Abstract
Buffelgrass is a forage grass that reproduces mainly by apomixis. In species with this reproduction mode, in vitro selection allows the incorporation of alternatives in a breeding program. The aims were to define a protocol for in vitro selection, provide a molecular and morphological characterization of the progenies of regenerated plants, and evaluate them under water stress conditions. In the embryogenic callus induction medium (IM), the highest values of the variables fresh weight of embryogenic calli, proportion of embryogenic calli and number of regenerated seedlings (NRS) were obtained in the 25 mM mannitol treatment. The remaining concentrations of the osmotic agent (50, 75, 100 and 150 mM) had a negative effect on these variables. In the regeneration medium (RM), NRS was reduced at all mannitol concentrations. When embryogenic calli were induced and seedlings were regenerated maintaining mannitol concentrations in IM and RM, the highest NRS values were recorded at 25 mM mannitol. In vitro regenerated seedlings transplanted to an experimental plot exhibited different morphological characteristics from those of the anther donor plant. ISSR primers detected 22% of polymorphic bands and divergence between 0.20 and 0.37 in in vitro regenerated plants. Finally, water stress assays confirmed that S1 progenies exhibited a differential behavior from that of the parent material. Under 100 mM of mannitol used as selection pressure in IM or in both IM and RM, S1 progenies of two regenerated materials had higher height, fresh weight and dry weight at the end of water stress assay.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cenchrus ciliaris L. (syn. Pennisetum ciliare (L.) Link, buffelgrass) is a perennial forage grass used for cattle feeding in different arid and semiarid regions worldwide (Hanselka et al. 2004). In Argentina, the species is present mainly in the northwestern region, in environments with a marked dry season during a long part of the year. Accordingly, several of the forage grasses developing in those environments undergo water deficit periods (Pérez 2005); this stress situation becomes more marked to the west of the region, with the southwestern area, where precipitations fail to meet 70% of plant demands, being the driest area (Conti et al. 2009). Buffelgrass reproduces mainly by apomixis, through a mechanism known as apospory followed by pseudogamy (Jessup et al. 2003). The progeny of an apomictic plant is identical to the female parent; thus, individuals can be selected and propagated as clones (Hanna and Bashaw 1987). This type of reproduction can be used in a genetic breeding program because it allows the stabilization of superior genotypes (Bashaw and Hignight 1990); however, designing a breeding program in which conventional hybridization is not involved in embryo development is difficult.
Plant establishment through in vitro tissue culture has been often studied in buffelgrass. Somatic embryogenesis and organogenesis have been induced using different types of explants, such as immature inflorescences (Kumar et al. 2015), seeds (Bhat et al. 2001), apical buds (Kumar and Bhat 2012), mature embryos (Colomba et al. 2006) and anthers (Carloni et al. 2014). Regarding the embryogenic process, we observed somaclonal variation during the implementation of seedling regeneration protocols, both with mature embryos and anthers as explants (López Colomba et al. 2011; Carloni et al. 2014). Later, further studies demonstrated that somaclones exhibited a better behavior that the donor plant under salt stress (López Colomba et al. 2013).
Tissue culture offers the possibility of selecting individuals in vitro by adding different components acting as selective agents to the culture medium (Mohamed et al. 2000; Lu et al. 2007), either directly or gradually (Gangopadhyay et al. 1997; Hassan et al. 2004; Mohamed and Ibrahim 2012). These works are based on pre-existing genetic variation or variation induced and recovered during cell or tissue culture (Biswas et al. 2002; Matheka et al. 2008; Lu et al. 2009; Verma et al. 2013). The agent has been applied at different moments: during the callus induction process, during seedling regeneration or all throughout the in vitro culture stages (Biswas et al. 2002; Errabii et al. 2007; Aazami et al. 2010; Verma et al. 2013).
Drought stress conditions are usually simulated by adding compounds, such as mannitol, sorbitol or polyethylene glycol (PEG) (Leone et al. 1994; Joshi et al. 2011; Mahmood et al. 2012), which reduce the water potential of the medium. The responses of the different explants or in vitro regenerated plants can be influenced by secondary effects, either morphological or physiological, of the compounds used to simulate stress (Hohl and Schöpfer 1991; Verslues et al. 1998; Cha-um et al. 2012). For this reason, besides confirming the efficiency of in vitro selection, all selection processes should include an ex vitro assay to determine the exact measure of tolerance to the osmotic agent observed in the laboratory (Remotti 1998).
Tolerance to the selective agent can be measured by evaluating morphological characters, since plants growing under water stress exhibit responses that are typical of that situation (Moore et al. 2004; Verslues et al. 2006; Claeys et al. 2014). In buffelgrass, growth-related variables such as height, fresh weight and dry weight reveal the level of tolerance at the seedling stage under water, temperature and salt stress (Tommasino et al. 2012; Tommasino 2013). These assays allow early identification of individuals showing desirable agronomic traits and different from apomictic genetic materials (López Colomba et al. 2013). Accordingly, the aims of this work were to establish a protocol for in vitro selection, provide a morphological and molecular characterization of the progenies of regenerated plants, and evaluate them under water stress conditions.
Materials and methods
Plant material
Anther donor plants were obtained from the apomictic genotype register number (RN) 51, belonging to an active collection of buffelgrass apomictic genotypes located at the Instituto de Fisiología y Recursos Genéticos Vegetales (IFRGV), Córdoba, Argentina. Plants were cultivated in the experimental field of IFRGV (31°24′S; 61°11′W) from mid December 2011 to early March 2012.
In vitro culture
A protocol for whole plant regeneration via somatic embryogenesis was used as described Carloni et al. (2014). Briefly, anthers were cultivated in culture medium and after 45 days, calli were subcultured and maintained in embryogenic callus induction medium (IM) for 90 days. At the end of this period, the variables fresh weight of embryogenic calli (FWEC) and proportion of embryogenic calli (PEC) were measured. Then embryogenic calli were individually transferred to tubes containing regeneration medium (RM). Calli were subcultured every 60 days and maintained on the same medium for 10 months; at the end of this period, the number of regenerated seedlings (NRS) was counted.
