Abstract
The gram pod borer (Helicoverpa armigera Hubner) is the most serious insect pest of pigeonpea. It is highly susceptible to the insecticidal proteins of Bacillus thuringiensis (Bt). A codon-optimized chimeric Cry1Aabc gene of Bt driven by a constitutive promoter was introduced in pigeonpea (cv. Asha) to confer resistance against the insect. A total of eight transgenic plants could be established with transformation frequency of 0.06%. Two transgenic events were selected for advancement based on high insect mortality, single locus integration, protein expression and fertility status. Quantitative ELISA indicated high protein expression in different plant parts viz., leaves (pre and post flowering), flowers, pod walls and immature seeds. Analysis for the stable integration, expression and insect mortality (detached leaf and pod bioassay) led to identification of lines with high efficacy. These events were further advanced for the identification of a viable event by selfing to create homozygosity. The chimeric Cry1Aabc expressed in pigeonpea is effective against gram pod borer and can be utilized in transgenic variety development programme.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pigeonpea [Cajanus cajan (L.) Millsp.] or red gram is an important grain legume of the tropical and subtropical regions. It is an important source of dietary protein especially for the large vegetarian population in India. It is used in diverse ways as a source of food, feed and fertilizers. Globally, pigeonpea ranks fifth in production and area of grain legumes after soybean, common bean, peanut and chickpea. In India, it ranks second after chickpea in production and area. Pigeonpea is an annual, often cross pollinated (10–30%), diploid crop (2n = 2x = 22) with genome size of approx. 858 Mb. In India, pigeonpea is grown in an area of 4.6 m ha producing 3.0 m t (FAOSTAT 2013). Despite its significant nutritional value, its production and productivity are constrained by several biotic and abiotic stresses. Insect pests are the most important biotic constraint mainly pod borers (Helicoverpa armigera and Maruca vitrata) which attack pigeonpea during flower and pod development stages causing yield loss of up to 85%. Farmers rely on chemicals for control of these insect pests which are expensive and often have adverse effect on environment and human health. In the absence of management measures, pod borers can cause major crop losses. Conventional plant breeding has not been successful in generating resistant varieties, due to lack of resistance in cultivated varieties, and incompatibility with wild relatives (Nene and Sheila 1990).
Development of pod borer resistant varieties is apparently possible by biotechnological approaches like genetic engineering. For the production of transgenic plant, an in vitro regeneration system is a prerequisite. There are several successful reports of regeneration in pigeonpea using different varieties and explants (Das and Parida 2014; Gatti et al. 2016). The most common regeneration pathway reported is direct organogenesis of shoots from leaf explants (Eapen et al. 1998; Yadav and; Padmaja 2003; Dayal et al. 2003; Singh et al. 2002; Villiers et al. 2008), cotyledonary nodes (Sreenivasu et al. 1998; Singh et al. 2003) and shoot apices (Geetha et al. 1998; Naidu et al. 1995). Somatic embryogenesis in pigeonpea has also been reported (Anbazhagan and Ganapathi 1999; Aboshama 2011). Agrobacterium-mediated genetic transformation was demonstrated using explants such as shoot apex, cotyledonary node, embryonic axis, leaf, decapitated embryo, axillary meristem, axillary bud, plumule (Geetha et al. 1999; Lawrence and Koundal 2001; Prasad et al. 2004; Dayal et al. 2003; Verma and; Chand 2005; Sharma et al. 2006; Surekha et al. 2007; Raghavendra and Sudhakar 2014) and intact seedling (Rao et al. 2008). The success of Agrobacterium transformation is usually based on wound-induced response by the meristematic tissue of explants and media regimes, delivery and integration of T-DNA in the plant genome, precision in the selection of the transformed cells and characterization of the transgenic plants (Olhoft et al. 2004). Several other factors also influence the transformation efficiency such as genotype, age, explant, Agrobacterium strain, transformation vector, co-cultivation time and culture composition (Paz et al. 2004; Rohini and Rao 2000).
The crystal (cry) protein provides important source of resistance for large group of insects of Lepidoptera family. The cry proteins have three domains: a seven helix bundle (Domain I) involved in pore formation, a triple anti-parallel beta sheet domain (Domain II) for receptor binding and beta sheet sandwich (Domain III) for protease protection as well as receptor binding (Kumar et al. 1996). The synergistic activity between different cry proteins against insect pest is well demonstrated (Xue et al. 2014). Domain swapping and generation of fusion proteins to improve protection against insect pest is an emerging area of insect management (Saraswathy and Kumar 2004). In the present investigation, we report development of transgenic pigeonpea lines for improved plant protection against the gram pod borer (Helicoverpa armigera Hubner). New transgenic lines developed can be used for pyramiding with lines harbouring other insecticidal genes (like Vip) and use in integrated pest management programmes (Bhatnagar-Mathur and Sharma 2016).
Materials and methods
Plant Material and culture conditions
The breeder seeds of pigeonpea variety ICPL 87119 (Asha) were used for all the experiments in the present study. All media contained modified MS medium comprising of MS salts (Murashige and Skoog 1962), B5 Vitamins (Gamborg et al. 1968), 3% sucrose and 0.8% Agar (pH 5.8). The growth regulators, cytokinins [6-benzyl amino purine (BAP), 6-furfurylaminopurine (kinetin)], auxin [1-napthalene acetic acid (NAA)] and gibberellic acid (GA3) (Duchefa Biochemie, Haarlem, the Netherlands) were added to the medium after filter sterilization. The cytokinins (BAP and kinetin) and auxin (NAA) were used for dedifferentiation and shoot induction and GA3 for shoot elongation. The cultures were incubated at 25 ± 2 °C under a light regime of 16 h light (100 μE/s/m2) provided by cool white fluorescent lamps and 8 h dark.
