Abstract
Vitis vinifera L. cv. Cabernet Sauvignon cell suspension cultures were treated with ultraviolet C (UV-C), methyl jasmonate (MeJA) and salicylic acid (SA), alone or in combination, to investigate the effects on stilbene biosynthesis. The application of elicitors at the proper dosage or concentration did not exert a negative effect on cell growth. All treatments enhanced both stilbene production inside the cells and trans-resveratrol accumulation in the culture medium. UV-C irradiation for 20 min or MeJA at 100 μM was efficient in promoting stilbene accumulation. The combined treatment of UV-C and MeJA highly induced total intracellular stilbene production to the maximum of 2005.05 ± 63.03 μg g−1 DW, and showed a synergistic effect on extracellular trans-resveratrol accumulation to 3.96 ± 0.2 mg l−1. SA at 100 μM was less efficient than UV-C and MeJA in promoting stilbene production. However, the combined elicitation of UV-C and SA further promoted intracellular stilbene production to the maximum of 1630.93 ± 44.17 μg g−1 DW, and markedly increased extracellular trans-resveratrol accumulation to 2.33 ± 0.15 mg l−1. Intracellular total phenolics and total flavonoids contents also significantly increased after elicitations. Relative expression of genes involved in stilbene and flavonoid biosynthesis was up-regulated, and there was a synergistic effect of UV-C together with MeJA or SA on STS expression. The results suggested that the combined treatment of UV-C together with MeJA or SA can be used as an efficient method to enhance stilbene production in V. vinifera cell cultures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Grapes and wines are main sources of polyphenols for human diet. Phenolic compounds, including stilbenes and flavonoids, belong to a wide range of plant secondary metabolites. Stilbenes are a group of low molecular weight phytoalexins produced by Vitaceae, and play an important role in plant defense mechanisms (Jeandet et al. 2002). Trans-resveratrol (3,4′,5-trihydroxystilbene) is the basic structure of the stilbene family. Stilbenes also exist as glucosylated derivatives like piceid (3-O-β-d-glucoside of resveratrol) (Waterhouse and Lamuela-Raventós 1994) and oligomers like ε-viniferin (a cylic resveratrol dehydrodimer) (Langcake and Pryce 1977). In recent years, much attention has been devoted to stilbenes because of their biological benefits on human health (Vang et al. 2011), such as anticancer (Riha et al. 2014) and cardioprotection (Palomer et al. 2013).
Stilbenes are synthesized by the phenylpropanoid pathway. Phenylalanine ammonia lyase (PAL, EC 4.3.1.24), cinnamate 4-hydroxylase (C4H, EC 1.14.13.11) and 4-coumarate: CoA ligase (4CL, EC 6.2.1.12) catalyze phenylalanine to form 4-coumaroyl-CoA (Dixon and Paiva 1995). Stilbene synthase (STS, EC 2.3.1.95) catalyzes a condensation reaction of 4-coumaroyl-CoA and three molecules malonyl-CoA to synthesis resveratrol (Soleas et al. 1997). Chalcone synthase (CHS, EC 2.3.1.74) utilizes the same substrates as STS for the production of chalcone, the precursor of flavonoid metabolism (Winkel-Shirley 2001). 4CL, STS and CHS constitute the branching point of stilbene and flavonoid pathway.
Stilbenes can be induced by a number of stresses, such as ultraviolet irradiation (Wang et al. 2010b), pathogen attack (Pezet et al. 2004) and biotic or chemical elicitation including methyl jasmonate, polysaccharide, hydrogen peroxide and cyclodextrin (Belhadj et al. 2008; Cai et al. 2012; Nopo-Olazabal et al. 2014).
Plant cell suspension cultures represent a convenient model for studying the regulation of plant secondary mechanisms (Larronde et al. 1998). Plant cell cultures have been used for the production of secondary metabolites such as alkaloids and isoflavones under in vitro conditions (Zhao et al. 2001; Sansanelli et al. 2014). Precursor feeding and elicitor application are effective strategies for secondary metabolites accumulation in suitable plant cell cultures (Qu et al. 2011; Shumakova et al. 2011). Numerous studies have focused on Vitis vinifera cell cultures to investigate the production of stilbene (Tassoni et al. 2005) and the expression of genes involved in stilbene biosynthesis (Belhadj et al. 2008).
