or absence of IAA in the medium.
However, in SEG explants, the situation was more complex as explants lacked both the root system and apical buds. Here, IAA supplemented to the agar medium significantly promoted PT bending capacity. Therefore, bending of the Air + IAA in agar treatment (no extra water supplemented) was nearly two times greater than the plain Air treatment with no IAA or water supplemented. At the same time, Submersion + IAA in agar treatment produced a slightly greater bending capacity than the plain submersion treatment (no IAA), with the difference becoming pronounced only in the second half of the treatment. Thus, in SEG explants, IAA only slightly improved PT bending in comparison to the Submersion treatment. In the absence of both IAA and water supplementation (Air), PT bending remained low. By comparing the Air and Air + IAA in medium in treatments with SEG type explants, which both lack water supplementation, it is apparent that IAA enters the shoot segment and promotes PT bending.
Response of wounded explants grown under continuous light conditions
Continuous light conditions are known to impose drastic changes on the PT response of potato plants (Vinterhalter and Vinterhalter 2015). Therefore, trials in continuous light conditions were conducted to test the response of segments to water and IAA supplementation (Fig. 6).
Water submersion and IAA supplementation had little effect on the PT bending of intact plantlets grown in continuous light conditions. They maintained the prominent 50 min maximum of the FPT response followed by a small decline and then a slow response recovery. IAA supplementation did not improve PT bending of intact plantlets, as was the case for the LD long day photoperiod (Fig. 3). In segment explants, PT bending was slower but after the first 50 min of fast PT bending there was no decline, but rather a steady increase in the PT bending curvature. IAA supplementation promoted the PT bending of segments for the first 120 min, i.e., until the second, steady PT bending stage started (Vinterhalter and Vinterhalter 2015). After that, during the third hour, PT bending rates were similar both for intact plants and segments, irrespective of IAA supplementation. Data for segments submersed in IAA but grown under the LD photoperiod (Fig. 3d) are provided as a comparison in Fig. 6, indicating a prominent difference in the PT response of explants from plantlets grown in the two different photoperiods.
It is clear that the effect of continuous light conditions on PT bending of intact plantlets strongly resembles the bending of segment explants, apart from at the 50 min peak maximum. This indicates a photoperiod-imposed limitation that cannot be overcome by water and IAA supplementation.
Effect of sucrose and mineral nutrition
The availability of water to plantlets growing in vitro can be fine-tuned by adjusting the sucrose and mineral salt content of the medium. Carbohydrates (sucrose) and macro salts are the two components with the greatest contribution to the water potential of nutrient media. We maintained potato shoot cultures at a 3% sucrose concentration, which supported fast development of axillary buds into rooted plantlets that were used in our studies. Higher sucrose concentrations are known to affect the development of potato cultures; at a concentration of 8%, shoot elongation stops and only radial enlargement of shoots is possible as they continue to grow in the form of tubers (Xu et al. 1998).
The 70 µM m−2 s−1 irradiance in our growth chamber promoted primarily autotrophic growth of our potato plantlets. Under these conditions we maintained a potato clone on sucrose-free medium continuously for more than 3 years with routine subcultures of single node explants onto fresh sucrose-free medium. These autotrophic cultures had reasonable PT bending capacity, indicating that the presence of sucrose as the carbohydrate source was not an absolute requirement for the PT bending response of potato shoot cultures. However, sucrose still had a noticeable stimulating effect on the PT bending capacity (Fig. 7). Sucrose effects were observed on two types of media, containing either high (full strength; Fig. 7a) or low (1/10 strength; Fig. 7b) levels of MS macro salts.
