Abstract
The effects of proline (0, 50, 100, 200 and 500 mg l−1) and chitosan (0, 10, 20, 50 and 100 mg l−1) treatment at three incubation periods (1, 2 and 5 days) were assessed on the efficiency of microsopore embryogenesis induction and subsequently plantlet regeneration in rapeseed (Brassica napus L.) cv. “Hyola 401”. About 30–55 % increase in microspore embryogenesis was observed in the cultures exposed to 100 mg l−1 proline for 2 and 5 days. Higher levels were not advantageous so that, microspore embryogenesis significantly reduced when 200 and 500 mg l−1 proline were exogenously applied to the culture medium. High normal regeneration was achieved in the cultures treated with 50 and 100 mg l−1 proline for 2 and 5 days. In addition, callogenesis increased as proline level and duration of its treatment was increased in which the majority of microspore-derived embryos (78 %) underwent callusing in the cultures exposed to 500 mg l−1 proline for 5 days. In contrast to untreated cultures, the efficiency of microspore embryogenesis increased about 1.8-fold in response to 10 mg l−1 chitosan for 2 days. High levels of chitosan were detrimental to microspore embryogenesis so that embryogenesis was completely inhibited in the cultures exposed to 50 and 100 mg l−1 for 5 days. Chitosan treatment was not advantageous to the normal plantlet development at all levels and durations tested. High levels and durations of chitosan treatment resulted in the higher callusing so that the highest callusing (88 and 79 %) were observed in the presence of 100 mg l−1 for 1 day and 20 mg l−1 for 2 days, respectively. Efficiency of microspore embryogenesis induction and plantlet regeneration could be improved by proline and chitosan treatment when appropriate levels and durations of incubation were selected.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Isolated microspores cultured in vitro can reprogram their gametophytic developmental pathway towards embryogenesis, thereby producing embryos which can further give rise to haploid or double haploid (DH) plants, an important biotechnological tool in plant breeding and physiological studies (Shariatpanahi et al. 2006). The deviation from gametophytic to sporophytic developmental pathway has been induced by applying various stress pretreatments. In the genus Brassica, isolated microspores are often inducted by heat shock and its duration (Prem et al. 2005), mutagenic agents (Ahmadi et al. 2012), stress hormones (Ahmadi et al. 2014a), antibiotics or polyamines (Asif et al. 2013; Ahmadi et al. 2014b).
Plants accumulate large quantities of different types of compatible solutes upon stress treatment i.e. those applied for embryogenesis induction (Patnaik et al. 2005; Gupta and Huang 2014). Compatible solutes are low molecular weight, highly soluble organic compounds and are usually non-toxic at high cellular concentrations. These solutes e.g. proline, glycine betain, polyols and trehalose, provide protection to cells by contributing to osmotic adjustment, reactive oxygen species (ROS) detoxification, protection of membrane integrity and enzyme/protein stabilization (Kishor et al. 2005; Szabados and Savouré 2009). Proline, a proteinogenic amino acid with an exceptional conformational rigidity, is essential for stress tolerance, cell primary metabolism and growth and also has exhibited stimulatory effects on in vitro somatic embryogenesis induction and plant regeneration (Vikrant and Rashid 2002; Hita et al. 2003; Szabados and Savouré 2009; Hayat et al. 2012). When applied exogenously to plants exposed to stress, proline enhanced growth and other physiological characteristics of the treated plants (Hayat et al. 2012). Although, much is known about proline metabolism, the information about its regulatory role(s) in different aspects of somatic embryogenesis is limited. Formation of compact and yellow embryogenic calli and subsequently somatic embryogenesis were significantly increased in sugarcane when the induction medium was supplemented with proline (Suprasanna et al. 2005). Also, exogenously applied proline in a two-step culture method drastically increased somatic embryo formation, maturation and subsequently plant regeneration in Paspalum scorbiculatum L. (Ceasar and Ignasimuthu 2010). Also, the promotive effect of proline on androgenesis induction has been reported in many crop species (Yoshida et al. 1994; Redha et al. 1998; Hema and Murthy 2008). Significantly higher embryos were obtained from in vitro cultured anthers of niger when proline was added to the induction medium (Hema and Murthy 2008). Successful microspore embryogenesis requires a strict coordination of cell proliferation, cell differentiation, and cell-death programs. It has been indicated that proline acts as a positive regulator of programmed cell death (PCD) and its involvement in the inducing cell death has been reported in different species (Deuschle et al. 2004; Cecchini et al. 2011). PCD, an energy-dependant and genetically controlled mechanism that envisages the organized destruction of specific cell types and tissues, is involved in the elimination of unneeded structures within the cells and embryos and also is essential for correct embryo patterning (Lam 2004; Suarez et al. 2004; Choi 2013).