Ultraviolet C (UV-C) irradiation promotes the phenylpropanoid pathway and stimulates flavonoid synthesis (Dixon and Paiva 1995). UV-C irradiation is an effective method to enhance stilbene production in V. vinifera berries (Wang et al. 2013), V. vinifera leaves (Wang et al. 2010b) and grape calli of different genotypes (Liu et al. 2010).
Jasmonic acid (JA) is an important signal molecule of plant in response to wound and pathogen attack (Wasternack and Parthier 1997). JA and its more active derivative methyl jasmonate (MeJA) can induce the production of a wide range of plant secondary metabolites such as rosmarinic acid, terpenoid indole alkaloid and plumbagin in various cell cultures (Krzyzanowska et al. 2012; Almagro et al. 2014; Silja et al. 2014). JA and MeJA have been used as elicitors for stilbene biosynthesis in V. vinifera foliar cuttings (Belhadj et al. 2006), V. vinifera cell cultures (Tassoni et al. 2005) and V. rotundifolia hairy root cultures (Nopo-Olazabal et al. 2014). The addition of MeJA to V. vinifera cell cultures also promoted anthocyanin accumulation (Tassoni et al. 2012).
Salicylic acid (SA) plays a central role in plant defense mechanisms against pathogens (Durner et al. 1997). SA infiltration into entire V. vinifera berries enhanced the accumulation of PAL mRNA, as well as and the synthesis of new PAL protein and enzyme activities (Chen et al. 2006). Resveratrol accumulation in Arachis hypogaea leaves increased after SA treatment, and the expression levels of STS were also up-regulated (Chung et al. 2003).
In recent years, combined elicitors have been used to study the synergistic or antagonistic effects on stilbene production. The combined treatment of MeJA and cyclodextrins enhanced more extracellular trans-resveratrol accumulation in V. vinifera cell cultures than each elicitor used alone, but its production was lower when the elicitors were combined with UV-C irradiation (Belchí-Navarro et al. 2012). MeJA in combination with sucrose triggered more extracellular trans-resveratrol accumulation in V. vinifera cell cultures (Belhadj et al. 2008). The combined treatment of CaCl2 and UV-C irradiation significantly increased resveratrol content in V. viniferin × V. amurensis leaves and V. vinifera berries (Wang et al. 2013).
The aim of our present work was to investigate the effects of two biotic elicitors (MeJA and SA) and one abiotic elicitor (UV-C), alone or in combination, on stilbene accumulation in V. vinifera cell suspension cultures. We monitored the dynamic production of both intracellular stilbene and trans-resveratrol release into the culture medium over a 96-h period. We also analyzed the relative expression of five genes (PAL, C4H, 4CL, STS and CHS) involved in stilbene and flavonoid biosynthesis to investigate the relationship between stilbene accumulation and the regulation of gene expression.
Materials and methods
Grape cell suspension cultures and culture conditions
Grape calli were established from V. vinifera L. cv. Cabernet Sauvignon berries by Wang et al. (2010a) and maintained on solid B5 medium (Gamborg et al. 1968) supplemented with 3 g l−1 phytagel, 30 g l−1 sucrose, 250 mg l−1 casein hydrolysate, 0.1 mg l−1 α-naphthylacetic acid and 0.2 mg l−1 kinetin, adjusted to pH 5.9. Subcultures of the calli were carried out every 28 days. V. vinifera cell suspension cultures were established by inoculating cell aggregates in 150 ml flasks containing 60 ml of liquid medium as described above (without phytagel). Cell cultures were maintained in a rotary shaker at 110 rpm in continuous light (200 μmol m−2 s−1) at 26 ± 1 °C. Subcultures were performed every 7 days by transferring 15 ml of cell cultures into 45 ml of fresh medium.
Elicitor treatments on V. vinifera cell suspension cultures
Elicitation experiments were performed using 4-day-old V. vinifera cell cultures. A UV-C lamp (254 nm, Model ZQJ-254, China) at 8 cm above (output 10 W m−2) was used as the UV light source. MeJA (Sigma-Aldrich, USA) was dissolved in 50 % (v/v) ethanol at concentrations of 25, 50, 75 and 100 mM. SA (Sigma-Aldrich) was dissolved in methanol at concentrations of 25, 50, 75 and 100 mM.