In vitro selection
In vitro selection was made by adding mannitol to the culture medium (Sigma-Aldrich, St. Louis, MO) at different stages of somatic embryogenesis (induction, regeneration, and induction + regeneration). A lethal dose 50 (LD50), i.e., the dose at which 50% of the material survives (López Colomba 2011), was determined.
Determination of LD50
To determine LD50, different concentrations of mannitol 25, 50, 75, 100, and 150 mM, corresponding to water potentials of −0.62, −0.72, −0.85, −0.93 and −1.22 MPa, respectively, were added to the culture medium. The control group consisted of anthers cultivated without the addition of mannitol (−0.45 MPa). At the embryogenic calli induction stage (IS), 30 anthers (10 flowers) were placed in each Petri dish, with seven repetitions per treatment. The three anthers from each central flower (fertile anthecium) were grouped and considered an explant (10 explants). After 90 days, the variables FWEC and PEC were evaluated. The resulting embryogenic calli were individually transferred to tubes containing regeneration medium (RM) without mannitol. During the regeneration stage (10 months), the NRS was counted.
At the seedling regeneration stage (RS), mannitol was added after 90 days of the start of the induction period in RM. Twenty embryogenic calli were used per treatment and the NRS was counted.
LD50 at embryogenic calli IS and RS was determined using the protocol previously described, but maintaining the mannitol concentrations in both media (IM and RM). Thirty anthers per Petri dish were used, with five repetitions per treatment. The NRS was counted at the end of the regeneration stage.
Field selection and morphological and molecular characterization of buffelgrass somaclones
All the regenerated seedlings (R1) were placed in rooting medium and were hardened as described in Carloni et al. (2014). To identify the phenotypic changes, RN 51 was used as donor plant and all the in vitro regenerated plants were transferred to an experimental plot in a completely randomized design (Fig. 1). Plant materials were transplanted at 1-m distance between them and 12 morphological characters of leaf, stem and panicle were measured, as described in Quiroga et al. (2013), during two crop seasons. Seeds (S1) of the selected plant materials (R1) were collected.
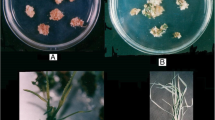
Protocol for in vitro selection during somatic embryogenesis for mannitol tolerance. a Anthers used as explant at the start of in vitro culture in induction medium (IM). b Embryogenic calli at the induction stage, 7 and 60 days (c) after the start of anther culture. d Induced embryogenic calli at the regeration stage. e Greenhouse plants after hardening period. f Experimental plots of in vitro regenerated buffelgrass plants (R1). g Water stress trial in buffelgrass progenies (S1), right pot: control (80% of soil water content, SWC) and left pot: drought treatment (30% of SWC). h Individual seedlings at 21 days after the end of the assay, left seedling: control; right seedling: drought treatment (80 and 30% of SWC, respectively)
Plant selection was made following different criteria. First, plants that were regenerated at the LD50 defined for each of the stages included in the process were selected. Then the R1 plants showing morphological characteristics different from those of RN 51 were identified. Finally, those plants that did not produce a sufficient number of seeds or did not resprout after winter were removed from the selection process.
In addition, R1 genetic materials selected in the field were analyzed using inter simple sequence repeat (ISSR) markers (Gupta et al. 1994; Zietkiewicz et al. 1994). For this, plant samples were processed and screened for polymorphisms using the ISSR primers UBC840A, ISSR8, UBC827 and UBC834 (Online Resource 1: Table 1), following the method described by Carloni (2016).
Evaluation of the response to water stress conditions of progenies (S1) of in vitro regenerated plants (R1)
Water stress assays were performed using the seeds (S1) collected from selected materials (R1), following the protocol described by Tommasino (2013). Briefly, approximately 250 seeds were sown in 17.6 L pots containing 2.76 kg sand and soil substrate (1:1) previously dried in heater at 105 °C for 48 h, and maintained in the greenhouse. The pots were then watered to saturation; once water was completely drained (4 h later) soil water content was determined using the gravimetric method. The obtained value was considered 100% moisture, corresponding to the maximum amount of water that can be retained by this substrate. Then, pots were watered daily to maintain 80% of soil water content (SWC). Between 30 and 45 days after sowing, the pots were transferred to a breeding chamber, maintaining the following conditions: temperature (28 ± 2 °C), photoperiod (16 h light and 8 h dark), humidity (40%) and light (250 μmol m−2 s−1).
Pots at 80% of SWC were considered control, whereas pots taken to 30% of SWC by interrupting irrigation were considered treatments (drought). This 30% SWC value was used because buffelgrass genotypes were found to show water stress symptoms at that SWC (Fig. 1) (Tommasino 2013). Two repetitions per plant material were performed for each experiment. At 21 days of reaching 30% SWC, 10 plants per pot were randomly selected and the following variables were evaluated: height, fresh weight (FW) and dry weight (DW) of aerial part.
Statistical analysis
The variables FWEC and PEC were determined at 90 days of the start of in vitro anther culture. PEC was calculated as the number of explants sown per Petri dish (10 explants). NRS was counted all throughout the regeneration period (10 months) and the value is expressed per Petri dish, except for the in vitro selection stage in RM, in which the mean NRS is reported (20 embryogenic calli per treatment).
To evaluate the efficiency of induction relative to FWEC, general linear and mixed models were fitted considering treatments as classification variables. To evaluate the efficiency of induction and regeneration relative to PEC and NRS, respectively, two generalized mixed linear models (GMLMs) were fitted, considering treatments as classification variables. The variable PEC was calculated via a binomial distribution with logit link function, whereas a Poisson distribution with log link function was used to calculate NRS. In both cases, differences between means for the treatments were evaluated using the Di Rienzo, Guzmán and Casanoves test (DGC) (Di Rienzo et al. 2002).