Binary vector, Agrobacterium strain and genetic transformation
The binary vector, pBinAR (Hofgen and Wilmitzer 1990) harbouring a chimeric Bt δ-endotoxin gene, Cry1Aabc and nptII as the selectable marker gene was used for genetic transformation (Fig S1). The Bt-cry1Aabc δ-endotoxin gene was constructed by taking nucleotide sequences coding for different domains from lepidopteran-specific Bt-toxins viz., Domain I of Cry1Aa, Domain II of Cry1Ab and domain III of Cry1Ac. The codon usage of higher plants was followed while designing the synthetic gene (Murray et al. 1989). The mRNA destabilizing features that exist in the native gene sequences have been identified (Hebsgaard et al. 1996; Liu et al. 2005; Chang et al. 2013; Dassi et al. 2014) and deleted while designing the codon modified version. The binary vector (14,350 bp) was mobilized into hypervirulent Agrobacterium tumefaciens strain EHA105 (Hood et al. 1986) and used for genetic transformation studies.
Genetic transformation was attempted in a popular pigeonpea cultivar, Asha (ICPL 87119). Agrobacterium culture harbouring the binary vector was grown overnight to OD 0.6–0.8 at 600 nm and used to inoculate axillary meristem explants for 10 min and then co-cultivated for 72 h in co-cultivation media. During co-cultivation, the explants were exposed to diffused light and temperature 25 ± 2 °C. Transformed shoots were selected on 100 mg/L kanamycin monosulphate for four to five sub-cultures, each of 10–14 days duration. Healthy green shoots surviving selection were grafted onto non-transgenic root-stocks and established in the Transgenic Containment Facility.
Confirmation of Cry1Aabc gene in putative transgenic plants
Total genomic DNA was extracted from the young leaves of putative transgenic pigeonpea plants using DNeasy Plant Kit (Qiagen, Hilden, Germany). The presence of the cry1Aabc gene in transgenic plants were confirmed by PCR using the primer set A4 F-5′-ccttgtacagaagacccttcaatatc-3′(forward) and A4R-5′-tctattctgaatgttatttccactgc-3′(reverse). The amplification was carried out in 20 µl volume using 1× Taq buffer, 200 µM dNTP mix, 10 pM each primer, 200 ng DNA template and 1 u Taq DNA polymerase (Merck Genie, Bangalore). PCR reaction was performed using DNA thermal cycler (BioRad, USA) and the PCR programme was set at 94 °C for 5 min, followed by 35 cycles of 94 °C for 60 s, 60 °C for 60 s and 72 °C for 45 s, with a final extension at 72 °C for 8 min. PCR products were resolved on 1.5% EtBr stained agarose gel in 1× TAE buffer. Gel images were documented using Gel Documentation system (BioRad Gel Doc XR, USA).
Total genomic DNAs were isolated from the young leaves of transgenic and wild-type (WT) plants (Dellaporta et al. 1983). Genomic DNAs were digested with HindIII (which restricts the T-DNA at one site near the termination of Cry1Aabc gene, the minimum expected size of band using Cry1Aabc probe is approximately 2.62 Kb) and double digested with EcoRI and HindIII (which release the entire Cry1Aabc cassette) separately. The digestion products of each line were electrophoresed in a 1% agarose gel and then blotted onto Hybond N+ membrane. The DIG labelled probe, specific to Cry1Aabc gene was synthesized using DIG DNA labelling kit. Labelling, hybridization and detection were performed using DIG labelling Kit with the chemiluminiscent substrate CDP-Star, according to manufacturer’s instructions (Roche Diagnostics GmbH, Mannheim, Germany). Hybridization was carried out at 65 °C overnight. Stringent washes were performed using a primary wash buffer at 65 °C for 20 min and a secondary wash buffer at 25 °C for 10 min. The signal was detected on an X-ray film after an exposure time of 10 min.
For reverse transcriptase PCR (RT-PCR), total cellular RNA was extracted from the young leaves of putative transgenic pigeonpea lines according to kit instructions (Plant RNA Isolation Kit, Sigma Aldrich, USA). cDNA synthesis was done using 1.0 µg of total RNA (Verso cDNA synthesis Kit, Thermo Fischer, USA). PCR was performed with house-keeping gene (Actin 2/7) specific primer (Act 2/7F: aattcacgagaccacctacaac & Act 2/7R: tgagccaccactaagaacaatg) and gene specific primers (A4F/A4R) based on PCR amplification protocol described earlier. RT-PCR product was electrophoresed in 2% EtBr stained agarose gel and documented.
Detection of Cry1Aabc protein in transgenic pigeonpea plants
For the detection of Cry1Aabc protein in transgenic pigeonpea progenies of different generations, total leaf protein was isolated using 50 mM bicarbonate extraction buffer (15 mM Sodium carbonate, 35 mM Sodium bicarbonate, 0.1% Triton X-100, 0.05% Tween 20, 0.5% Sodium azide, 1% PVP and 1 mM PMSF) and added to the wells of poly l-Lysine coated ELISA plates (Greiner Bio One, Germany) and kept overnight at 4 °C along with positive control, negative control and blank. The following day, the bound antigen was blocked by 1% BSA (Sigma Aldrich, USA) for 90 min followed by washing (thrice) with PBST (Incubated at 37 °C). Primary antibody (produced in Rabbits against Cry1Ac protein) diluted 1:10,000 in 1× PBST and 0.25% BSA then added to each well followed by washing (thrice) with PBST for 90 min (Incubated at 37 °C). Secondary antibody (Anti-Rabbit IgG whole molecule- alkaline phosphatase, antibody produced in goat) was diluted 1:20,000 in 1× PBST and 0.25% BSA then added to each well followed by thrice washing with PBST for 90 min (Incubated at 37 °C). After 90 min of incubation in 37 °C the unbound secondary antibody was washed twice with PBST and once with PBS for removing the traces of Tween 20. Specific substrate pNPP (Sigma Aldrich, USA) was dissolved in 200 mM Tris pH 9.5, 0.5 mM MgCl2 and incubate the plate at 37 °C for 30 min. After 30 min of incubation of substrate, a yellow coloured reaction was obtained due to interaction between enzyme conjugated secondary antibody and substrate in positive samples and positive control. The absorbance was measured at 405 nm in ELISA reader (Spectramax, Molecular Devices, USA).