Five individual sub experiments were designed. All treatments were performed using three biological replicates. (1) UV-C irradiation. Cell cultures were transferred into petri dishes and irradiated with UV-C for durations of 10, 20 or 30 min (total exposure dosages were 6, 12 or 18 kJ m−2, respectively), and then cell cultures were transferred back into the flasks and incubated at 110 rpm in continuous light (200 μmol m−2 s−1) at 26 ± 1 °C. Cell cultures without UV-C irradiation were used as the control group. The most effective dosage of UV-C irradiation for stilbene synthesis was chosen according to the results. (2) MeJA elicitation. Filter-sterilized MeJA solution at each concentration was added into cell cultures to obtain the final concentration of 50, 100, 150 or 200 μM. Untreated cell cultures and cell cultures treated with equal volumes of filter-sterilized 50 % (v/v) ethanol (final concentration was 0.1 %, v/v) were used as the control groups. The most effective concentration of MeJA for stilbene synthesis was chosen according to the results. (3) SA elicitation. Filter-sterilized SA solution at each concentration was added into cell cultures to obtain the final concentration of 50, 100, 150 or 200 μM. Untreated cell cultures and cell cultures treated with equal volumes of filter-sterilized methanol (final concentration was 0.2 %, v/v) were used as the control groups. The most effective concentration of SA for stilbene synthesis was chosen according to the results. (4) Combined treatment of UV-C and MeJA. Cell cultures were incubated with MeJA (100 μM) for 30 min at 110 rpm in continuous light (200 μmol m−2 s−1) at 26 ± 1 °C, and then cell cultures were transferred into petri dishes and irradiated with UV-C for 20 min. Untreated cell cultures and cell cultures treated with 0.1 % (v/v) ethanol were used as the control groups. (5) Combined treatment of UV-C and SA. Cell cultures were incubated with SA (100 μM) for 30 min at 110 rpm in continuous light (200 μmol m−2 s−1) at 26 ± 1 °C, and then cell cultures were transferred into petri dishes and irradiated with UV-C for 20 min. Untreated cell cultures and cell cultures treated with 0.2 % (v/v) methanol were used as the control groups. All of the cell cultures subjected to elicitations and control cell cultures were incubated at 110 rpm in continuous light (200 μmol m−2 s−1) at 26 ± 1 °C. Samples were collected at 0, 6, 12, 24, 36, 48, 72 and 96 h after treatment. Cells were harvested by vacuum filtration, rapidly washed, weighed to determine fresh weight (FW), frozen in liquid nitrogen and stored at −70 °C until analysis. The culture medium was collected, and then centrifuged at 5,000 rpm for 10 min to remove cell residues and stored at −40 °C until analysis.
Determination of cell growth
The growth of cell cultures was monitored by determining dry weight (DW). About 1 g FW of filtered cells were placed at 105 °C overnight until reaching a constant weight, and then they were weighed to determine DW. Cell viability was measured as described by Larkins (1976). Briefly, 0.5 ml of cell cultures was introduced in a test tube, and then fluorescein diacetate solution (5 mg ml−1 in acetone) was added to give a final concentration of 100 μg ml−1. After 5 min at room temperature, the cells were observed under fluorescence. The results were expressed as percentage of living cells.
Extraction and quantification of phenolic compounds
All measurements of phenolic compounds were performed in triplicate.
Stilbenes were extracted from the cells and culture medium as described by Tassoni et al. (2005). Briefly, 1 g FW of cells were ground into powder in liquid nitrogen and homogenized with 5 ml of 95 % (v/v) methanol. The homogenate was incubated for 1 h in the dark at room temperature in a rotatory shaker at 100 rpm, and then filtered under vacuum. The extract was concentrated to an aqueous phase under vacuum for 15 min at 40 °C. Stilbenes were extracted from the aqueous phase by adding 5 ml of 5 % (w/v) sodium bicarbonate, 10 ml of ethyl acetate and energic vortexing for 1 min, and then the ethyl acetate phase was vacuum-dried at 40 °C. Stilbenes were extracted from 20 ml of culture medium after addition of 10 ml of 5 % (w/v) sodium bicarbonate, 20 ml of ethyl acetate and energic vortexing for 1 min, and then the ethyl acetate phase was vacuum-dried at 40 °C. The dried samples (cells or medium extractions) were re-dissolved in 1 ml of methanol and stored at −40 °C until analysis.