Chitosan, a polymeric deacetylated derivative of chitin, is naturally present in some fungi cell walls that combines a unique set of versatile physicochemical and biological characteristics which allows for a wide range of applications (Raafat et al. 2008; El Hadrami et al. 2010). Chitosan is used primarily as a natural seed treatment and plant growth enhancer, as an ecologically friendly biopesticide substance that boosts the innate ability of plants to defend themselves against fungal infections and also as a plant cell division and growth stimulator (Nge et al. 2006; Uthairatanakij et al. 2007). Conversion of orchid’s meristematic explants into the protocorm-like bodies (PLBs) was accelerated up to 15 times in the presence of shrimp and fungal chitosan in the liquid medium (Nge et al. 2006). Although its exact mode of action is still unknown, but many studies have indicated that exogenously applied chitosan rapidly induces oxidative stress, releases cytochrome c and further increases the activity of caspase3-like proteases, all of which lead to the PCD which is required for proper embryo formation (Zuppini et al. 2003; Zhang et al. 2012; Mejía-Teniente et al. 2013).
Despite many studies in microspore embryogenesis induction, the effects of proline and chitosan have not been well explored. In this study, the effects of various levels and durations of incubation of proline and chitosan treatment on the efficiency of microspore embryogenesis induction and subsequently regeneration of the derived embryos were assessed in the isolated microspores of B. napus L.
Materials and methods
Donor plants and growth conditions
Spring rapeseed (B. napus) cv. ‘Hyola 401’ was used as the test plant. Seeds were sown in 25 cm-diameter plastic pots (1 seed per pot) filled with a mix of sand, green manure, and a clay field soil (1:1:1, by volume). Donor plants were grown in a growth chamber at a day/night temperature of 15/10 °C with a 16-h photosynthetic photon flux density (PPFD) of 350 µE m−2 s−1 and relative humidity of 45–65 %. Donor plants were irrigated two times a week.
Isolated microspore culture
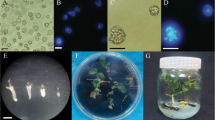
Floral buds measuring 2.5–3.5 mm in length containing a mixed population of mid to late-uninucleate microspores were harvested from the main and lateral branches of donor plants that had reached anthesis after about 90–110 days. These buds were surface sterilized by sodium hypochlorite (3 %) with gentle shaking for 10 min and washed three times (each 5 min) with cold sterile distilled water. Approximately, 180–200 sterilized buds were placed in a glass tube and were gently macerated into 25 ml of liquid NLN-13 (Lichter 1982) medium supplemented with 13 % (w/v) sucrose (Merck, Darmstadt, Germany) using a sterile glass rod. The crude suspension was filtered through a 40 µm metal mesh (Damavand Tes Sieve Ltd, Tehran, Iran), collected into two 15 ml centrifuge tubes and the volume was adjusted with fresh NLN-13 medium to 12 ml. The filtrate was centrifuged at 100×g for 4 min. The supernatant was decanted and the pellet was rinsed in fresh NLN-13 medium. This procedure was repeated twice. Finally the plating density was adjusted to 4 × 104 microspores ml−1 using a hemocytometer (Precicolor, Germany). Microspore suspension (5 ml) was dispensed into 6 cm sterile plastic Petri dishes (Farazbin, Tehran, Iran) then, the cultures were incubated at 30 ± 0.5 °C in the dark for 14 days. Once embryos were visible to the naked eye (Fig. 1a, b), the Petri dishes were transferred onto a rotary shaker in the dark at 30 rpm at 25 °C.