Pigeonpea progenies identified based on qualitative assay were further subjected to the quantitative ELISA (Envirologix, USA). Expression of the protein was quantified at different stages from different target tissues (leaves, flowers, pod walls and immature seeds). Tissue specific and stage specific expression of Cry1Aabc protein was also assessed using quantitative ELISA in both the selected pigeonpea events at T5 generation at pre-flowering: 92 days after sowing (DAS) and post-flowering (160 DAS). Only high expressing progenies of both events were selected for analysis of the tissue specific expression of protein. Total protein was extracted from young leaves, flowers, pod walls and immature seeds (188 DAS), and subjected to quantitative ELISA (Envirologix, USA).
For western blot hybridization, total crude protein was extracted from young leaves (300 DAS), using 50 mM bicarbonate buffer (15 mM Sodium carbonate, 35 mM Sodium bi carbonate, 0.1% Triton X-100, 0.05% Tween 20, 0.5% Sodium azide, 1% PVP, 1 mM PMSF, 1% PPIC). The quantification of total soluble protein was performed with Bradford reagent (Sigma Aldrich, USA) (Bradford 1976). 30 µg protein was fractionated on 15% acrylamide gel with 10% SDS and blotted on to nitrocellulose membrane (BIO-RAD, USA) by wet transfer. Primary antibody used was raised in rabbits against Cry1Ac (Envirologix, USA) and horse radish peroxidase conjugated secondary antibody raised in goats against rabbit IgG (Sigma Aldrich, USA) were used for detection. The blot was developed in X-ray films using luminol (Roche Diagnostics GmbH, Mannheim, Germany) chemiluminescence substrate in developer and fixer (Kodak, Germany).
Insect bioassay
After confirmation of cry1Aabc gene expression in selected events, insect bioassay with gram pod borer (Helicoverpa armigera) was performed using leaves and pods under no-choice condition. The larvae were reared on artificial diet (Armes et al. 1992) and 5 days old larvae were used for detached leaf bioassay. The third fully expanded leaf from the top of the pigeonpea plant was selected for the bioassay. Bioassays were performed in 50 ml sample containers with 10 ml of a 2% agar and 0.1% (w/v) sorbic acid solution (as a preservative) per container. One trifoliate leaf was placed in each container with one larva. The containers were closed with the lid having 5 min pinholes for air movement and then placed inside a BOD incubator maintained at 25 °C and 44–55% relative humidity. Similarly, young tender pods (20 days old) of selected lines were used for detached pod assay using 7 days old larvae. All the treatments were replicated ten times. Observations on larval mortality were recorded up to 7 days post larval release. The weight of diet consumed by the larvae was measured by taking the initial and final weight of the pod/leaf. The percent reduction in defoliation was calculated, based on the formula given below:
The experiment was conducted in Completely Randomized Design (CRD) and data analyzed by PROC-ANOVA and Duncan’s Multiple Range Test (DMRT) was used to test the significance using SAS 9.2 software (SAS Institute Inc., Cary, NC, USA).
Results
Construction of chimeric Bt gene and production of transgenic plants
A plant codon-optimized chimeric Bt δ-endotoxin gene Cry1Aabc was constructed with nucleotide sequences that encode three different domains I, II and III from three different δ-endotoxins viz., Cry1Aa, Cry1Ab and Cry1Ac, respectively (Fig. S2). The chimeric protein differs from Cry1Ac protein (truncated) with respect to five amino acids in the first two domains (I and II) (Adang et al. 1985) (Table 1). The codons were optimized for plant expression, A + T content in nucleotide base composition and elimination of sequences like destabilization, inappropriate polyadenylation, degradation and presence of RNA splice sites etc (Table S1).
A total of 11,839 axillary meristem explants were co-cultivated with Agrobacterium tumefaciens harbouring chimeric cry1Aabc gene and 11 putative transgenics were established in the Containment Facility. All the established transformants were screened through PCR using gene specific primers, and only 8 plants were found to be positive, with the transformation frequency of 0.06% (data not shown). Seeds from all eight plants were harvested and planted in the Containment facility for generation advancement. The T1 seeds harvested from all eight PCR positive lines were sown and screened for the presence and transmission of gene based on PCR analysis using gene specific primers. Molecular characterization (protein expression data) and insect bioassay (100% larval mortality) of the transgenic pigeonpea lines (T2 generation onwards) confirmed two promising events viz., IPCc1 and IPCc2, amongst the generated transgenic pigeonpea lines. These two events were further advanced for the identification of a viable event. Molecular characterization (PCR, ELISA, Western blot and Southern blot hybridization) of the events were performed in T3 and T4 generations.
Molecular characterization of transgenic lines
PCR and Alkaline phosphatase (ALP) chemistry based direct antigen coating (DAC) ELISA (qualitative ELISA) was performed in all progenies of pigeonpea derived from two T0 events. In T4 generation, 203 progenies derived from both the events were analysed of which 31 progenies have been identified as positive. These progenies were further advanced to T5 generation. In T5 generation, 2013 progenies derived from two T0 events were analysed and 84 progenies were identified as positive from both the events.PCR analyses of all pigeonpea progenies show the presence of gene, confirming to expression data (Fig. S3).
Horse Radish Peroxidase (HRP) chemistry based Double Antibody Sandwich ELISA (quantitative ELISA) were performed in all qualitative ELISA and PCR positive pigeonpea progenies for the quantification of δ-endotoxin protein. An Envirologix kit was used according to the manufacturer’s instructions. In T4 generation, a total 31 pigeonpea progenies (identified positive in PCR and qualitative ELISA) derived from two T0 events were analysed. This assay was performed at 120 DAS using the leaf tissue (40 mg). All the thirty-one progenies were identified as positive and the δ-endotoxin expression range was 15.82–44.89 ng/mg of total soluble protein (TSP). These higher expressing progenies with high protein expression were further analysed in T5 generation.