Chromatographic separation was performed using a DIKMA column (Inertsil ODS-3, 250 × 4.6 mm, 5 μm partical size) protected by a guard column of the same material (DIKMA, Japan). Analysis of stilbenes was carried out on a Waters 2695 HPLC system fitted with a 2996 Photodiode Array Detector (Waters, USA). Stilbenes were quantified as described by Pezet et al. (2003). The injection volume was 10 μl. Separation was performed at a flow rate of 1 ml min−1 with the mobile phases consisting of acetonitrile (A) and ultra pure water (B). The solvent gradient was as follows: 0–1 min with 20 % of solvent A; 1–31 min with 20–75 % of solvent A; 31–33 min with 75–100 % of solvent A; 33–36 min with 100 % of solvent A; 36–37 min with 100–20 % of solvent A; and 37–41 min with 20 % of solvent A. The column temperature was room temperature for HPLC.
Stilbene standards, including trans-resveratrol, trans-piceid and ε-viniferin, were purchased from Sigma-Aldrich and dissolved in methanol. Cis-resveratrol and cis-piceid standards were obtained by UV-C irradiation (output 10 W m−2) for 30 min (total exposure dosage was 18 kJ m−2) on the mixed solution of trans-resveratrol and trans-piceid standards. The conversion coefficients were 37.1 % for trans-resveratrol and 42.6 % for trans-piceid, respectively. HPLC detection of stilbenes was at 307 nm, and calibration curves were calculated for each stilbene on the basis of six different concentrations from 0.05 to 100 μg ml−1. Total stilbene content indicates the sum of trans-piceid, cis-piceid, trans-resveratrol, cis-resveratrol and ε-viniferin contents.
Total phenolics were measured according to the Folin-Ciocalteu reagent method as described by Pastrana-Bonilla et al. (2003). Briefly, 1 g FW of cells were ground into powder in liquid nitrogen and then extracted in 2 % (v/v) HCl in methanol for 24 h in the dark and at room temperature. After centrifugation at 12,000 rpm for 20 min at 4 °C, the supernatant was diluted with the same solvent used for extraction to a suitable concentration for analysis. Two hundred microliters of sample extract was introduced in a test tube, 1 ml of Folin-Ciocalteu reagent (Sigma-Aldrich) and 0.8 ml of 7.5 % (w/v) sodium carbonate were added, and the contents were mixed and allowed to stand for 30 min. Absorption at 765 nm was measured in a Shimadzu UV–Vis spectrophotometer (Shimadzu UV-1601). Total phenolics content was expressed as gallic acid in milligrams per gram DW of sample, using a standard curve generated with 50, 100, 150, 200, 250, 300, 350, 400, 450 and 500 mg l−1 of gallic acid (Sigma-Aldrich).
Total flavonoids content was measured using a colorimetric method as described by Wolfe et al. (2003). Briefly, a volume of 0.25 ml of a known dilution of total phenolics extract was added to a test tube containing 1.25 ml of distilled water. To the mixture was added 0.075 ml of 5 % (w/v) sodium nitrite solution, and this was allowed to stand for 5 min. Then, 0.15 ml of 10 % (w/v) aluminum chloride was added. After 6 min, 0.5 ml of 1 M sodium hydroxide was added, and the mixture was diluted with another 0.275 ml of distilled water. Absorption at 510 nm was measured in a Shimadzu UV–Vis spectrophotometer (Shimadzu UV-1601). Total flavonoids content was expressed as catechin equivalent in milligrams per gram DW of sample, using a standard curve generated with 50, 100, 150, 200, 250, 300 mg l−1 of catechin (Sigma-Aldrich).
RNA isolation, cDNA preparation and quantitative real-time polymerase chain reaction (qPCR) analysis
Total RNA was isolated from cells using the Column Plant RNAOUT 2.0 Kit (Tiandz, China), and cDNA was prepared using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fischer Scientific, USA), according to the manufacturer’s protocol. The amplification of Actin1 was used as the internal reference gene to normalize the expression of selected genes. PAL, C4H, STS, CHS and Actin1 primers sequences were designed based on V. vinifera nucleotide sequences deposited in GenBank, using Primer Premier 5 and Oligo 7, and 4CL primers have been described by Wang et al. (2013). All primer pairs are given in Table 1.