Microspore embryogenesis of B. napus L. c.v. “Hyola 401” in the presence of a proline (100 mg l−1 for 5 days), b chitosan (10 mg l−1 for 2 days), c cotyledonary MDE, d distal half of the cotyledons was sliced off after GA3 treatment, e regenerated plant which was transferred to the pots containing sterile pit and perlite in the greenhouse condition, f normal plantlet regeneration, g callogenesis, h abnormal MDEs were produced in the presence of 100 mg l−1 chitosan (for 1 day)
Proline and chitosan treatment
Chitosan (Sigma-Aldrich) was dissolved (100 mg in 5 ml of doubled distilled water) using gentle shaking for stock preparation. The pH was adjusted at 6.0 with 1 N NaOH and 1 N HCl and maintained in a refrigerator at 4 °C until needed (stock solution was kept for maximum 1 or 2 days and used as soon as possible). After determining plating density (4 × 104 microspores ml−1) and dispensing microspore suspension into the Petri dishes, different levels of filter-sterilized (0.22 μm filter) chitosan (0, 10, 20, 50 and 100 mg l−1) were added to the induction medium for three time periods (1, 2 and 5 days) and incubated at 30 °C in the dark. Each Petri dish contains 5 ml of microspore suspension and in the case of 10 mg l−1 for instance, each Petri dish requires 0.05 mg of chitosan to be added so that, 2.5 µl (contains 0.05 mg chitosan and is equal to 10 mg l−1) from stock solution is needed. Therefore, 0 µl (equal to 0 mg l−1 chitosan as the control), 2.5 µl (equal to 10 mg l−1), 5 µl (equal to 20 mg l−1), 12.5 µl (equal to 50 mg l−1) and finally 25 µl (equal to 100 mg l−1) from stock solution was added to each Petri dish. In the case of proline (Duchefa Biochime) 250 mg was dissolved in 5 ml of doubled distilled water for stock preparation. Similarly, each Petri dish contains 5 ml of microspore suspension and various levels of proline (0, 50, 100, 200 and 500 mg l−1) were tested. In the case of 50 mg l−1 for instance, each Petri dish needs 0.25 mg proline so that, 5 µl (contains 0.25 mg proline and is equal to 50 mg l−1) from stock solution is needed. Therefore, 0 µl (equal to 0 mg l−1 proline as the control), 5 µl (equal to 50 mg l−1), 10 µl (equal to 100 mg l−1), 20 µl (equal to 200 mg l−1) and 50 µl (equal to 500 mg l−1) from stock solution was added to each Petri dish. Proline or chitosan treated and also untreated cultures were centrifuged at 150×g for 4 min in order to decanting the residual. The plating density was adjusted to 4 × 104 microspores ml−1 and the suspension was dispensed into the same Petri dishes and incubated at 30 °C.
Microspore-derived embryo (MDE) conversion to plantlet
Fully developed MDEs (Fig. 1c) were transferred onto the B5 medium (Gamborg et al. 1968) containing 0.1 mg l −1 gibberellic acid (GA3, Fluka, Buchs, Switzerland), 2.5 % sucrose, pH 5.8 and 0.7 % agar (Duchefa Biochemie) and after incubating at 4 ± 0.5 °C in the dark for 1 week, the Petri dishes were maintained at 25 ± 1 °C under a 16-h photoperiod with light intensity of 40 µE m−2 s−1 for 1 week. Subsequently, the distal half of the cotyledons was sliced off (Fig. 1d) and the MDEs were placed onto hormone-free B5 medium containing 2 % sucrose, pH 5.8, 0.6 % agar for plantlet development (Ahmadi et al. 2012).
Plant regeneration
Regenerated plantlets were transferred to the pots containing sterile pit and perlite and maintained in a growth chamber at 20 ± 1 °C under a 16-h photoperiod with light intensity of 100 µE m−2 s−1 for 3 weeks. Then, gradual adaptation was followed to greenhouse conditions (Fig. 1e).
Experimental design and statistical analysis
The experiments were conducted in a factorial experiment based on a completely randomized design (CRD) to evaluate the effect of different factors. Entire experiments were repeated three times. Each treatment had five replications (Petri dishes). Data analyses were performed using SPSS software version 17 and the means were compared using Duncan’s multiple range (DMRT) test at α = 0.01 following analysis of variance.