To confirm phenotypic variability in R1 in vitro regenerated materials with respect to the donor plant RN 51, morphological variables were compared using t tests for independent samples. Moreover, a cluster analysis was performed using the unweighted pair group method with arithmetic mean (UPGMA) by applying Euclidean distance as proximity measure.
To code the molecular profiles obtained with the ISSR primers, a binary data matrix was performed for each primer and for each individual, with 1 and 0 indicating presence and absence of bands, respectively. The molecular profile of the donor plant RN 51 was used to define a pattern. Genetic associations among individuals were evaluated using the sqrt(1-S) transformation of Jaccard similarity index. The distance matrix was subjected to the UPGMA hierarchical clustering method.
Finally, to detect differences in growth responses in the water stress assay, the following biomass variables were evaluated using t tests: plant height, FW and DW of the progenies (S1) of the selected genetic materials (R1) with respect to RN 51.
Statistical analyses were performed using InfoStat statistical software (Di Rienzo et al. 2014), except for the binary matrix analyses, which were performed using Info-Gen software (Balzarini and Di Rienzo 2013).
Results
In vitro selection
Buffelgrass genotype RN 51 was able to induce embryogenic calli and regenerate seedlings (Fig. 1), as described previously (Carloni et al. 2014). However, the addition of osmotic agent to the medium produced different responses in the variables fresh weight of embryogenic calli (FWEC), proportion of embryogenic calli (PEC) and number of regenerated plants (NRS). At the induction stage of embryogenic calli (IS), the analysis included natural log transformation of the observed FWEC values, due to the best fit of the model. The highest values for this variable were obtained at a mannitol concentration of 25 mM (Fig. 2a), with no differences from control (0 mM), but with differences from the remaining mannitol concentrations (P < 0.001). Regarding PEC (Fig. 2b), interestingly, the lowest mannitol concentration (25 mM) at IS stimulated the induction response of embryogenic calli compared to control (P < 0.001). For the variable NRS, the highest number of seedlings was obtained at 25 mM, with values differing statistically only from values of the treatment containing 150 mM mannitol (P < 0.001) (Fig. 2c).
Response of growth parameters of in vitro cultured buffelgrass anthers in an embryogenic calli induction medium (IM) at different mannitol concentrations. Embryogenic callus induction medium supplemented with 25, 50, 75, 100 and 150 mM mannitol or not supplemented with mannitol (0 mM). a Fresh weight of embryogenic calli (FWEC). b Proportion of embryogenic calli (PEC). c Number of regenerated seedlings (NRS). For the variable NRS, embryogenic calli were regenerated in regeneration medium without mannitol. Mean values represent three dates of tiller collection. Different letters within a column indicate significant differences (P ≤ 0.05) according to the Di Rienzo, Guzmán and Casanoves (DGC) means comparison test. Cut-off line on the “y” axis indicates the LD50
At the seedling regeneration stage (RS) no response was observed at concentrations above 100 mM mannitol. A similar result was obtained when mannitol was maintained in both media (IM and RM). For this reason, only values obtained from plants regenerated at concentrations ranging between 25 and 100 mM of mannitol and the control (0 mM mannitol) were considered. Figure 3a shows that when embryogenic calli were induced in the IM without mannitol, all the concentrations of the osmotic agent in RM caused a decrease in NRS (P < 0.001).
Number of regenerated seedlings (NRS) obtained from in vitro culture of buffelgrass anthers. a Embryogenic calli were induced in mannitol-free induction medium (IM) and then placed in seedling regeneration medium (RM) containing 0, 25, 50, 75 and 100 mM of mannitol. b Embryogenic calli were induced in IM and seedlings were regenerated in RM, both media containing 0, 25, 50, 75 and 100 mM of mannitol. Different letters within a column indicate significant differences (P ≤ 0.05) according to the Di Rienzo, Guzmán and Casanoves (DGC) means comparison test. Cut-off line on the “y” axis indicates the LD50
When the embryogenic calli were induced and seedlings were regenerated maintaining the mannitol concentrations (IM and RM) (Fig. 3b), the GMLMs indicated that maximum NRS values were obtained at a 25 mM (P < 0.001). This value differed significantly from values obtained with the remaining treatments, and even from values of the control medium.
Determination of LD50
The results of the embryogenic callus induction stage indicate that the highest values of the three variables were obtained at 25 mM of mannitol (Fig. 2). The values that were closer to 50% of the total obtained (LD50) were obtained in treatment at 100 mM of mannitol. The FWEC value obtained was 0.0890 ± 0.0137 g, representing 57% of the maximum value obtained. The value obtained for PEC, 0.34% ± 0.04 represents a reduction of 65%. The NPR values that were closer to the LD50 were obtained at 100 mM of manitol (58% of the total). While these values are not statistically different from treatment with 25 mM, the next concentration in the series (150 mM) regenerated only one seedling, which did not produce seeds. According to these results, seedlings (R1) regenerated from IM with 100 mM of mannitol were considered suitable for selection using embryogenic callus induction.
According to the observed NRS responses, when the embryogenic calli are in an IM without mannitol (Fig. 3a), approximately 1.25 seedlings should be regenerated at the LD50 in the RM. The treatment that was closest to that value was a concentration of 25 mM mannitol. However, when mannitol concentrations were maintained in both media (IM and RM), the treatment with 25 mM stimulated a higher NRS (Fig. 3b). During the selection stage in IM and RM, all the treatments differed from 25 mM mannitol treatment; however, no value represents the percentage required to reach the LD50, since all of them increased selection pressure. Accordingly, considering the NRS and the aim of this work, we decided to increase selection pressure at both stages of in vitro culture (regeneration and induction + regeneration with mannitol, respectively). For this, we selected R1 plants that regenerated in RM at 75 and 100 mM of mannitol. When the osmotic agent concentration was maintained in both (IM and RM), we selected R1 seedlings that regenerated in the 100 mM mannitol treatment.