Two-step qPCR was performed using a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, USA). Each reaction was performed in triplicate with a reaction volume of 20 μl containing: 1 μl of template (50 ng cDNA), 1 μl of each primer (400 nM, final concentration), 10 μl of iTaq Universal SYBR Green Supermix (Bio-Rad, USA) and 7 μl of nuclease-free sterile water (Amresco, USA). The following thermal cycling protocol was used for each qPCR essay: 95 °C for 5 min, followed by 40 cycles (95 °C for 5 s and 60 °C for 30 s). The melting curve analysis was performed from 65 to 95 °C. Data were analyzed using the CFX Manager Software 1.6 (Bio-Rad, USA). Relative mRNA ratios were calculated using the 2−ΔΔCT method (Livak and Schmittgen 2001). Untreated cells (at time zero) were considered as the reference sample. For each gene, the reference sample was defined as expression = 1, and results were expressed as the fold-changes compared to the reference sample. Values represented the average of three biological replicates.
Statistical analysis
All experiments were performed using three biological replicates, and all measurements were analyzed in triplicate. The obtained data were expressed as mean ± SE. Graphs of the experimental data were developed using OriginPro 8.1 software for Windows (OriginLab Corporation, USA). Statistical analysis was performed by analysis of variance using Student’s t test and Duncan’s multiple range test, using SPSS version 20 (SPSS Inc, USA). Differences at p < 0.05 were considered statistically significant.
Results
Growth kinetics of V. vinifera cell cultures under elicitations
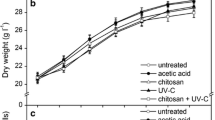
Vitis vinifera L. cv. Cabernet Sauvignon cell suspension cultures were treated with UV-C, MeJA and SA, alone or in combination. The effect of elicitations on cell growth was monitored by dry weight (Fig. 1). The DW in cells treated with UV-C for 10 or 20 min showed no significant difference from in untreated cell cultures, but UV-C for 30 min triggered a significant DW reduction (Fig. 1a). The cell growth was not significantly affected by the addition of 0.1 % (v/v) ethanol or 0.2 % (v/v) methanol, and the addition of MeJA or SA at lower concentrations (50 and 100 μM) (Fig. 1b, c). However, MeJA or SA at higher concentrations (150 and 200 μM) caused a significant decline of DW. The optimal dosage/concentration for stilbene induction in V. vinifera cell cultures were chosen as described below; and UV-C (20 min) together with MeJA (100 μM) or SA (100 μM) were applied to investigate the effect of combined elicitation on stilbene biosynthesis. As shown in Fig. 1d, cell cultures treated by the combined elicitation of UV-C together with MeJA or SA showed a similar trend of DW increase, which was not significantly different from untreated cell cultures. As shown in Fig. 2, treatments at the optimal dosage/concentration, alone or in combination, did not significantly influence cell viability (p > 0.05) with more than 80 % of living cells.
Growth curves of V. vinifera cell cultures treated with UV-C irradiation (a), MeJA elicitation (b), SA elicitation (c) and the combined elicitation of UV-C together with MeJA or SA (d). *Statistically significant difference (Student’s t test, p < 0.05) between cells under elicitation and untreated cells at each time point. Values are mean ± SE (n = 3)
Effect of individual elicitation of UV-C, MeJA or SA on stilbene production in V. vinifera cell cultures
Vitis vinifera cell cultures were treated with different dosages of UV-C irradiation and different concentrations of MeJA or SA in order to determine the optimal elicitor dosage/concentration for the induction of stilbene production. Trans-piceid, cis-piceid and trans-resveratrol were observed inside all cells, and ε-viniferin appeared only after elicitations. Cis-resveratrol was undetectable during the whole experimental period. Only trans-resveratrol was detected in the culture medium after elicitations.
The changes of stilbene content in V. vinifera cell cultures subjected to UV-C irradiation are shown in Fig. 3. Stilbene content of untreated cells maintained low and constant during the experimental period. UV-C irradiation significantly promoted intracellular stilbene accumulation. Trans-piceid content (Fig. 3a) increased gradually from 24 h to the maximum at 96 h. Cis-piceid content (Fig. 3b) peaked at 24 h and declined. Trans-resveratrol content (Fig. 3c) started to increase at 12 h and peaked at 48 h, and then declined rapidly. ε-viniferin (Fig. 3d) was not observed until 24 h, and its content increased gradually to the maximum between 48 and 72 h. Total intracellular stilbene content (Fig. 3e) in cell cultures treated with UV-C for 10, 20 and 30 min reached the maximum between 72 and 96 h (1171.78 ± 37.41, 1400.32 ± 55.53 and 1365.06 ± 48.25 μg g−1 DW, respectively), which was 2.21-, 2.62- and 2.55-fold higher than in untreated cells, respectively. Extracellular trans-resveratrol (Fig. 3f) appeared since 24 h, and its concentration peaked at 72 h (0.8 ± 0.06, 1.49 ± 0.09 and 1.15 ± 0.06 mg l−1, respectively, in the culture medium subjected to UV-C irradiation for 10, 20 and 30 min). UV-C irradiation for 20 or 30 min induced a higher accumulation of stilbene than that for 10 min, but 30 min irradiation caused a significant biomass reduction (Fig. 1a). Therefore, 20 min was chosen as the optimal dosage for UV-C irradiation.