Results
Proline treatment
Microspore embryogenesis was significantly affected by applied proline levels and also duration of its application (Table 1). Approximately, 30–55 % increase in the embryo formation was observed in the cultures exposed to 100 mg l−1 proline for 2 and 5 days. Lower level (50 mg l−1) was not advantageous to embryogenesis induction when compared with the untreated cultures at all durations tested. When treated with 100 mg l−1 proline, embryogenesis increased as the duration of incubation increased, so that the highest embryogenesis was achieved in the cultures incubated for 5 days (296.8 embryos Petri dish−1), 2 days (258.2 embryos Petri dish−1) and 1 day (202.6 embryos Petri dish−1) respectively. Higher levels were detrimental to microspore embryogenesis and embryogenesis significantly reduced when 200 and 500 mg l−1 proline were exogenously applied to the culture medium. Proline treatment also affected the path of MDE conversion into the plantlet i.e. via normal regeneration (Fig. 1f) or callusing (Fig. 1g). High normal regeneration was observed in the presence of 50 and 100 mg l−1 proline for 2 and 5 days (Table 2). Callogenesis increased as proline level was increased in which the majority of the MDEs (78 %) underwent callusing in the cultures exposed to 500 mg l−1 proline for 5 days.
Chitosan treatment
Microspore embryogenesis was significantly enhanced (about 1.8-fold) in response to 10 mg l−1 chitosan incubated for 2 days in contrast to the untreated cultures (Table 3). Chitosan at 20 mg l−1 also improved embryogenesis induction when maintained for 1 day in the liquid culture medium but higher durations of maintenance significantly decreased embryo formation. Moreover, microspore embryogenesis drastically decreased as chitosan level and also duration of its exposure were increased, so that embryogenesis was completely inhibited in the cultures exposed to 50 and 100 mg l−1 for 5 days. Abnormal embryos (Fig. 1h) were also produced in the presence of 100 mg l−1 chitosan incubated for 1 day, many of which (88 %, Table 4) failed to regenerate normally when transferred onto the regeneration medium. The mode of MDE regeneration was also affected by chitosan treatment and its duration of incubation. The rate of normal regeneration drastically reduced in the cultures exposed to 100 mg l−1 chitosan for 1 day. Also, callogenesis increased as duration of chitosan treatment was increased and 79 % of transplanted of MDEs underwent callusing in the presence of 20 mg l−1 chitosan for 5 days.
Discussion
Plants accumulate an array of metabolites and amino acids particularly proline, when exposed to stressful conditions. The accumulation of compatible solutes is often regarded as a basic strategy for the protection and survival of cells under stressful conditions, including the stresses used for embryogenesis induction (Patnaik et al. 2005; Chen et al. 2007; Redha and Suleman 2013). According to our results, proline was not advantageous to microspore embryogenesis when maintained for 1 day in the induction medium, but a 30–55 % increase in the embryo formation was observed in response to 100 mg l−1 proline in the cultures incubated for 2 and 5 days. Proline proves an organic form of nitrogen (reduced state), which is readily metabolized by plant cells, stimulating faster cell growth and development. Working on anther culture in Oryza sativa L. Gill et al. (2003) reported that the N6 medium supplemented with 560 mg l−1 proline resulted in higher callusing and subsequently higher plantlet regeneration. Also, significantly higher androgenic embryos were achieved from in vitro cultured anthers of niger when 230 mg l−1 proline was added to the induction medium (Hema and Murthy 2008). However, proline treatment was not favorable to embryogenesis induction and plantlet regeneration from cultured anthers of Capsicum anuum L. (Özkum and Tipirdamaz 2011). It has been indicated that the ROS generated by inductive stresses can modify the effectiveness of microspore embryogenesis, influencing both cell viability and cell metabolism (Żur et al. 2009, 2014) and proline can function as non-enzymatic antioxidant by scavenging the ROS produced by inductive stresses and reestablish redox homeostasis (Szabados and Savouré 2009). The enhancing effect of proline on somatic embryogenesis induction has also been observed in several species (Ceasar and Ignacimuthu 2010; Nhu et al. 2012). Patnaik et al. (2005) applied polyethylene glycol (PEG-8000), mannitol and air drying treatment for somatic embryogenesis induction in T.aestivem L. Surprisingly, they observed twofold to fivefold increase in the endogenous level of proline content in embryogenic explants compared with non-embryogenic explants (untreated cultures). Comparing the effects of three different amino acids i.e. proline, serine and glutamine on somatic embryogenesis in P. scorbiculatum L., Ceasar and Ignacimuthu (2010) noted that the MS medium containing 25 mg l−1 proline was superior to that containing serine and glutamine or untreated cultures in the term of somatic embryo formation and maturation. In addition, significant increase in the number of somatic embryos produced from in vitro cultured main root transverse thin cell layers of Panax vietnamensis was observed when 300 mg l−1 proline was exogenously applied to the induction medium (Nhu et al. 2012). However, our results indicated that proline treatment is detrimental to microspore embryogenesis induction at high levels (200 and 500 mg l−1) and longer durations (5 days).