Field selection and morphological and molecular characterization of buffel grass somaclones
As indicated at the IS, we used with R1 plants that regenerated in an IM with 100 mM mannitol. We identified a total of 18 plants, which allowed us to perform a divergence tree using 12 morphological characters and to make a first selection of five materials (Online Resource 2: Fig. 1). At the RS we used 7 plants, of which we selected 4 from the treatments with 75 and 100 mM of mannitol (3 and 1, respectively) (Online Resource 2: Fig. 2). At the selection stage using mannitol in both IM and RM, we characterized morphologically four plants regenerated in vitro in media supplemented with 100 mM of manitol, and selected three of them (Online Resource 2: Fig. 3).
Since the phenomenon of somaclonal variation is random (Larkin and Scowcroft 1981), it is possible to find somaclonal variants regardless of the culture medium used. Therefore, with the aim of interpreting the results obtained after fine-tuning each stage of the selection process, we included genetic material that were regenerated in vitro without using mannitol. We characterized morphologically a total of 20 plants (Online Resource 2: Fig. 4), of which we selected two. We also included two somaclones (R1−t4 and R1−t32), which showed morphological differences as well as genetic variations with respect to RN 51 donor plant (Carloni et al. 2014).
The external morphological characters of leaves, stems and panicles evaluated in the different materials selected and used for the water stress assays are described in Table 1. The t test for independent samples applied to the in vitro regenerated plants (R1) revealed significant differences (P < 0.05) for most of the morphological characters. Variability was observed in 9 of the 12 morphological characters with respect to RN 51; the three variables that did not show variability were flag leaf length (FLL), number of branches (NBR) and number of flowers/branch (FL/BR).
All the ISSR molecular markers were polymorphic (Online Resource 1: Table 1) and generated both types of data expected (absence and presence of allele) in the DNA samples extracted from in vitro regenerated buffelgrass plants. The amplification profiles generated with ISSSRs UBC840A and ISSR8 are displayed as an example in Fig. 4. The results show 22.6% of the polymorphic bands (Online Resource 1: Table 1) and the distance coefficients vary from 0.20 to 0.37 between in vitro regenerated and RN 51 (Online Resource 1: Table 2). The dendrogram generated by UPGMA is shown in Fig. 5. The cophenetic correlation coefficient yielded by this analysis was 0.92.
Detection of genetic variability using ISSR molecular markers in in vitro regenerated buffelgrass plants. Agarose gel electrophoresis (2%) of PCR products obtained using the primer UBC840A (a, b) or ISSR8 (c, d). Seedlings regenerated in vitro (R1) in induction medium (IM) with 100 mM (R1−17.2.1.1, R1−4.3.1.1.2, R1−4.3.3.1, R1−6.6.1 and R1−6.6.4) of mannitol, in IM and regeneration media (RM) with 100 mM (R1−11.2.2, R1−4.6.1 and R1−6.1.1) of mannitol, in RM with 75 (R1−61.1, R1−70.4, R1−75.1) or 100 mM (R1–92.1) of mannitol or mannitol-free culture media (R1−17.3, R1−10.1.2, R1−t32 and R1−t4). RN 51: DNA of donor plant used as pattern. Mix: Negative control (a reaction containing all PCR components except the DNA template of the studied plant). MM: molecular weight marker (100 pb Plus DNA Ladder, Thermo Scientific)
Dendrogram obtained with the average linkage UPGMA method through the analysis of four ISSR molecular markers, by applying the sqrt(1-S) transformation of the Jaccard similarity index as a proximity measure. R1: Seedlings regenerated in vitro in induction medium (IM) with 100 mM (R1−17.2.1.1, R1−4.3.1.1.2, R1−4.3.3.1, R1−6.6.1 and R1−6.6.4) of mannitol, in regeneration medium (RM) with 75 (R1−61.1, R1−70.4, R1−75.1) or 100 mM (R1−92.1) of mannitol, in IM and RM with 100 mM (R1−11.2.2, R1−4.6.1 and R1−6.1.1) of mannitol or mannitol-free culture media (R1−17.3, R1−10.1.2, R1−t32 and R1−t4). RN 51: DNA of donor plant used as pattern
Evaluation of tolerance to water stress in the progeny (S1) of in vitro regenerated plants (R1)
The behavior of S1 progenies of the material selected during the embryogenic callus induction stage (IS) under water stress is shown in Table 2. The t test for independent samples showed significant differences (P < 0.05) in some biomass characters of these progenies with respect to the donor plant RN 51. In the control treatment, with pots maintained at 80% of SWC, one of the selected plants (S1−6.6.4) exhibited lower height than the donor plant. In the water stress treatment (30% SWC), three of the evaluated materials exhibited variability with respect to RN 51. Two of them showed a decrease in all the variables analyzed (S1−17.2.1.1 and S1−6.6.4), and material S1−4.3.1.1.2 presented a better behavior in height, fresh weight and dry weight than the donor plant.
The analysis of S1 progenies of materials selected during the callus regeneration stage (RS) showed significant differences (P < 0.05) in most of the biomass characters analyzed at the end of the assay (Table 3). However, none of the evaluated materials showed a better behavior than that of RN 51.
The S1 progenies of the materials selected in the callus induction and regeneration stages (IS and RS) also showed significant differences in biomass characters (P < 0.05) from those of RN 51. In the control treatment, the three plants exhibited lower height, two of them lower fresh weight (S1−4.6.1 and S1−6.1.1) one of them lower dry weight (S1−4.6.1) (Table 4). In the water stress treatment, two of the materials (S1−11.2.2 and S1−4.6.1) did not show any differences in any of the variables with respect to RN 51. However, the three evaluated materials had higher dry weight and one of them (S1−6.1.1) differed statistically from RN 51.