A total 84 T5 pigeonpea progenies (derived from 2 T0 events) were analysed pre-flowering and post flowering stage using quantitative ELISA. For both the events, the expression at pre-flowering (av. 28.73 ng/mg TSP) is higher than post flowering stage (av. 17.04 ng/mg TSP). Tissue specific expression in flowers, pod walls and immature seeds 188 DAS, indicated higher expression in pod walls (av. 13.01 ng/mg TSP), as compared to the flowers (av. 11.71 ng/mg TSP) and immature seeds (av. 9.84 ng/mg TSP). Expression range of the two events in both the generations is described (Table 2).
To detect the stable integration of Cry1Aabc gene in two lines, Southern blot hybridization was done in three subsequent generations (T3 generation onwards). For this purpose, DNA from pooled progenies of both the events showed the presence and integration of single copy of cry1Aabc gene. Single site restricted samples exhibited single band corresponding to a unique position in the genome of pigeonpea (ca. 4.91 kb for IPCc2 and 3.63 kb for IPCc1). Double site restriction for gene cassette release showed that the 2.62 kb of recombinant gene integrated in the genome (Fig. 1a).
Molecular analysis of two transgenic pigeonpea lines. a Southern blot analysis of two events with cry1Aabc specific probe [L: DIG labelled mol wt marker; IPCc2CN: Event IPCc2; IPCc1CN: Event IPCc1; IPCc2CR: Event IPCc2; IPCc1CR: Event IPCc1 WT: Non-transgenic (wild-type) Asha; P positive control (binary plasmid digested with HindIII); P CR Positive control (binary plasmid)] CN copy number estimation, CR casettee release. b RT-PCR of pigeonpea lines derived from two pigeonpea events [L1 1 kb DNA Ladder, P positive control (binary plasmid); lanes 3–5 Progenies from transgenic event (IPCc1); lanes 6–9 Progenies from transgenic event (IPCc2); WT Wild-type (Asha), NS null segregant, RT RNA template, NTC no template control, L2 100 bp DNA ladder]. c Western blot analysis of transgenic pigeonpea lines [lanes 1–3 Progenies from transgenic event IPCc1, lanes 4–7 Progenies from transgenic event IPCc2, WT wild type (Asha), P positive control (purified cry protein)]
RT-PCR with gene specific primers detected the presence of 440 bp amplification product of both the events indicating the transcription of Cry1Aabc in leaf tissues, with actin as internal control gene (Fig. 1b). The Cry1Aabc protein was detected in all the high expressing progenies of two events using Western hybridization. In all, a clear ~66 kDa band was observed in leaf samples harvested after 200 days of sowing, without any degradation (Fig. 1c).
Insect bioassay
Two pigeonpea lines IPCc1 and IPCc2 were subjected to insect (Helicoverpa armigera) bioassay using 5 days and 7 days old larvae and young trifoliate leaves and pods, respectively. WT (non-transformed) Asha was used as control (Table 3). Larval mortality between the controls did not differ significantly. In leaf bioassay, there was significantly greater defoliation in control lines than in transgenic pigeonpea lines (Fig. 2a). Similarly in detached pod assay, mortality was observed up to 90 and 100%, respectively in T4 and T5 generation (Fig. 2b, c). The mean larval mortality values are significantly different in transgenic lines as compared to that of control. Significantly higher amount of leaf was consumed in non-transformed lines (0.048 g) compared to transgenic lines (0.012–0.015 g) in T4 stage. Considerable reduction in defoliation was observed in the transgenic lines at T4 (78.4%) and T5 stages (83.4%). Similarly, there was significant reduction in pod consumption also in IPCc1 (0.18 g), compared to WT (0.99 g). Further, larvae found surviving on the transgenic lines exhibited retarded growth, compared to non-transgenic control. Correlation was observed between expression of Bt-protein in transgenic lines and larval mortality based on detached leaf bioassay (Table 4).
Insect bioassay of transgenic pigeonpea lines. a Detached leaf assay (no choice condition) of transgenic pigeonpea line (IPCc1) and non-transgenic Asha (WT). b Detached pod bioassay of transgenic pigeonpea lines (IPCc1 and IPCc2) and non-transgenic Asha (WT) and c. Opened up pods of transgenic pigeonpea lines (IPCc1 and IPCc2) and non-transgenic Asha (WT)
Discussion
Grain legumes are important component of sustainable agriculture. Genetic engineering is a potential approach to address problems where plant breeding interventions have limited success. Two successful examples of genetically engineered grain legume are herbicide (glyphosate) tolerant soybean and golden mosaic virus common bean (Jacob et al. 2016). Pigeonpea is an important grain legume grown across 22 countries worldwide. The gram pod borer, Helicoverpa armigera, a lepidopteran pest is the most serious and widespread pest of pigeonpea, particularly short duration and medium duration varieties. Heavy consumption of pesticides along with the development of resistance by the pest has led to the need for alternate approaches for the management of Helicoverpa armigera (Sharma et al. 2006). Sources of resistance to the insect pest have not been identified in pigeonpea germplasm, which limited the approach of conventional breeding to develop resistant varieties (Nene and Sheila 1990). Genetic engineering of crops by utilizing the genes encoding insecticidal crystal proteins or δ-endotoxins of Bt (Bacillus thuringiensis) was shown to impart resistance to this class of insect pests in soybean, cotton, maize and other crops (Kumar et al. 1996; James 2014).