The effect of sucrose on PT bending capacity was closely correlated with its effect on shoot elongation (results not presented). On full strength MS macro salts, PT bending increased from 0 to 5% sucrose, but then rapidly declined with further increases in sucrose (Fig. 7a). At sucrose concentrations of 8% and higher, PT bending was absent. Trials showed that full strength MS macro salts in the medium was supraoptimal for PT bending. To demonstrate this effect, we tested PT bending at various sucrose concentrations in the presence of 1/10 strength MS macro salts (Fig. 7b). At equal sucrose concentrations, 1/10 strength MS mineral salt concentrations always produced greater PT bending values than treatments performed using media with full strength MS macro salts. Thus, although sucrose strongly affected the PT bending capacity of potato shoots, its action was further modified and coordinated with the action of macro mineral salts. Media containing 1/10 strength MS salts and lower sucrose concentrations (1–3%) had high PT bending capacities. Both the effects of sucrose and macro mineral salts were long-term effects, characterising the PT bending capacity of cultures grown for 10–14 days on tested media. Cultures therefore had ample time to adjust to the specific growth conditions.
Osmotic shock
We previously demonstrated that high water availability provided by submersion had no deleterious effects on the PT bending capacity of potato shoots and that water significantly improved PT bending in wounded shoots. Cultures could also adjust to different water availability conditions caused by the long-term presence of medium components providing high osmotic values to media (carbohydrates and mineral salts). What remained was to investigate whether an abrupt decrease in water availability induced by osmotic shock could trigger rapid changes in the PT bending response of potato plantlets.
For this experiment, osmotic shocks were applied by adding 15–20 ml of concentrated carbohydrate solution with a pipette to the top of the media in culture flasks. PT stimulation was started 55 min prior to the application of osmotic shock. The osmotic compounds layered on top of the medium immediately absorbed water from the medium, decreasing the water available to roots for upward translocation. Water in the culture flask, in the same way as in plants growing naturally, is considered to be organised in a modified SPAC continuum stretching from the medium into the plantlets’ roots, then up into the shoots, ending finally as water vapour surrounding the plantlets. SPAC denotes soil, plant and atmosphere continuum proposed by Philip (1966). Any obstruction of water movement made in any part of this system should be instantly transmitted to all parts of the system. Therefore, a decrease in the amount of water available to the roots is instantly transmitted upward, resulting in a decrease in PT bending rate that is visible 5–10 min after the beginning of osmotic shock treatments (Fig. 8).
The osmotic shock treatments presented here should be considered as a demonstration rather then an accurate, quantitative technique. In these experiments there were too many different factors affecting water availability for plantlets in their culture vessels. The results could have been affected by media preparation, autoclaving procedures, culture vessel storage and shelf life, and by the level of root and shoot development of individual plantlets in the culture vessel. Still, we believe this experiment was sufficient for demonstrating the importance of water for plantlets to conduct their PT bending responses.
Discussion
Light is an important factor that affects various aspects of plantlet development under in vitro culture conditions and not only the PT bending. Although cool-white light of fluorescent lamps is considered as sufficient to maintain potato cultures (Seabrook 2005), large efforts are made to improve light conditions by incorporation of LED lights focused at certain wavebands. Recently Edesi et al. 2014 found that a mixture of light from red and blue LEDs improved shoot regeneration in all of the studied potato cultivars following cryopreservation treatments.
The structure of shoots developing from single node explants into potato shoot cultures strongly differs from the structure of shoots present in seedlings. Seedlings are characterised by the presence of fast hypocotyl development. However, the hypocotyl is not a true shoot but a transition zone between root and shoot that exhibits mixed features visible in the vascular system layout. On the other hand, potato shoot cultures are epicotyl derivatives, and they should be compared to shoots of homologous structure and origin. Therefore, their responses should be compared with those of Arabidopsis flower stalks but not with the responses of seedlings.
Potato shoots in their PT bending zone have nearly circumferential rings of vascular elements, with a xylem ring positioned between external and internal rings of phloem. In lower positioned (older) internodes vascular elements are concentrated in three large and three small bicollateral bundles (McCauley and Evert 1988). As a consequence of this strong and complex vasculature the PT response of potato shoots is fairly accurate (Vinterhalter et al. 2016) and it is not dependent on the side from which shoots are irradiated, as it is the case with seedlings of other species (Britz and Galston 1983; Khurana et al. 1989).