Changes of stilbene content in V. vinifera cell cultures treated with UV-C irradiation over a 96-h time course. Intracellular trans-piceid (a), intracellular cis-piceid (b), intracellular trans-resveratrol (c), intracellular ε-viniferin (d) and extracellular trans-resveratrol (f) contents were determined by HPLC, and total intracellular stilbene content (e) indicates the sum of a, b, c and d. Different letters indicate the statistically significant differences (Duncan’s multiple range test, p < 0.05) among different treatments at each time point. Values are mean ± SE (n = 3)
The changes of stilbene content in V. vinifera cell cultures subjected to MeJA elicitation are shown in Fig. 4. The addition of 0.1 % (v/v) ethanol did not significantly affect stilbene production during the experimental period. MeJA highly enhanced intracellular stilbene production, and the effects depended on MeJA concentrations. Trans-piceid (Fig. 4a) and cis-piceid (Fig. 4b) contents increased gradually to the maximum at 96 h. Trans-resveratrol content (Fig. 4c) was induced to two peaks at 12 h and 48 h, and then declined. ε-viniferin (Fig. 4d) appeared since 48 h in cells treated with MeJA at 50 μM or since 12 h in cells treated with MeJA at 100, 150 and 200 μM, and reached the maximal content within 72–96 h. Total intracellular stilbene content (Fig. 4e) increased gradually and remained stable after 48 h. The maximum of total intracellular stibene content in cells treated with MeJA at 50, 100, 150 and 200 μM was obtained between 48 and 96 h (868.98 ± 24.57, 1253.39 ± 29.77, 1222.79 ± 23.54 and 1159.4 ± 31.52 μg g−1 DW, respectively), which was 1.62-, 2.34-, 2.3- and 2.11-fold higher than in untreated cells, respectively. Extracellular trans-resveratrol (Fig. 4f) was first detected at 24 h, and its concentration increased gradually to the maximum within 72–96 h (0.53 ± 0.03, 1.74 ± 0.02, 1.63 ± 0.09 and 1.55 ± 0.12 mg l−1, respectively, in the culture medium treated with MeJA at 50, 100, 150 and 200 μM). Stilbene accumulation in cell cultures treated with a MeJA concentration of 100 or 150 μM was significantly higher than other groups, but MeJA at 150 μM caused a significant biomass reduction (Fig. 1b). Therefore, 100 μM was chosen as the optimal concentration for MeJA elicitation.
Changes of stilbene content in V. vinifera cell cultures treated with MeJA over a 96-h time course. Intracellular trans-piceid (a), intracellular cis-piceid (b), intracellular trans-resveratrol (c), intracellular ε-viniferin (d) and extracellular trans-resveratrol (f) contents were determined by HPLC, and total intracellular stilbene content (e) indicates the sum of a, b, c and d. Different letters indicate the statistically significant differences (Duncan’s multiple range test, p < 0.05) among different treatments at each time point. Values are mean ± SE (n = 3)
The changes of stilbene content in V. vinifera cell cultures subjected to SA elicitation are shown in Fig. 5. Only intracellular stilbene was detected, and ε-viniferin was not observed along the time course. The addition of 0.2 % (v/v) methanol did not significantly affect stilbene production during the experimental period. Trans-piceid (Fig. 5a) and cis-piceid (Fig. 5b) contents increased gradually to the maximum within 72–96 h. Trans-resveratrol content (Fig. 5c) peaked at 48 h and decreased. Total intracellular stilbene content (Fig. 5d) in cells treated with SA at 50, 100, 150 and 200 μM reached the maximum between 72 and 96 h (617.6 ± 7.86, 709.82 ± 18.08, 711.19 ± 16.18 and 700.18 ± 16.61 μg g−1 DW, respectively), which was 1.15-, 1.34-, 1.33- and 1.31-fold higher than in untreated cells, respectively. Stilbene production showed no significant difference in cells treated with SA at 100, 150 and 200 μM, and SA at 50 μM was less effective. However, SA at 150 or 200 μM caused a significant biomass reduction (Fig. 1c). Therefore, 100 μM was chosen as the optimal concentration for SA elicitation.