Reports of pigeonpea transformation are abundant since last decade. Transgenic pigeonpea plants expressing oat arginine decarboxylase gene (Sivamani et al. 2001), hemagglutinin (H) gene (Satyavathi et al. 2003), rice chitinase (Kumar et al. 2004), HN gene of PPRV (Prasad et al. 2004), feedback inhibition insensitive dhdps-r1 (Thu et al. 2007), P5CSF129A (Surekha et al. 2014), insecticidal Bt-genes viz. cryI E-C (Surekha et al. 2005), cryIA (b) (Verma and Chand 2005), cryIAb (Sharma et al. 2006), cryIAcF (Ramu et al. 2011) has been reported earlier. Our report is first on using chimeric Bt gene, Cry1Aabc derived from three different Bt genes (Cry1Aa, Cry1Ab and Cry1Ac) for improving the level of resistance. Among the lepidopteran specific δ-endotoxins of Bt, Cry1A class is the most effective toxin against Helicoverpa armigera (Chakrabarti et al. 1998). Attempts to improve the level of toxicity of Cry1Ac by domain shuffling have led to the finding that a chimera of Cry1Aa, Cry1Ab and Cry1Ac was more effective than Cry1Ac (Kumar, unpublished data). Similar, attempts to use chimeric cry1AcF gene (possessing cry1Ac and cry1F domain) with anticipated increase in the tolerance was reported earlier (Ramu et al. 2011).The chimeric protein differs from Cry1Ac protein (truncated) with respect to five amino acids in Domains I and II. A plant codon-optimized chimeric gene cry1Aabc was constructed and sub-cloned in an Agrobacterium tumefaciencs based binary vector. Agrobacterium tumefaciens mediated genetic transformation of pigeonpea cv. Asha was carried out to develop transgenic lines expressing the chimeric gene. The protocol entails organogenetic (shoot) regeneration system and selection based on antibiotic Kanamycin monosulphate (100 mg/L) from axillary meristem explants. Healthy shoots surviving four-five cycles of selection (10–14 days each) were grafted onto WT root stock to establish mature fertile plant. Transformation frequency of 0.06% was obtained in the present study based on our data of advancing positive lines across generations. In pigeonpea, variable transformation frequency (less than 1.0%–more than 50%) has been reported based on transgene presence upto T2 generation. Variable transformation frequency is dependent on genotype, type of explants, osmotic treatment of explants, Agrobacterium cell density, co-cultivation time, antibiotics, temperature, media composition etc. (Opabode 2006).
Based on detailed molecular characterization and insect bioassay, two promising events were selected for further study (T2 generation onwards). These events show single locus integration in the genome, high expression (15.82–44.89 ng/mg of TSP) and high insect mortality (95–100%) based on detached leaf assay across three generations. Results of PCR and Southern hybridization confirmed stable integration of cry1Aabc gene in the progenies of the two events. Presence of single copy of T-DNA across both the generations indicates its stable integration in the pigeonpea genome (Fig. 1a, lanes 2, 3). Release of the entire cry1Aabc gene from cassette indicates integration of the gene of interest in the genome of both the events (Fig. 1a lanes 4, 5).
Quantitative ELISA performed in the progenies of two pigeonpea events using different plant tissues at different developmental stages indicated expression variation as reported earlier in other crops (Acharjee et al. 2010; Dong and Li 2007). Expression in leaf tissues at pre-flowering stage was found to be higher in all the progenies tested, as compared to the post flowering stage. The expression of Cry1Aabc protein in different plant tissues viz. flower, pod wall and immature seeds were also assessed since pod borer larvae feed on these organs, during various developmental stages. Lower expression was observed as compared to the leaves both pre- and post-flowering stages. Higher expression was observed in pod wall in comparison to immature seeds and flowers. Similar results of decreasing Cry1Ac levels consistently throughout the growing season were also reported, attributing part of the decline in expression to reduction in the levels of mRNA production (Finnegan et al. 1998). The content of foreign protein in the same tissue decreased along with the growth of transgenic plants because of the decrease in full length Bt toxin gene transcripts. The higher expression of Cry gene at earlier stages led to the gene regulation at the post-transcriptional level and contributed to the consequent gene silencing that was developmentally regulated (Dong and Li 2007). Lower expression level of the Bt insecticidal gene at late developmental stages was also correlated with changes in the methylation state of the 35S promoter region (Xia et al. 2005). Lower expression in floral bud on maturity was also attributed to protein degradation (Szwacka et al. 2009).
Detailed characterization of the progenies of two pigeonpea events include quantitative ELISA coupled with insect bioassay to establish efficacy of the transgenic lines. The variable mortality levels among the plants corroborated mostly with the expression levels in the progenies. However, alterations were also obtained in few progenies having higher expression but lower larval mortality. This may be due to change in the level of secondary compounds such as phenolics and terpenoids as the plant matures (Zummo et al. 1984). Further, secondary compounds alter the toxicity of Bt-proteins against lepidopteran larvae either negatively (Olsen and Daly 2000; Zhang and Guo 2000) or positively (Zhang et al. 2002). Further, elevated CO2 concentration has also been implicated to affect efficacy studies in cotton tissues (Chen et al. 2005).
Typically, transgene with single copy usually segregates in 3:1 ratio among the progenies. Based on the segregation analyses of the progenies of both the events, it appears the lines are hemizygous, with distorted segregation ratio (<75% positive progenies) (Table S2). Non-Mendelian segregation of the transgene has been reported in transgenic rice (Datta et al. 1990; Peng et al. 1995) soybean (Christou et al. 1989), wheat (Srivastava et al. 1996) and cotton (Sachs et al. 1998). This may be ascribed to the lower viability of transgenic pollen resulting in lower fertilization ability (Zhang et al. 1996), transgene inactivation (Spencer et al. 1992; Walters et al. 1992), or the recessive lethal (Scott et al. 1998) gene action or other intra genomic conflicts. Failure to pass the transgene to the next generation through pollen (pollen lethality) is the reason for abnormal segregation (Christou et al. 1989). For the fixing of transgene locus, forced selfing might help attaining homozygosity in often cross-pollinated pigeonpea, or backcrossing with recipient genome. However, further research is required to understand the segregation distortion pattern of pigeonpea progenies.
Insect bioassay was conducted exhaustively using two target tissues viz., leaf and pod of the selected progenies with higher expression, utilizing the first instar and third instar larvae from homogeneous brood. Adult insects were collected from pigeonpea field and bred artificially to raise homogenous population for the bioassay, obviating external factors. Larval mortality was recorded in the progenies after 7 days of incubation. Larvae fed on transgenic plants stopped feeding and most of the tissues remained unaffected, whereas the larvae on untransformed plants fed voraciously. The larvae surviving on the transgenic lines were deformed with significantly reduced body weight and hardly advancing beyond moulting stage. However, for more effective control of the pod borer, tissue specific promoter may be utilized in future.