The wounding of potato shoots and shoot segment preparation are not as detrimental as in seedlings. A potato shoot segment, although wounded by excision at both ends, retains several axillary buds providing good prospects for recovery. When placed on fresh medium, these buds may regenerate a whole plant in the same way as single node explants used in the present study for multiplication and maintenance of shoot culture stocks.
Potato shoots are insensitive to red, amber and yellow light and there seems to be no pigment crosstalk comparable to that observed in Arabidopsis (Pedmale et al. 2010). When grown under a long day photoperiod (16/8 h light to darkness) they also never manifest nutational or circumnutational movement. Therefore, phototropic bending of potato shoots seems to be a simple and straightforward response to unilateral blue light irradiation, likely due to decreased lateral water translocation at the illuminated side of the shoot in the zone responsive to PT bending. Such an explanation fits well with the McIntyre (1980) hypothesis, which assumes that tropic responses are induced by asymmetries in the lateral water distribution within the shoot. None of the treatments employed here can address lateral water translocation but our study generally indicates that water is a strong requirement for PT bending in potato shoots.
Submersion
The ability of potato plantlets to undergo PT bending movement during short-term submergence enabled intact shoots and their wounded explant types to be grown temporarily under conditions of high water availability. Intact cultures submerged in distilled water were able, in the same way as non-submerged cultures, to demonstrate reversible PT movement following lateral 180° relocation of the light source (Vinterhalter et al. 2016). Submersion also enabled easy and uniform supplementation of IAA to plants, offering a new way for the entry of IAA into the plant body. The experiments presented in Figs. 5, 6 and 7 showed that IAA promoted PT bending in shoot segments, irrespective of the side of the shoot at which IAA entered the plant body. Long-term exposure of cultures to IAA could not be tested, as IAA in plants exhibits strong morphogenetic effects inducing adventitious rooting.
IAA concentration
IAA supplementation to shoot segments (Fig. 3) showed that strong promotive effect on PT bending capacity over a wide range of IAA concentrations, unexpectedly implying that even water alone can promote PT bending. Further clarification of this observation is presented in Figs. 5, 6 and 7, showing that IAA had a strong stimulatory effect only in decapitated explants (DEC and SEG) while in CUT explant types, IAA was redundant. Water, on the other hand, had a high stimulatory effect on the recovery of the PT bending response in all types of wounded shoot explants, indicating that the action of water and IAA in the restoration of PT bending capacity of shoots could be synergistic.
The preparation of SEG and DEC explants included shoot excision just below the apical leaf whorl, presumably damaging the shoot elongation zone that generally coincides with the PT bending zone. The PT bending zone in these explants was re-allocated to a new, lower position and it is possible that IAA is involved in and required for this re-allocation. This would mean a novel role for IAA, whereby it determines the shoot position at which PT bending occurs. This would also explain why there is a promotive effect of IAA supplementation on decapitated shoots and shoot segments but not on stem cuttings or intact plants.
Continuous light conditions
The studies performed on shoot segments excised from plantlets cultured under continuous light conditions confirm previous findings that a high PT bending capacity of shoots requires well spaced dark periods, repeated regularly on a daily basis (Vinterhalter et al. 2015), indicating circadian regulation. The involvement of circadian reguIation enables potato shoots grown in long day conditions to anticipate the imminent change of the light regime with adjustments visible as daily changes in the capacity of PT bending. In continuous light conditions diurnal rhithmicity of the PT response gradually declines and the somewhat lower PT response becomes constant at any time point in treatments done in continuous light (Vinterhalter and Vinterhalter 2015) High water availability and IAA supplementation cannot improve PT bending under continuous light conditions and the overall response of shoot segments is similar to the response of intact plants. Shoot segments fall short only in the initial fast phototropic response, as they do not have a prominent maximum, while in the later, steady stage phototropic response this shortcoming is compensated. Responses of plants grown in continuous light conditions are insufficiently studied and additional studies may have positive implications both for fundamental and applied science (Velez-Ramirez et al. 2011).