The S1 progenies obtained from in vitro regenerated materials that were not subjected to mannitol treatment as selection agent also showed significant differences from RN 51 (P < 0.05) (Table 5). Only three variables of three different plants did not show differences from the donor plant, and none of the plants showed an improved behavior.
Discussion
Our results show that during the embryogenic callus induction stage, the water potential generated at a mannitol concentration of 25 mM (~−0.62 MPa) did not affect FWEC. However, as observed in other species (Hassan et al. 2004; Matheka et al. 2008; Verma et al. 2013), a decrease in water potential and FWEC occurs with increasing osmotic agent concentrations. A reduction in FWEC may be attributed to a delayed growth as a result of the loss of turgor observed in cells that develop under stress conditions (Errabii et al. 2007; Joshi et al. 2011). In addition, such reduction depends on the plant material and the species (Biswas et al. 2002; Ahmad et al. 2007; Aazami et al. 2010). Our results, based on FWEC, suggest that the negative effect of water stress on callus growth starts at water potentials ~−0.72 MPa (50 mM of mannitol).
Accordingly, mannitol provides an additional source of carbon to the IM (MS + 3% sucrose + 6 mg/l 2-4D + mannitol). If mannitol were metabolized, an increase in explant fresh weight should be expected (Gulati and Jaiwal 1993; Lipavská and Vreugdenhil 1996; Mohamed and Ibrahim 2012). This behavior was not recorded in our work. Similar results were mentioned for other species, in which mannitol did not produce favorable responses compared to other sugar forms available in the medium (Hilae and Te-chato 2005; Yaseen et al. 2009). Yaseen et al. (2013) suggest that the capacity of cells or tissues to grow in a mannitol-supplemented medium is related to the species’ capacity to form and assimilate this polyol. The lack of use of mannitol as a carbon source may be due to the inability of buffelgrass to metabolize that sugar. Therefore, complementary studies would be useful to confirm this hypothesis (Vítová et al. 2002; Rejšková et al. 2007).
The response of PEC is closely related to the reduction of water potential of the medium and the generated stress. First, treatment with 25 mM of mannitol produced a higher number of embryogenic calli. Similar effects were observed in other regeneration systems using mannitol as osmotic agent (Nadel et al. 1989; Biahoua and Bonneau 1999). Some authors suggested that the decrease in osmotic potential in the culture medium exposes the explant to a stress condition necessary for stimulating the embryogenic response (Kamada et al. 1993; Aoshima 2005). Therefore, the increased PEC observed at the lowest mannitol concentration may be due to the stimulus generated by water stress (Zavattieri et al. 2010). Indeed, the increase in PEC observed in some buffelgrass genotypes exposed to other stressors in tillers agrees with that hypothesis (Carloni et al. 2014).
Nevertheless, the embryogenic response to the different explants cultivated in vitro depends on the stress level, among other factors (Karami and Saidi 2010). In C. sinensis a threshold of osmotic potential with mannitol to stimulate embryogenic calli formation has been mentioned, since that effect does not occur at concentrations above the optimum ones (Aoshima 2005). Here, the mentioned observations agree with treatments above 25 mM of mannitol, since at 50, 75 and 100 mM there were no positive responses in PEC. In addition, the low or nil response of PEC observed at 150 mM or higher concentrations (200 and 300 mM, data not shown), can be related to inhibition in embryogenic response, as mentioned for crops that cannot tolerate stress conditions (Matheka et al. 2008; Mahmood et al. 2012).
Interestingly, the response of NRS differed with the presence or absence of mannitol in the induction medium. When the osmotic agent was not present during the regeneration stage, the stimulus observed in PEC with 25 mM of mannitol in IM did not translate into a higher NRS. A positive effect on NRS was observed only when mannitol concentration (25 mM) was maintained in both culture media (IM and RM). In other species, treatment with PEG improves the capacity of somatic embryos to develop into plants (Svobodová et al. 1999). Osmotic stress conditions in the medium induced with PEG are necessary to increase storage protein deposition (Misra et al. 1993) and the level of transcripts involved in differentiation of somatic embryos (Stasolla et al. 2003). The increase in NRS observed when mannitol was maintained in both media may be related to the requirement of an osmotic potential for somatic embryo maturation at the regeneration stage (Svobodová et al. 1999).
On the contrary, when mannitol treatments were applied only during the RS, NRS was reduced in all treatments with respect to control. This behavior might be related to a lack of acclimation reported in cell or plant cultures maintained in a medium with some level of stress for some time (Leone et al. 1994; Gangopadhyay et al. 1997; Claeys et al. 2014). Accordingly, when mannitol was added only in the RM, NRS was reduced at all concentrations. By contrast, in those calli induced during 90 days under osmotic stress conditions (IM with mannitol), the lowest number of regenerated plants in RM with mannitol was observed in treatments supplemented with more than 25 mM of mannitol. Stress conditions imposed by low mannitol concentrations during IS may have generated equilibrium in cellular metabolism (Leone et al. 1994), which allowed somatic embryo to adapt to the negative effects of osmotic stress at the RS (Mohamed et al. 2000). By contrast, when mannitol is added only in the RM, an osmotic shock is likely to occur in cells (Gangopadhyay et al. 1997), affecting somatic embryos and their further regeneration (Matheka et al. 2008).
Regarding selection and evaluation of the in vitro regenerated material, it is well known that plants generated and recovered from cell or tissue culture exhibit changes with respect to the donor plant (Larkin and Scowcroft 1981). This phenomenon was previously demonstrated using the same in vitro regeneration system in buffelgrass (Carloni et al. 2014). The present results also show changes in morphology of leaves, stems or panicle of the in vitro regenerated plants. In addition, the use of ISSR markers allowed us to detect different molecular profiles in R1 plants, suggesting the existence of genetic variability (Gupta et al. 1994; Zietkiewicz et al. 1994). Finally, the water stress assays confirmed that the S1 progeny of these selected materials (R1) exhibited a differential behavior with respect to that of the anther donor genotype, both in control and water stress conditions. Our present results, as well as those previously performed by our work group (Lopez Colomba et al. 2011, 2013), confirm that somaclonal variation is a phenomenon that can be applied to obtain genetic variability in apomictic buffelgrass genotypes.