Fully-developed cotyledonary MDEs were transferred onto the B5 medium containing 0.1 mg l−1 GA3 in order to induce embryo axis elongation and primary root formation, then, transferred to hormone-free B5 medium for subsequent plantlet regeneration. Upon transferring to the regeneration medium, many MDEs do not develop directly into plantlets (normal regeneration) but usually undergo abnormal development called callogenesis (Ahmadi et al. 2014a). According to our results, higher vigorous and normal plantlets were achieved in the presence of 50 and 100 mg l−1 proline at all durations tested. More recently, flow cytometric analysis of endoreduplication and mitotic cycle in classic mutant proline responding 1 (pro1, which catalyses the biosynthesis of proline from glutamic acid) of Zea mays indicated that the G1/S transition is arrested and thus cell proliferation suppressed in pro1 mutant plantlets. Gene expression profile analyses also showed that transcripts of cell cycle related genes, including cyclins, nucleosome assembly genes, and DNA replication-related genes, were significantly down-regulated in the pro1 plantlets (Wang et al. 2014) indicating the important role(s) of proline during the normal plant growth. According to our results, proline was not effective on normal plantlet regeneration at high levels so that, the majority of MDEs (78 %) underwent callusing in the cultures exposed to 500 mg l−1 proline for 5 days.
Chitosan, a biodegradable polymer, has been reported to act as a plant growth stimulator in some plant species, however the information about the regulatory mechanism(s) through which chitosan exerts an effect(s) is too limited (Kim et al. 2005; Nge et al. 2006; Mathew and Sankar 2012). According to our results, chitosan (10 mg l−1 for 2 days) caused a 1.8-fold increase in microspore embryogenesis induction relative to untreated cultures. Significant improvements in growth also have been reported in Vitis vinifera L. (Ait et al. 2004), soybean sprouts (Lee et al. 2005), Ocimum basilicum L. (Kim et al. 2005), as well as ornamental crops, such as Gerbera (Wanichpongpan et al. 2000) and Dendrobium orchids (Chandrkrachang 2002; Nge et al. 2006). It has been indicated that chitosan plays an important role in the enhancing growth and development by auxin biosynthesis pathway via tryptophan-independent pathway (Uthairatanakij et al. 2007). The phytohormone auxin regulates nearly all aspects of plant growth and development including embryogenesis. More recently, Wang et al. (2015) indicated that tryptophan-independent auxin biosynthetic pathway and auxin synthesized through this spatially and temporally regulated pathway contributes significantly to the establishment of the apical–basal axis, which profoundly affects the early embryogenesis in Arabidopsis. However, our results indicated that higher doses and durations of incubation are detrimental to MDE formation so that embryogenesis was completely inhibited in the cultures exposed to 50 and 100 mg l−1 chitosan for 5 days. Working on in vitro formation of PLBs in Thai orchid (Garmmatophyllum speciosum), Sopalun et al. (2010) noted that high level of chitosan (100 mg l−1) not only inhibited the growth of PLBs but also killed the in vitro cultured explants. Amborabé et al. (2008) observed that the reduction in growth and also eventual loss of viability in both plant and fungal cells following high dose of chitosan treatment (200 mg l−1) is related to the destruction of cell organization, induced leakage of electrolytes, UV-absorbing materials, and proteins. According to our results, abnormal MDEs were also produced in the presence of 100 mg l−1 chitosan, all failed to regenerate normally upon transferring onto the B5 regeneration medium.