Fine tuning of water availability
We have shown that the presence of compounds that affect the osmotic potential (water potential) of the medium also affects the PT bending capacity of plantlets. Sucrose and inorganic salts strongly contribute to the build-up of osmotic potential of the medium (George et al. 2008); however, it is usually difficult to determine whether some of the observed plant responses are imposed by nutritional or osmotic effects of the medium. Luckily, potato shoot cultures can be grown on sucrose-free medium, where they manifest a respectable PT bending capacity, indicating that the sucrose effect on PT bending capacity is of osmotic and not of nutritional nature. Single node explants placed on sucrose-free or any other type of medium at sub-culture have ample time prior to PT treatments to adapt to the nutritional conditions of the employed medium.
The effect of sucrose on the development of potato shoot cultures has been presented in numerous studies. The work of Xu et al. (1998) is noteworthy because they showed that 8% sucrose was the limiting concentration up to which shoot development in potato shoot cultures was still possible. Although the medium with 8% or more sucrose contains ample water, it is not available to the cultures as the high osmotic potential of the medium prevents uptake. Cultures growing on low sucrose and low inorganic salt media—conditions of high water availability—all manifest high PT bending capacities. Sucrose on its own also promoted PT bending with a combination of 5% sucrose and 1/10 strength MS inorganic salts, providing the greatest PT bending response, as measured by the rate and magnitude of PT bending. At sucrose concentrations greater than 5%, shoot elongation was decreased or arrested and PT bending could not be measured. Similarly, on sucrose-free medium with 1/10 strength MS macro inorganic salts, shoots developed and were well elongated but they could not maintain a vertical growth habit, thereby preventing PT bending measurement.
Osmotic shock studies
Cell enlargement, which is the basis of shoot elongation and tropic bending, is inhibited if there is inadequate water (Nonami and Boyer 1990). In hyperosmotic solutions, root elongation is known to decelerate and stop almost immediately, followed by a short 1–30 min period of adjustment (Frensch 1997). Therefore, osmotic shock treatments were performed with the purpose of slowing down the transport of water from the medium to the sub-apical shoot region where PT bending occurs. As the locations of water entry into the plant body and the shoot bending zone are positioned far from each other, and as the osmotic shock response itself is fast, the nature of the osmotic compound employed and its putative detrimental effects are of little importance. In contrast to sucrose, which is the major media supplement promoting the growth of cultures, mannitol entry into potato tissues can have an adverse effect (Lipavska and Vreugdenhil 1996). However, the effects of sucrose and mannitol on the PT bending of potato plantlets were still similar (Fig. 7).
Water and its movement as a continuum can be simultaneously the signal for the change in the rate of water supply and the quantitative change in the available building blocks required for shoot elongation. The fact that osmotic shock can trigger a fast decline in PT bending far from its place of action should be considered as firm and direct proof that water translocation along the root to shoot axis is a major component of PT bending. Molecular techniques registered a large number of different proteins involved in water translocation in potato and other plant species. Among them special care in the future should be paid to aquaporins (Venkatesh et al. 2013) and dehydrins (Charfeddine et al. 2017) as they may be shown to affect and modulate photo- and gravitropic responses.
Conclusions
Classic phototropic studies on seedlings are focused on phototropism as a phenomenon that occurs in the apical shoot portion. The studies presented here offer a different, extended view in which phototropic bending appears as a more complex process. It starts with water from the medium entering plantlet roots or shoots at a cut surface of shoot explants with upward translocation to the apical shoot portions. As water moves in a continuum (SPAC), changes in the water translocation rate are almost instantly transposed into the changes of PT bending rate. Thus, plant–water relations need to be considered as a factor in the execution of tropic responses. The standardisation and accurate control implemented in the use of seedling germination under strictly defined conditions does not mean that water is not an important factor in PT bending. Leaving the effect of water out of this picture simplifies the studies but unfortunately it also affects the overall process functioning. In this study we have shown that in vitro culture technique can be used as a model system to study tropic bending. This technique was used to demonstrated that water is a major factor that affects phototropic shoot bending of light-grown plants. Our results are a step towards an explanation of McIntyre’s (1980) hypothesis, although none of the presented treatments were designed to demonstrate lateral water movement across the shoots. The role of exogenous IAA supplementation was limited to wounded explants only, and even in this instance, IAA did not affect shoots excised from the root system and those with an excised apical shoot bud in the same way. However, water translocation and IAA distribution may be related, implying that IAA gradients could participate in water partitioning, regulating the position of the shoot elongation zone and restoring damaged tissue.