Descriptors such as height, fresh weight and dry weight are the most important morphological characters to evaluate and characterize the different buffelgrass genotypes (Griffa et al. 2010). In addition, dry weight is the most agronomically important character and the main yield component in forage pastures (Moore et al. 2004). The materials regenerated in culture media without mannitol and evaluated in the water stress assay did not show improvement in behavior for any of the biomass variables mentioned. By contrast, the S1 progenies from the materials R1−4.3.1.1.2 and R1−6.1.1 selected from media with mannitol had a higher dry weight in the water stress treatment. The better behavior of these materials than that of the donor plant suggests tolerance in their response to growth under stress conditions (Munns 2002; Verslues et al. 2006). Therefore, these results indicate that in vitro selection with mannitol allows regeneration of materials with improved response to water stress, as observed in other species (Mohamed et al. 2000).
Regarding the most suitable stage for performing the selection process, S1−4.3.1.1.2 was in contact with the osmotic agent during the IS and S1−6.1.1 in both IS and RS. None of the materials selected during RS showed improved behavior, suggesting that selection pressure with mannitol should be performed at least during induction of embryogenic calli. Although any source or morphogenetic process is susceptible to somaclonal variation (Bairu et al. 2011), our results may be attributed to processes occurring in the explant. Accordingly, when anthers are placed in the IM, dedifferentiation and disorganized proliferation of cells that grow to form a callus is observed (Carloni et al. 2014). These are key processes in somaclonal variation, since they can induce cell alterations, generating instability (Karp 1995), accordingly, it has been suggested that some of the genetic changes observed in in vitro regenerated plants are originated in the callus phase (Phillips et al. 1994).
Abbreviations
- IM:
-
Induction medium
- RM:
-
Regeneration medium
- FWEC:
-
Fresh weight of embryogenic calli
- PEC:
-
Proportion of embryogenic calli
- NRS:
-
Number of regenerated seedlings
- IS:
-
Induction stage
- RS:
-
Regeneration stage
- R1 :
-
Regenerated seedlings
- S1 :
-
Progenies of in vitro regenerated plants
- ISSR:
-
Inter simple sequence repeat
References
Aazami MA, Torabi M, Jalili E (2010) In vitro response of promising tomato genotypes for tolerance to osmotic stress. Afr J Biotechnol 9:4014–4017.
Ahmad MSA, Javed F, Ashraf M (2007) Iso-osmotic effect of NaCl and PEG on growth, cations and free proline accumulation in callus tissue of two indica rice (Oryza sativa L.) genotypes. Plant Growth Regul 53:53–63
Aoshima Y (2005) Efficient embryogenesis in the callus of tea (Camellia sinensis) enhanced by the osmotic stress or antibiotics treatment. Plant Biotechnol 22:277–280
Bairu MW, Aremu AO, Van Staden J (2011) Somaclonal variation in plants: causes and detection methods. Plant Growth Regul 63:147–173
Balzarini MG, Di Rienzo JA (2013) InfoGen versión 2013. FCA, Universidad Nacional de Córdoba, Argentina. http://www.info-gen.com.ar
Bashaw EC, Hignight KW (1990) Gene transfer in apomictic buffelgrass through fertilization of an unreduced egg. Crop Sci 30:571–575
Bhat V, Dalton SJ, Kumar S, Bhat BV, Gupta MG, Morris P (2001) Particle-inflow-gun-mediated genetic transformation of buffelgrass (Cenchrus ciliaris L.): optimizing biological and physical parameters. J Appl Genet 42:405–412
Biahoua A, Bonneau L (1999) Control of in vitro somatic embryogenesis of the spindle tree (Euonymus europaeus L.) by the sugar type and the osmotic potential of the culture medium. Plant Cell Rep 19:185–190
Biswas J, Chowdhury B, Bhattacharya A, Mandal AB (2002) In vitro screening for increased drought tolerance in rice. In Vitro Cell Dev Plant 38:525–530
Carloni E (2016) Cultivo in vitro de anteras como estrategia para el mejoramiento genético de buffelgrass (Cenchrus ciliaris L). Tesis Doctorado. FCA – Universidad Nacional de Córdoba. https://rdu.unc.edu.ar/bitstream/handle/11086/2729/Carloni.%20Cultivo%20in%20vitro%20de%20anteras%20como%20estrategia%20para%20el%20mejoramiento%20gen%C3%A9tico%20de%20buffelgrass...%20.pdf?sequence=1
Carloni E, Ribotta A, Colomba EL, Griffa S, Quiroga M, Tommasino E, Grunberg K (2014) Somatic embryogenesis from in vitro anther culture of apomictic buffel grass genotypes and analysis of regenerated plants using flow cytometry. Plant Cell Tissue Organ Cult 117:311–322
Cha-um S, Takabe T, Kirdmanee C (2012) Physio-biochemical responses of oil palm (Elaeis guineensis Jacq.) seedlings to mannitol and polyethylene glycol-induced iso-osmotic stresses. Plant Prod Sci 15:65–72
Claeys H, Van Landeghem S, Dubois M, Maleux K, Inzé D (2014) What is stress? Dose-response effects in commonly used in vitro stress assays. Plant Physiol 165:519–527
Colomba EL, Grunberg K, Griffa S, Ribotta A, Mroginski L, Biderbost E (2006) The effect of genotype and culture medium on somatic embryogenesis and plant regeneration from mature embryos of fourteen apomictic cultivars of buffelgrass (Cenchrus ciliaris L.). Grass Forage Sci 61:2–8