Chitosan treatment also affected the type of MDE regeneration in a dose-dependent manner. High callusing was observed in the cultures treated with high dose of chitosan i.e. 100 mg l−1 for 1 day. The enhancing effect of chitosan on in vitro shoot and plantlet regeneration has been observed in some species (Kanchanapoom et al. 2012; Dastjerd et al. 2013). However, according to our results, chitosan was not advantageous to conversion of the MDEs into the plantlets when transferred onto the regeneration medium.
Conclusion
The production of DH plants from isolated microspores is an important technique used in plant breeding and basic researches. DH technology is a rapid method for developing homozygous lines, which can be used to accelerate crop improvement programs. In addition, isolated microspore culture is routinely used for establishing genetic mapping and for production of desired traits through the mutagenesis and selection. However, all these applications depend heavily on the efficiency of microspore embryogenesis and the regeneration of derived MDEs. Our results indicated that, proline at 100 mg l−1 for 2 and 5 days increases the efficiency of microspore embryogenesis (about 30–55 %) and normal regeneration of derived MDEs. Also, chitosan treatment (10 mg l−1 for 2 days) significantly enhanced embryo formation (approximately 1.8-fold) in contrast to the untreated cultures. Efficiency of microspore embryogenesis induction and plantlet regeneration could be improved by proline and chitosan treatment when appropriate levels and durations of incubation were selected.
References
Ahmadi B, Alizadeh K, Teixeira da Silva JA (2012) Enhanced regeneration of haploid plantlets from microspores of Brassica napus L. using bleomycin, PCIB, and phytohormones. Plant Cell Tiss Org Cult 109:525–533
Ahmadi B, Shariatpanahi ME, Teixeira da Silva JA (2014a) Efficient induction of microspore embryogenesis using abscisic acid, jasmonic acid and salicylic acid in Brassica napus L. Plant Cell Tiss Org Cult 116:343–351
Ahmadi B, Shariatpanahi ME, Ojaghkandi MA, Heydari AA (2014b) Improved microspore embryogenesis induction and plantlet regeneration using putrescine, cefotaxime and vancomycin in Brassica napus L. Plant Cell Tiss Org Cult 118:497–505
Ait BE, Eullaffroy P, Clément C, Vernet G (2004) Chitosan improves development, and protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Rep 22:608–614
Amborabé BE, Bonmort J, Fleurat-Lessard P, Roblin G (2008) Early events induced by chitosan on plant cells. J Exp Bot 59(9):2317–2324
Asif M, Eudes F, Randhawa H, Amundsen E, Yanke J, Spaner D (2013) Cefotaxime prevents microbial contamination and improves microspore embryogenesis in wheat and triticale. Plant Cell Rep 32:1637–1646
Ceasar SA, Ignacimuthu S (2010) Effects of cytokinins, carbohydrates and amino acids on induction and maturation of somatic embryos in kodo millet (Paspalum scorbiculatum Linn.). Plant Cell Tiss Org Cult 102:153–162
Cecchini NM, Monteoliva MI, Alvarez ME (2011) Proline dehydrogenase is a positive regulator of cell death in different kingdoms. Plant Signal Behav 6(8):1195–1197
Chandrkrachang S (2002) The applications of chitin in agriculture in Thailand. Adv Chitin Sci 5:458–462
Chen Z, Cuin TA, Zhou M, Twomey A, Naidu BP, Shabala S (2007) Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. J Exp Bot 58(15–16):4245–4255
Choi CQ (2013) The fate of the plant embryo’s suspensor: balancing life and death. PLoS Biol 11(9):e1001656. doi:10.1371/journal.pbio.1001656
Dastjerd ZH, Jabbarzadeh Z, Marandi RJ (2013) Interaction effects of chitosan, benzyladenin, and gibberellic acid on in vitro proliferation of M26 apple rootstock. Hort Environ Biotechnol 54(6):538–547