In the present investigation, we demonstrated that the chimeric Cry1Aabc protein is effective against Helicoverpa armigera larvae in insect bioassays carried across generations. Molecular characterization and insect bioassays confirmed two superior events in pigeonpea. These events will be further tested to identify a tangible elite event that can be incorporated in breeding programmes for the development of insect resistant pigeonpea.
References
Aboshama HMS (2011) Somatic embryogenesis, proliferation, maturation and germination in Cajanus cajan. World J Agric Sci 7(1):86–95
Acharjee S, Sarmah BK, Kumar PA, Olsen K, Mahon R, Moar WJ, Moore A, Higgings TJV (2010) Transgenic chickpeas (Cicer arietinum L.) expressing a sequence-modified cry2Aa gene. Plant Sci 178:333–339
Adang MA, Staver MJ, Rocheleau TA, Leighton J, Barker RF, Thompson DV (1985) Characterized full-length and truncated plasmid clones of crystal protein of Bacillus thuringiensis subsp Kurstaki HD-73 and their toxicity to Manduca sexta. Gene 36: 289–300
Anbazhagan VR, Ganapathi A (1999) Somatic embryogenesis in cell suspension cultures of pigeonpea (Cajanus cajan). Plant Cell Tiss Organ Cult 56(3):179–184
Armes NJ, Bond GS, Cooters RJ (1992) The laboratory culture and development of Helicoverpa armigera. Natural Resources Institute Bulletin No. 57. Chatam. Natural Resource Institute, UK
Bhatnagar-Mathur P, Sharma KK (2016) Genetic transformation of pigeonpea: An overview. Leg Perspect 11:35–36
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 112:195–203
Chakrabarti SK, Mandaokar A, Kumar PA, Sharma RP (1998) Toxicity of lepidopteran specific delta endotoxins of Bacillus thuringiensis towards neonate larvae of Helicoverpa armigera. J Inverteb Pathol 72:336–337
Chang TH, Huang HY, Hsu JB, Weng SL, Horng JT, Huang HD (2013) An enhanced computational platform for investigating the roles of regulatory RNA and for identifying functional RNA motifs. BMC Bioinformatics 14(2):S4
Chen FJ, Wu G, Ge F, Parajulee MN, Shreshtha RB (2005) Effects of elevated CO2 and transgenic Bt cotton on plant chemistry, performance and feeding of an insect herbivore, the cotton bollworm. Entomol Exp Appl 115:341–346
Christou P, Swain WF, Yang NS, McCabe DE (1989) Inheritance and expression of foreign gene in transgenic soybean. Proc Nat Acad Sci USA 86:7500–7504
Das A, Parida SK (2014) Advances in biotechnological applications in three important food legumes. Plant Biotechnol Rep 8:83–99
Dassi E, Re A, Leo S, Tebaldi T, Pasini L, Peroni D, Quattrone A (2014) AURA 2: Empowering discovery of post-transcriptional networks. Translation 2(1):e27738
Datta SK, Peterhans A, Datta K, Potrykus I (1990) Genetically engineered fertile indica-rice plants recovered from protoplasts. Biotechnol 8:736–740
Dayal S, Lavanya M, Devi P, Sharma KK (2003) An efficient protocol for shoot regeneration and genetic transformation of pigeonpea (Cajanus cajan L. Millisp) by using leaf explants. Plant Cell Rep 21:1072–1079
Dellaporta S, Wood J, Hicks JB (1983) A plant DNA mini preparation: version II. Plant Mol Biol Rep 1:19–21
Dong HJ, Li WJ (2007) Variability of endotoxin expression in Bt transgenic cotton. J Agron Crop Sci 193(1):21–29
Eapen S, George L (1993) Plant regeneration from leaf discs of peanut and pigeonpea: influence of benzyl-adenine, indole acetic acid and indole acetic acid-amino acid conjugates. Plant Cell Tiss Organ Cult 35(3):223–227
Eapen S, Livareker S, George L (1998) Thiadizuron-induced shoot regeneration in pigeonpea (Cajanus cajan L). Plant Cell Tiss Organ Cult 53(3):217–220
FAOSTAT (2013) Food and agricultural organization (http://faostat3.fao.org/)
Finnegan EJ, Genger RK, Peacock WJ, Dennis ES (1998) DNA methylation in plants. Ann Rev Plant Physiol Plant Mol Biol 49:223–247
Gamborg OL, Miller RA, Ojima O (1968) Nutrient requirements of suspension culture of soybean root cell. Exp Cell Res 50:151–158
Gatti I, Guindon F, Bermeja C, Cspasito A, Cointry E (2016) In vitro tissue culture in breeding programs of leguminous pulses: use and current status. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-016-1082-6
Geetha N, Venkatachalam P, Prakash V, Lakshmi Sita G (1998) High frequency induction of multiple shoots and plants regeneration from seedling explants of pigeonpea (Cajanus cajan L.). Curr Sci 17:1036–1041
Geetha N, Venkatachalam P, Lakshmi Sita G (1999) Agrobacterium-mediated genetic transformation of pigeonpea (Cajanus cajan L.) and development of transgenic plants via direct organogenesis. Plant Biotech 16:213–218
Hebsgaard SM, Korning PG, Tolstrup N, Engelbrecht J, Rouze P, Brunak S (1996) Splice site prediction in Arabidopsis thaliana DNA by combining local and global sequence information. Nucl Acid Res 24:3439–3452
Hofgen R, Willitzer L (1990) Biochemical and genetic analysis of different patatin isoforms expressed in various organs of potato (Solanum tuberosum). Plant Sci 66:221–230