Conti H, Cazenave G, Giagnoni R (2009) Características climáticas de las provincias de Santiago del Estero, Chaco y Formosa. Flora Chaqueña, Asteraceae. Colecc Ci Inst Tecnol Agropecu 23:9–26
Di Rienzo JA, Guzmán AW, Casanoves F (2002) A multiple comparisons method based on the distribution of the root node distance of a binary tree. J Agric Biol Environ Stat 7:1–14
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2014) Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina
Errabii T, Gandonou CB, Essalmani H, Abrini J, Idaomar M, Senhaji NS (2007) Effects of NaCl and mannitol induced stress on sugarcane (Saccharum sp.) callus cultures. Acta Physiol Plant 29:95–102
Gangopadhyay G, Basu S, Gupta S (1997) In vitro selection and physiological characterization of NaCl-and mannitol-adapted callus lines in Brassica juncea. Plant Cell Tissue Organ Cult 50:161–169
Griffa S, Ribotta A, López Colomba E, Tommasino E, Carloni E, Luna C, Grunberg K (2010) Evaluation seedling biomass and its components as selection criteria for improving salt tolerance in Buffel grass genotypes. Grass Forage Sci 65:358–361
Gulati A, Jaiwal PK (1993) Selection and characterization of mannitol-tolerant callus lines of Vigna radiata (L.) Wilczek. Plant Cell Tissue Organ Cult 34:35–41
Gupta M, Chyi YS, Romero-Severson J, Owen JL (1994) Amplification of DNA markers from evolutionarily diverse genomes using single primers of simple-sequence repeats. Theor Appl Genet 89:998–1006
Hanna WW, Bashaw EC (1987) Apomixis: its identification and use in plant breeding. Crop Sci 27:1136–1139
Hanselka CW, Hussey MA, Ibarra F (2004) Buffelgrass. In: Moser LE, Burson BL, Sollenberger LE (eds) Warm-season (C4) grasses. American Society of Agronomy, Madison, pp 477–502
Hassan NS, Shaaban LD, Hashem ESA, Seleem EE (2004) In vitro selection for water stress tolerant callus line of Helianthus annus L. Cv. Myak. Int J Agric Biol 6:1–13
Hilae A, Te-chato S (2005) Effect of carbon sources and strength of MS medium on germination of somatic embryos of oil palm (Elaeis guineensis Jacq.). Songklanakarin J Sci Technol 27:629–635.
Hohl M, Schopfer P (1991) Water relations of growing Maize coleoptiles comparison between mannitol and polyethylene glycol 6000 as external osmotica for adjusting turgor pressure. Plant Physiol 95:716–722
Jessup RW, Burson BL, Burow O, Wang YW, Chang C, Li Z, Paterson AH, Hussey MA (2003) Segmental allotetraploidy and allelic interactions in buffelgrass (Pennisetum ciliare (L.) Link syn. Cenchrus ciliaris L.) as revealed by genome mapping. Genome 46:304–313
Joshi R, Shukla A, Sairam RK (2011) In vitro screening of rice genotypes for drought tolerance using polyethylene glycol. Acta Physiol Plant 33:2209–2217
Kamada H, Ishikawa K, Saga H, Harada H (1993) Induction of somatic embryogenesis in carrot by osmotic stress. Plant Tissue Cult Lett 10:38–44
Karami O, Saidi A (2010) The molecular basis for stress-induced acquisition of somatic embryogenesis. Mol Biol Rep 37:2493–2507
Karp A (1995) Somaclonal variation as a tool for crop improvement. Euphytica 85:295–302
Kumar S, Bhat V (2012) High-frequency direct plant regeneration via multiple shoot induction in the apomictic forage grass Cenchrus ciliaris L. In Vitro Cell Dev Plant 48:241–248
Kumar S, Sahu N, Singh A (2015) High-frequency in vitro plant regeneration via callus induction in a rare sexual plant of Cenchrus ciliaris L. In Vitro Cell Dev Plant 51:28–34
Larkin PJ, Scowcroft SC (1981) Somaclonal variation: a novel source of variability from cell culture for plant improvement. Theor Appl Genet 60:197–214
Leone A, Costa A, Tucci M, Grillo S (1994) Adaptation versus shock response to polyethylene glycol-induced low water potential in cultured potato cells. Physiol Plant 92:21–30
Lipavská H, Vreugdenhil D (1996) Uptake of mannitol from the media by in vitro grown plants. Plant Cell Tissue Organ Cult 45:103–107
López Colomba E (2011) Inducción de variabilidad genética para tolerancia a estreses abióticos mediante técnicas de cultivo in vitro en Cenchrus ciliaris L. Tesis Maestría. Universidad Internacional de Andalucía. http://dspace.unia.es/bitstream/handle/10334/1681/0229_Lopez.pdf?sequence=1
López Colomba E, Prina A, Griffa S, Ribotta AN, Carloni E, Tommasino E, Luna C, Biderbost E, Grunberg K (2011). Obtaining new germplasm in Cenchrus ciliaris L. through induced-mutation and in vitro selection. Phyton Int J Exp Bot 80:59–64
López Colomba E, Tommasino E, Luna C, Griffa S, Carloni E, Ribotta A, Quiroga M, Grunberg K (2013) Differential salt-stress response during germination and vegetative growth in in vitro selected somaclonal mutants of Cenchrus ciliaris L. S Afr J Bot 87:157–163
Lu S, Peng X, Guo Z, Zhang G, Wang Z, Wang C, Pang C, Fan Z, Wang J (2007) In vitro selection of salinity tolerant variants from triploid bermudagrass (Cynodon transvaalensis × C. dactylon) and their physiological responses to salt and drought stress. Plant Cell Rep 26:1413–1420
Lu S, Chen C, Wang Z, Guo Z, Li H (2009) Physiological responses of somaclonal variants of triploid bermudagrass (Cynodon transvaalensis × Cynodon dactylon) to drought stress. Plant Cell Rep 28:517–526