Deuschle K, Funck D, Forlani G, Stransky H, Biehl A, Leister D, van der Graaff E, Kunze R, Frommer WB (2004) The role of D1-pyrroline-5-carboxylate dehydrogenase in proline degradation. Plant Cell 16:3413–3425
El Hadrami A, Adam LR, El Hadrami I, Daayf F (2010) Chitosan in plant protection. Mar Drugs 8:968–987
Gamborg OL, Miller RA, Ojima L (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gill R, Kaur N, Sindhu AS, Bharaj TS, Gosal SS (2003) Improved methods for anther and pollen culture in rice. In: Kush GS, Brar DS, Hardy B (eds) Advances in rice genetics. International Rice Research Institute (IRRI), Philippines, pp 503–505
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genom 2014:1–18
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7(11):1456–1466
Hema BP, Murthy HN (2008) Improvement of in vitro androgenesis in niger using amino acids and polyamines. Biol Plant 52(1):121–125
Hita O, Gallego P, Villalobos N, Lanas I, Blazquez A, Martin JP, Fernandez J, Martin L, Guerra H (2003) Improvement of somatic embryogenesis in Medicago arborea. Plant Cell Tiss Org Cult 72:13–18
Kanchanapoom K, Pimolthai P, Kanchanapoom K (2012) The effect of chitosan on regeneration of Lily (Lilium longiflorum Thunb. ‘Ester Lily’). Propag Ornam Plant 12(2):127–129
Kim HJ, Chen F, Wang X, Rajapakse NC (2005) Effect of chitosan on the biological properties of sweet basil (Ocimum basilicum L.). J Agric Food Chem 53:3696–3701
Kishor PBK, Sangam S, Amrutha RN, Laxmi PS, Naidu KR, Rao KRSS, Rao S, Reddy KJ, Theriappan P, Sreenivasulu N (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88(3):424–438
Lam E (2004) Controlled cell death, plant survival and development. Nat Rev Mol Cell Biol 5:305–315
Lee YS, Kim YH, Kim SB (2005) Changes in the respiration, growth, and vitamin C content of soybean sprouts in response to chitosan of different molecular weights. Hort Sci 40:1333–1335
Lichter R (1982) Induction of haploid plants from isolated pollens of Brassica napus. Z Pflanzenphysiol 105:427–434
Mathew R, Sankar PD (2012) Effect of methyl jasmonate and chitosan on growth characteristics of Ocimum basilicum L., Ocimum sanctum L. and Ocimum gratissimum L. cell suspension cultures. Afr J Biotechnol 11(21):4759–4766
Mejía-Teniente L, Durán-Flores FDD, Chapa-Oliver AM, Torres-Pacheco I, Cruz-Hernández A, González-Chavira MM, Ocampo-Velázquez RV, Guevara-González RG (2013) Oxidative and molecular responses in Capsicum anuum L. after hydrogen proxide, salicylic acid and chitosan foliar applications. Int J Mol Sci 14:10178–10196
Nge KL, Nwe N, Chandrkrachang S, Stevens WF (2006) Chitosan as a growth stimulator in orchid tissue culture. Plant Sci 170:1185–1190
Nhut DT, Vinh BVT, Hien TT, Huy NP, Nam NB, Chien HX (2012) Effects of spermidine, proline and carbohydrate sources on somatic embryogenesis from main root transverse thin cell layers of Vietnamese ginseng (Panax vietnamensis Ha et. Grushv.). Afr J Biotechnol 11(5):1084–1091
Özkum D, Tipirdamaz R (2011) Effects of l-proline and cold treatment on pepper (Capsicum annuum L.) anther culturen. In: Gökçekus S et al (eds) Survival and sustainability, environmental earth sciences. Springer, Berlin, pp 137–143
Patnaik D, Mahalakshmi A, Khurana P (2005) Effect of water stress and heavy metals on induction of somatic embryogenesis in wheat leaf base cultures. Ind J Exp Bot 43:740–745
Prem D, Gupta K, Agnihotri A (2005) Effect of various exogenous and endogenous factors on microspore embryogenesis in Indian mustard (Brassica juncea L. Czern and Coss). In Vitro Cell Dev Biol Plant 41:266–273
Raafat D, von Bergen K, Haas A, Sahl HG (2008) Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microbiol 74:3764–3773