Hood EE, Helmer GL, Fraley RT, Chilton M-D (1986) The hypervirulence of Agrobacterium tumefaciens A 281 is encoded in a region of pTiBo542 outside of T DNA. J Bacteriol 168:1291–1301
Jacob C, Carrasco B, Schwember AR (2016) Advances in breeding and biotechnology of legume crops. Plant Cell Tiss Organ Cult. doi:10.1007/s11240-016-1106-2
James C (2014) Global status of commercialized biotech/GM crops: 2014. ISAAA Brief No. 49. ISAAA, Ithaca, NY
Kumar PA, Malik VS, Sharma RP (1996) Insecticidal proteins of Bacillus thuringiensis. Adv Appl Microbiol 42:1–43
Kumar SM, Kumar BK, Sharma KK, Devi P (2004) Genetic transformation of pigeonpea with rice chitinase gene. Plant Breed 123(5):485–489
Lawrence PK, Koundal KR (2001) Agrobacterium tumefaciens-mediated transformation of pigeonpea (Cajanus cajan L. Millisp) and molecular analysis of regenerated plants. Curr Sci 80:1428–1432
Liu H, Han H, Li J, Wong L (2005) DNAFSMiner: a web-based software toolbox to recognize two types of functional sites in DNA sequences. Bioinformatics 21:671–673
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Phys Plant 15:473–497
Murray EE, Lotzer J, Eberle M (1989) Codon usage in plant genes. Nucl Acid Res 17:477–498
Naidu RB, Kulkarni DD, Krishnamurhty KV (1995) Genotype dependent morphogenetic potentiality of various explants of a food legume, the pigeonpea (Cajanus cajan L. Millisp). In vitro Cell Dev Biol Plant 31:26–30
Nene YL, Sheila VK (1990) Pigeonpea: geography and importance. In: Nene YL, Hall SD, Sheila VK (eds) The pigeonpea. CAB International, UK, pp 1–14
Olhoft PM, Flagel LE, Somers DA (2004) T-DNA locus structure in a large population of soybean plants transformed using the Agrobacterium-mediated cotyledonary-node method. J Plant Biotechnol 2:289–300
Olsen KM, Daly JC (2000) Plant-toxin interactions in transgenic Bt cotton and their effects on mortality of Helicoverpa armigera. Entomol Society America 93:1293–1299
Opabode JT (2006) Agrobacterium mediated transformation of plants: emerging factors that influence efficiency. Biotech Mol Bio Rev 1:12–20
Paz MM, Shou HX, Guo ZB, Zhang ZY, Banerjee AK, Wang K (2004) Assessment of conditions affecting Agrobacterium-mediated soybean transformation using the cotyledonary node explant. Euphytica 136:167–179
Peng JY, Wen SJ, Lister RL, Hodges TK (1995) Inheritence of gusA and neo genes in transgenic rice. Plant Mol Biol 27:91–104
Prasad V, Satyavathi VV, Sanjaya, Valli KM, Khandelwal A, Shaila MS, Lakshmi Sita G (2004). Expression of biologically active hemagglutinin-neuraminidase protein 4 of Peste despetits ruminants virus in transgenic pigeonpea (Cajanus cajan L. Millisp). Plant Sci 166:199–205
Raghavendra T, Sudhakar P (2014) Agrobacterium-mediated transformation of pigeonpea (Cajanus cajan L.) var. LRG 41 from axillay bud. Int JAppl Biol Pharm Tec 5(1):274–282
Ramu SV, Rohini S, Keshavareddy G, Neelima MG, Shanmugam NB, Kumar ARV, Sarangi SK, Kumar PA, Udaykumar M (2011) Expression of a synthetic cryIAcF gene in transgenic pigeonpea confers resistance to Helicoverpa armigera. J Appl Entomol. doi:10.1111/j.1439-0418.2011.01703.x
Rao KS, Rohini S, Sharma PD, Keshamma E, Udaykumar M (2008) In planta transformation of pigeonpea: a method to overcome recalcitrancy of the crop to regeneration in vitro. Physiol Mol Biol Plants 14(4):321–328
Rohini VK, Rao KS (2000) Embryo transformation, a practical approach for realizing transgenic plants of safflower (Carthamus tinctorius L.) Ann Bot 86:1043–1049
Sachs ES, Benedict JH, Stelly DM, Taylor JF, Altman DW, Berberich SA, Davis SK (1998) Expression and segregation of genes encoding Cry1A insecticidal proteins in cotton. Crop Sci 38:1–11
Saraswathy N, Kumar PA (2004) Protein engineering of δ-endotoxins of Bacillus thuringiensis. Electron J Biotechnol 7(2):178–188
Satyavathi VV, Prasad V, Khandelwal A, Shaila MS, Lakshmi Sita G (2003) Expression of hemagglutinin protein of Rinderpest virus in transgenic pigeonpea [Cajanus cajan L. (Millsp.)] plants. Plant Cell Rep 21:651–658
Scott A, Woodfield D, White DWR (1998) Allelic composition and genetic background effects on transgene expression and inheritance in white clover. Mol Breed 4:479–490
Sharma KK, Lavanya M, Anjaiah V (2006) Agrobacterium-mediated production of transgenic pigeonpea (Cajanus cajan L. Millsp.) expressing the synthetic BT cry1Ab gene. In vitro Cell Dev Biol Plant 42(2):165–173
Singh ND, Sahoo L, Neera BS, Jaiwal PK (2002) In vitro shoot organogenesis and plant regeneration from cotyledonary node and leaf explants of pigeonpea (Cajanus cajan L. Millisp). Physiol Mol Biol Plant 8:133–140
Singh ND, Sahoo L, Neera BS, Jaiwal PK (2003) The effect of TDZ on organogenesis and somatic embryogenesis in pigeonpea (Cajanus cajan L. Millisp). Plant Sci 164:341–347
Sivamani E, Bassie L, Christou P, Capell T (2001) Development of a novel gene transfer system for Cajanus cajan and expression of a monocot arginine decarboxylase cDNA in transformed cell lines. Plant Physiol Biochem 39: 575–582
Spencer TM, O’Brien JV, Start WG, Adams TR, Gordon-Kamm WJ, Lemaux PG (1992) Segregation of transgenes in maize. Plant Mol Biol 18:201–210