References
Britz SJ, Galston AW (1983) Physiology of movements in stems of seedling Pisum sativum L. cv Alaska. III Phototropism in relation to gravitropism, nutation and growth. Plant Physiol 71:313–318
Bruinsma J, Karssen CM, Benschop M, van Dort JB (1975) Hormonal regulation of phototropism in the light-grown sunflower seedling, Helianthus annuus L. Immobility of endogenous indoleacetic acid and inhibition of hypocotyl growth by illuminated cotyledons. J Exp Bot 26:411–418
Charfeddine S, Charfeddine M, Saïdi MN, Jbir R, Bouzid RG (2017) Potato dehydrins present high intrinsic disorder and are differentially expressed under ABA and abiotic stresses. Plant Cell Tiss Organ Cult 128:423–435
Chen R, Rosen E, Masson PH (1999) Gravitropism in higher plants. Plant Physiol 120:343–350
Christian M, Lüthen H (2000) New methods to analyse auxin-induced growth I: classical auxinology goes Arabidopsis. Plant Growth Regul 32:107–114
Christie JM, Murphy AS (2013) Shoot phototropism in higher plants: new lights through old concepts. Am J Bot 100:35–40
Debergh P, Cohen D, Grout B, von Arnold S, Zimmerman R, Ziv M (1992) Reconsideration of the term ‘vitrification’ as used in micropropagation. Plant Cell Tiss Organ Cult 30:135–140
Edesi J, Kotkas K, Pirttilä AM, Häggman H (2014) Does light spectral quality affect survival and regeneration of potato (Solanum tuberosum L.) shoot tips after cryopreservation? Plant Cell Tissue Organ Cult 119:599–607
Evans ML (1991) Gravitropism: interaction of sensitivity modulation and effector redistibution. Plant Physiol 95:1–5
Frensch J (1997) Primary responses of root and leaf elongation to water deficits in the atmosphere and soil solution. J Exp Bot 48:985–999
Fukaki H, Fujisawa H, Tasaka M (1996a) Gravitropic response of inflorescence stems in Arabidopsis thaliana. Plant Physiol 110:933–943
Fukaki H, Fujisawa H, Tasaka M (1996b) SGR1, SGR2, and SGR3: novel genetic loci involved in shoot gravitropism in Arabidopsis thaliana. Plant Physiol 110:945–955
George EF, Hall MA, De Klerk G-J (2008) Plant propagation by tissue culture procedure – volume 1. The Background. Springer, Dordrecht
Goyal A, Szarzynska B, Fankhauser C (2013) Phototropism: at the crossroads of light-signaling pathways. Trends Plant Sci 18:393–401
Hager A (2003) Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. J Plant Res 116:483–505
Hager A, Menzel H, Krauss A (1971) Versuche und hypothese zur Primärwirkung des Auxins beim Zellstreckungswachstum (experiments and hypothesis concerning the primary action of auxin in elongation growth). Planta 100:47–75
Hussey G, Stacey NJ (1981) In vitro propagation of potato (Solanum tuberosum L.). Ann Bot 48:787–796
Iino M (1995) Gravitropism and phototropism of maize coleoptiles: evaluation of the Cholodny-Went theory through effects of auxin application and decapitation. Plant Cell Physiol 36:361–367
Kaldenhoff R, Iino M (1997) Restoration of phototropic responsiveness in decapitated maize coleoptiles. Plant Physiol 114:267–1272
Khurana JP, Best TR, Poff KL (1989) Influence of hook position on phototropic and gravitropic curvature by etiolated hypocotyls of Arabidopsis thaliana. Plant Physiol 90:376–379
Kutschera U (1994) The current status of the acid-growth hypothesis. New Phytol 126:549–569
Kutschera U, Niklas KJ (2013) Cell division and turgor-driven stem elongation in juvenile plants: a synthesis. Plant Sci 207:45–56
Lipavska H, Vreugdenhil D (1996) Uptake of mannitol from the media by in vitro grown plants. Plant Cell Tiss Org Cult 45:103–107