Redha A, Suleman P (2013) Assessment of polyamines and trehalose in wheat microspores culture for embryogenesis and green regenerated plants. Am J Plant Sci 4:2218–2226
Redha A, Attia T, Büter B, Stamp P, Schmid JE (1998) Single and combined effects of colchicine, l-proline and post-inoculation low temperature on anther culture of wheat, Triticum aestivum L. Plant Breed 4:335–340
Shariatpanahi ME, Bal U, Heberle-Bors E, Touraev A (2006) Stresses applied for the re-programming of plant microspores towards in vitro embryogenesis. Physiol Plant 127:519–534
Sopalun K, Thammasiri K, Ishikawa K (2010) Effects of chitosan as the growth stimulator for Grammatophyllum speciosum in vitro culture. World Acad Sci Eng Technol 4:11–29
Suarez MF, Filonova LH, Smertenko A, Savenkov EI, Clapham DH, von Arnold S, Zhivotovsky B, Bozhkov PV (2004) Metacaspase-dependent programmed cell death is essential for plant embryogenesis. Curr Biol 14:339–340
Suprasanna P, Choudhary RS, Desai NS, Bapat VA (2005) Regulation of somatic embryogenesis by plant growth regulators in sugarcane. Sugar Technol 7(4):123–128
Szabados L, Savouré A (2009) Proline: a multifunctional amino acid. Trends Plant Sci 15(2):89–97
Uthairatanakij A, Teixeira da Silva JA, Obsuwan K (2007) Chitosan for improving orchid production and quality. Orchid Sci Biotechnol 1(1):1–5
Vikrant A, Rashid A (2002) Somatic embryogenesis from immature and mature embryos of a minor millet Paspalum scrobiculatum L. Plant Cell Tiss Org Cult 69:71–77
Wang G, Zhang J, Wang G, Fan X, Sun X, Qin H, Xu N, Zhong M, Qiao Z, Tang Y, Song R (2014) Proline responding 1 plays a critical role in regulating general protein synthesis and the cell cycle in maiz. Plant Cell 26:2582–2600
Wang B, Chu J, Yu T, Xu Q, Sun X, Yuan J, Xiong G, Wang G, Wang Y, Li J (2015) Tryptophan-independent auxin biosynthesis contributes to early embryogenesis in Arabidopsis. PNAS 12(5):4821–4826
Wanichpongpan P, Suriyachan K, Chandrkrachang S (2000) Effects of chitosan on the growth of Gerbera flower plant (Gerbera jamesonii). In: Uragami T, Kurita K, Fukamizo T (eds) Chitin and chitosan in life science. Proceedings of the eighth international chitin and chitosan conference and fourth Asia Pacific chitin and chitosan symposium, Yamaguchi, Japan, pp 198–201, 21–23 Sept 2000
Yoshida KT, Fujii S, Sakata M, Takeda G (1994) Control or organogenesis and embryogenesis in rice calli. Breed Sci 4:355–360
Zhang H, Wang W, Yin H, Zhao X, Du Y (2012) Oligochitosan induces programmed cell death in tobacco suspension cells. Carbohydr Polym 87(3):2270–2278
Zuppini A, Baldan B, Millioni R, Favaron F, Navazio L, Mariani P (2003) Chitosan induces Ca2+-mediated programmed cell death in soybean cells. New Phytol 161:557–568
Żur I, Dubas E, Golemier F, Szechyńska-Hebda M, Gołębiowska G, Wędzony M (2009) Stress-related variation in antioxidative enzymes activity and cell metabolism efficiency associated with embryogenesis induction in isolated microspore culture of triticale (×Triticosecale Wittm.). Plant Cell Rep 28:1279–1287
Żur I, Dubas E, Krzewska M, Janowiak F, Hura K, Pociecha E, Bączek-Kwinta R, Płażek A (2014) Antioxidant activity and ROS tolerance in triticale (×Triticosecale Wittm.) anthers affect the efficiency of microspore embryogenesis. Plant Cell Tiss Org Cult 119:79–94
Acknowledgments
This research was supported by grants from Agricultural Biotechnology Research Institute of Iran (ABRII) Project No. 1-05-05-8601.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmadi, B., Shariatpanahi, M.E. Proline and chitosan enhanced efficiency of microspore embryogenesis induction and plantlet regeneration in Brassica napus L.. Plant Cell Tiss Organ Cult 123, 57–65 (2015). https://doi.org/10.1007/s11240-015-0814-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0814-3