Sreenivasu K, Kumar PA, Sharma RP (1998) Plant regeneration via somatic embryogenesis in pigeonpea (Cajanus cajan L. Millsp). Plant Cell Rep 17:294–297
Srivastava B, Vasil V, Vasil IK (1996) Molecular characterization of the fate of transgene in transformed wheat (Triticum aestivum L.) Theor Appl Genet 92:1031–1037
Surekha Ch, Beena MR, Arundhati A, Singh PK, Tuli R, Dutta-Gupta A, Kirti PB (2005) Agrobacterium-mediated genetic transformation of pigeon pea (Cajanus cajan (L.) Millsp.) using embryonal segments and development of transgenic plants for resistance against Spodoptera. Plant Sci 169:1074–1080
Surekha Ch, Arundhati A, Rao GS (2007) Differential response of Cajanus cajan varieties to transformation with different strains of Agrobacterium. J Biol Sci 7:176–181
Surekha Ch, Kumari KN, Aruna LV, Suneetha G, Arundhati A, Kishore PBK (2014) Expression of the Vigna aconitifolia P5CSF129A gene in transgenic pigeonpea enhances proline accumulation and salt tolerance. Plant Cell Tiss Organ Cult 116(1):27–36
Szwacka M, Siedlecka E, Zawirska-Wojtasiak R, Wisniewski L, Malepszy S (2009) Expression pattern of the pre-prothaumatin II gene under the control of the CaMV35S promoter in transgenic cucumber (Cucumis sativus L.) flower buds and fruits. J Appl Genet 50:9–16
Thu TT, Dewade E, Trung LQ, Clays H, Jacobs H, Angenon G (2007) Increasing lysine levels in pigeonpea (Cajanus cajan L Millsp) seeds through genetic engineering. Plant Cell Tiss Organ Cult 91(2):135–143
Verma AK, Chand L (2005) Agrobacterium-mediated transformation of pigeonpea (Cajanus cajan L.) with uidA and cry1Ab genes. Physiol Mol Biol Plant 11:99–109
Villiers SD, Emongor Q, Njeri R, Gwata E, Hoisington D, Njagi I, Silim S, Sharma K (2008) Evaluation of shoot regeneration response in tissue culture of pigeonpea (Cajanus cajan L. Millisp) varieties adapted to eastern and southern Africa. Afr J Biotechnol 7:587–590
Walters DA, Vetsch CS, Potts DE, Lundquist RC (1992) Transformation and inheritance of a hygromycin phosphotransferase gene in maize plants. Plant Mol Biol 18:189–200
Xia L, Xu Q, Guo S (2005) Bt insecticidal gene and its temporal expression in transgenic cotton plants. Acta Agronomica Sin 31:197–202
Xue C, Wang BC, Yu Z, Sun M (2014) Structural insights into Bacillus thuringiensis cry, cyt and parasporin toxins. Toxins (Basel) 6(9):2732–2770
Yadav PBS, Padmaja V (2003) Shoot organogenesis and plantlet regeneration from leaf segments of pigeonpea. Plant Cell Tiss Organ Cult 73(2):197–200
Zhang Y, Guo Y (2000) Interactions between condensed tannin and Bt crystal protein in cotton. Acta Gossypii Sin 12:294–297
Zhang S, Warkentin D, Sun B, Zhong H, Sticklen N (1996) Variation in the inheritance of expression among subclones for unselected (uidA) and selected (bar) transgenes in maize (Zea mays L.) Theor Appl Genet 92:752–761
Zhang Y, Yang J, Guo Y, Wu K (2002) Study on the interactions between exogenous Bt-ICP and cotton terpenoids chemicals. Scientia Agric Sin 35:514–519
Zummo GR, Segers JC, Benedict JH (1984) Seasonal phenology of allelochemicals in cotton and resistance to bollworm (Lepidoptera: Noctuidae). Environ Entomol 13:1287–1290
Acknowledgements
The authors are thankful to Network Project on Transgenics in Crops (NPTC) and National Agricultural Science Fund (NASF) for financial support. Thanks are due to Dr Deepak Singh, for helping us with statistical analyses.
Author contributions
AD, SD, SGK and NPS: planned the experiments; AD, SD, SGK, MK, AKS, ARP, AS, JA, ST, MK, MP and LF: conducted the experiments; AD, SGK, AS, PAK and NPS: analyzed the data and compiled the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11240_2016_1131_MOESM1_ESM.ppt
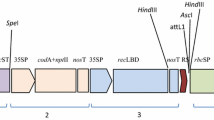
Fig S1.T-DNA of pBinAR containing cry1Aabc gene [RB: Right Border; Pnos: nopaline synthase promoter; nptII: neomycin phosphotransferase II; nosA: nopaline synthase terminator; ocsA: octopine synthase terminator; cry1Aabc: Domain shuffled Bt gene; 35S P: Cauliflower Mosaic Virus 35S promoter; LB: Left Border] (PPT 101 KB)
11240_2016_1131_MOESM3_ESM.ppt
Fig S3. PCR analysis of transgenic pigeonpea progenies [L1: 1 kb DNA Ladder; IPCc1: Progenies from IPCc1; IPCc2: Progenies from IPCc2; N: No Template Control; WT: Wild-type (non-transgenic line); P: Positive Control (Plasmid); L2: 1 kb DNA Ladder] (PPT 604 KB)
11240_2016_1131_MOESM4_ESM.doc
Table S1. Comparison of different negative regulatory elements present in the native and codon optimized sequences of Insecticidal Crystal Protein gene (DOC 32 KB)
Rights and permissions
About this article
Cite this article
Das, A., Datta, S., Sujayanand, G.K. et al. Expression of chimeric Bt gene, Cry1Aabc in transgenic pigeonpea (cv. Asha) confers resistance to gram pod borer (Helicoverpa armigera Hubner.). Plant Cell Tiss Organ Cult 127, 705–715 (2016). https://doi.org/10.1007/s11240-016-1131-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-1131-1