McCauley MM, Evert RF (1988) Morphology and vasculature of the leaf of potato (Solanum tuberosum). Am J Bot 75(3):377
McIntyre GI (1980) The role of water distribution in plant tropisms. Aust J Plant Physiol 7:401–413
McIntyre GI (2001) Control of plant development by limiting factors: a nutritional perspective. Physiol Plant 113:165–175
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nonami H, Boyer JS (1990) Primary events regulating stem growth at low water potentials. Plant Physiol 94:1601–1609
Pedmale UV, Celaya BR, Liscum E (2010) Phototropism: mechanisms and outcomes. Arabidopsis Book 8:e0125
Phillip JR (1966) Plant water relations: some physical aspects. Ann Rev Plant Physiol 17:245–268
Preuten T, Hohm T, Bergmann S, Fankhauser C (2013) Defining the site of light perception and initiation of phototropism in Arabidopsis. Curr Biol 23:1934–1938
Rayle DL, Cleland R (1970) Enhancement of wall loosening and elongation by acid solutions. Plant Physiol 46:250–253
Rayle DL, Cleland RE (1992) The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol 99:1271–1274
Seabrook JEA (2005) Light effects on the growth and morphogenesis of potato (Solanum tuberosum) in vitro: a review. Am J Potato Res 82:353–367
Spalding EP (2013) Diverting the downhill flow of auxin to steer growth during tropisms. Am J Bot 100:203–214
Vandenbrink JP, Kiss JZ, Herranz R, Medina FJ (2014) Light and gravity signals synergize in modulating plant development. Front Plant Sci 5:563
Velez-Ramirez AI, van Leperen W, Vreugdenhil D, Millenaar FF (2011) Plants under continuous light. Trends Plant Sci 16:310–318
Venkatesh J, Yu J-W, Park SW (2013) Genome-wide analysis and expression profiling of the Solanum tuberosum aquaporins. Plant Physiol Biochem 73:392–404
Vinterhalter D, Vinterhalter B (2015) Phototropic responses of potato under conditions of continuous light and subsequent darkness. Plant Growth Regul 75:725–732
Vinterhalter D, Vinterhalter B, Orbović V (2012) Photo- and gravitropic bending of potato plantlets obtained in vitro from single-node explants. J Plant Growth Regul 31:560–569
Vinterhalter D, Vinterhalter B, Miljuš-Djukić J, Jovanović Ž, Orbović V (2015) Daily changes in the competence for photo- and gravitropic response by potato plantlets. J Plant Growth Regul 34:440–450
Vinterhalter D, Savić J, Stanišić M, Jovanović Ž, Vinterhalter B (2016) Interaction with gravitropism, reversibility and lateral movements of phototropically stimulated potato shoots. J Plant Res 129:759–770
Whippo CW, Hangarter RP (2006) Phototropism: bending towards enlightenment. Plant Cell 18:1110–1119
Xu X, van Lammeren AAM, Vermeer E, Vreugdenhil D (1998) The role of gibberellin, abscisic acid and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol 117:575–584
Yamamoto K, Suzuki T, Aihara Y, Haga K, Sakai T, Nagatani A (2014) The phototropic response is locally regulated within the topmost light-responsive region of the Arabidopsis thaliana seedling. Plant Cell Physiol 55:497–506
Acknowledgements
Research was sponsored by the Serbian Ministry of Education, Science and Technological Development, Project No. 173015 and by a grant from Swiss National Science Foundation, SCOPES project 152221.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Richard E. Veilleux.
Rights and permissions
About this article
Cite this article
Vinterhalter, D., Vinterhalter, B. Phototropic bending of intact and wounded potato shoots. Plant Cell Tiss Organ Cult 130, 393–404 (2017). https://doi.org/10.1007/s11240-017-1235-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1235-2