Mahmood I, Razzaq A, Hafiz IA, Kaleem S, Khan AA, Qayyum A, Ahmad M (2012) Interaction of callus selection media and stress duration for in vitro selection of drought tolerant callus of wheat. Afr J Biotechnol 11:4000–4006
Matheka JM, Magiri E, Rasha AO, Machuka J (2008) In vitro selection and characterization of drought tolerant somaclones of tropical maize (Zea mays L.). Biotechnology 7:641–650
Misra S, Attree SM, Leal I, Fowke LC (1993) Effect of abscisic acid, osmoticum, and dessication on synthesis of storage proteins during the development of white spruce somatic embryos. Ann Bot 71:11–22
Mohamed MAH, Ibrahim TA (2012) Enhanced in vitro production of Ruta graveolens L. coumarins and rutin by mannitol and ventilation. Plant Cell Tissue Organ Cult 111:335–343
Mohamed MH, Harris PJC, Henderson J (2000) In vitro selection and characterisation of a drought tolerant clone of Tagetes minuta. Plant Sci 159:213–222
Moore KJ, Boote KJ, Sanderson MA (2004) Physiology and developmental morphology. In: Moser LE, Burson BL, Sollenberger LE (eds) Warm-season (C4) grasses. American Society of Agronomy, Madison, pp 179–216
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Nadel BL, Altman A, Ziv M (1989) Regulation of somatic embryogenesis in celery cell suspensions. Plant Cell Tissue Organ Cult 18:181–189
Pérez H (2005) Características de las especies forrajeras adaptadas a las condiciones del Noroeste del país. Forrajes 33–41. http://www.produccionanimal.com.ar/produccion_y_manejo_pasturas/pasturas_cultivadas_megatermicas/68-caforra.pdf
Phillips RL, Kaeppler SM, Olhoft P (1994) Genetic instability of plant tissue cultures: breakdown of normal controls. Proc Natl Acad Sci USA 91:5222–5226
Quiroga M, Grunberg K, Ribotta A, López Colomba E, Carloni E, Tommasino E, Luna C, Griffa S (2013) Obtaining sexual genotypes for breeding in buffel grass. S Afr J Bot 88:118–123
Rejšková A, Patková L, Stodůlková E, Lipavská H (2007) The effect of abiotic stresses on carbohydrate status of olive shoots (Olea europaea L.) under in vitro conditions. J Plant Physiol 164:174–184
Remotti PC (1998) Somaclonal variation and in-vitro selection for crop improvement. In: Jain SM, Brar DS, Ahloowali BS (eds) Somaclonal variation and induced mutations in crop improvement. Kluwer Academic Publishers, Dordrecht, pp 169–201
Stasolla C, van Zyl L, Egertsdotter U, Craig D, Liu W, Sederoff RR (2003) The effects of polyethylene glycol on gene expression of developing white spruce somatic embryos. Plant Physiol 131:49–60
Svobodová H, Albrechtová J, Kumstýřová L, Lipavská H, Vágner M, Vondráková Z (1999) Somatic embryogenesis in Norway spruce: anatomical study of embryo development and influence of polyethylene glycol on maturation process. Plant Physiol Biochem 37:209–221
Tommasino E (2013) I Workshop sobre Estresses Abióticos em Plantas Forrageiras. Disertación: Fenotipificación de Genotipos de Cenchrus ciliaris para tolerancia al estrés hídrico. http://cloud.cnpgc.embrapa.br/weapf2013/files/2012/12/ENSAYOS-DE-ESTR%C3%89S-POR-SEQU%C3%8DA-FINAL.pdf
Tommasino E, Griffa S, Grunberg K, Ribotta A, López Colomba E, Carloni E, Quiroga M, Luna C (2012) Malondialdehyde content as a potential biochemical indicator of tolerant Cenchrus ciliaris L. genotypes under heat stress treatment. Grass Forage Sci 67:456–459
Verma D, Ansari MW, Agrawal GK, Rakwal R, Shukla A, Tuteja N (2013) In vitro selection and field responses of somaclonal variant plants of rice cv PS113 for drought tolerance. Plant Signal Behav 8:23519
Verslues PE, Ober ES, Sharp RE (1998) Root growth and oxygen relations at low water potentials. Impact of oxygen availability in polyethylene glycol solutions. Plant Physiol 116:1403–1412
Verslues PE, Agarwal M, Katiyar Agarwal S, Zhu J, Zhu JK (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 45:523–539
Vítová L, Stodůlková E, Bartoníčková A, Lipavská H (2002) Mannitol utilisation by celery (Apium graveolens) plants grown under different conditions in vitro. Plant Sci 163:907–916
Yaseen M, Ahmad T, Abbasi NA, Hafiz IA (2009) Assessment of apple rootstocks M 9 and M 26 for in vitro rooting potential using different carbon sources. Pak J Bot 41:769–781
Yaseen M, Ahmad T, Sablok G, Standardi A, Hafiz IA (2013) Review: role of carbon sources for in vitro plant growth and development. Mol Biol Rep 40:2837–2849
Zavattieri MA, Frederico AM, Lima M, Sabino R, Arnholdt-Schmitt B (2010) Induction of somatic embryogenesis as an example of stress-related plant reactions. Electron J Biotechnol 13:1–9
Zietkiewicz E, Rafalski A, Labuda D (1994) Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 20:176–183
Acknowledgements
This work was supported financially by The Instituto Nacional de Tecnología Agropecuaria (INTA), Argentina, Project PNPA-1126072 and CONICET PIP 112 201101 0031.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carloni, E., Tommasino, E., López Colomba, E. et al. In vitro selection and characterization of buffelgrass somaclones with different responses to water stress. Plant Cell Tiss Organ Cult 130, 265–277 (2017). https://doi.org/10.1007/s11240-017-1220-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1220-9