Changes of stilbene content in V. vinifera cell cultures treated with SA over a 96-h time course. Intracellular trans-piceid (a), intracellular cis-piceid (b) and intracellular trans-resveratrol (c) contents were determined by HPLC, and total intracellular stilbene content (d) indicates the sum of a, b and c. Different letters indicate the statistically significant differences (Duncan’s multiple range test, p < 0.05) among different treatments at each time point. Values are mean ± SE (n = 3)
Effect of the combined elicitation of UV-C together with MeJA or SA on stilbene production in V. vinifera cell cultures
The changes of stilbene content in V. vinifera cell cultures subjected to the combined elicitation of UV-C (20 min) together with MeJA (100 μM) or SA (100 μM) are shown in Fig. 6. In cells treated with 0.1 % (v/v) ethanol or 0.2 % (v/v) methanol, stilbene content maintained low and constant throughout the experimental period and showed no significant difference from untreated cells.
Changes of stilbene content in V. vinifera cell cultures treated with the combined elicitation of UV-C together with MeJA or SA over a 96-h time course. Intracellular trans-piceid (a), intracellular cis-piceid (b), intracellular trans-resveratrol (c), intracellular ε-viniferin (d) and extracellular trans-resveratrol (f) contents were determined by HPLC, and total intracellular stilbene content (e) indicates the sum of a, b, c and d. For a, b, c and e, different letters indicate the statistically significant differences (Duncan’s multiple range test, Student’s t test, p < 0.05) among different treatments at each time point. For d and f, *statistically significant difference (Student’s t test, p < 0.05) between the two treatments at each time point. Values are mean ± SE (n = 3)
The combined elicitation of UV-C and MeJA triggered more intracellular stilbene accumulation than UV-C (Fig. 3) and MeJA (Fig. 4) individual treatments. Trans-piceid content (Fig. 6a) increased gradually to the maximum at 96 h, and cis-piceid, trans-resveratrol and ε-viniferin contents (Fig. 6b–d) reached the maximum within 36–48 h. Total intracellular stilbene content (Fig. 6e) increased to the maximum at 48 h (2005.05 ± 63.03 μg g−1 DW), which was 3.65-fold higher than in untreated cells. Extracellular trans-resveratrol (Fig. 6f) appeared since 12 h, and its concentration accumulated to the maximum at 72 h (3.96 ± 0.2 mg l−1), which was 2.65- and 2.27-fold higher than in UV-C and MeJA individual treatments, respectively. The combined treatment of UV-C and MeJA led to a higher extracellular trans-resveratrol accumulation than that obtained for the sum of both individual treatments, so this result can be considered as a synergistic effect.
Stilbene response in cell cultures subjected to the combined treatment of UV-C and SA was similar to that in the case of the combined treatment of UV-C and MeJA. Total intracellular stilbene content (Fig. 6e) reached the maximum at 72 h (1630.93 ± 44.17 μg g−1 DW), which was 3.07-fold higher than in untreated cells. Extracellular trans-resveratrol (Fig. 5f) appeared since 24 h, and its concentration reached the maximum at 72 h (2.33 ± 0.15 mg l−1), which was 1.56-fold higher than in UV-C individual treatment.
Effect of UV-C, MeJA and SA, alone or in combination, on total phenolics and total flavonoids contents in V. vinifera cell cultures
The changes of intracellular total phenolics and total flavonoids contents in cells treated with UV-C (20 min), MeJA (100 μM) and SA (100 μM), alone or in combination, are shown in Fig. 7. The addition of 0.1 % (v/v) ethanol or 0.2 % (v/v) methanol did not significantly affect total phenolics and flavonoids contents.
Changes of intracellular total phenolics (a) and total flavonoids (b) contents in V. vinifera cell cultures treated with UV-C, MeJA and SA, alone or in combination, over a 96-h time course. Different letters indicate the statistically significant differences (Duncan’s multiple range test, p < 0.05) among different treatments at each time point. Values are mean ± SE (n = 3)
As shown in Fig. 7a, total phenolics content in cells subjected to UV-C and MeJA individual treatments reached the maximum at 48 h, which was 1.49- and 1.39-fold higher than in untreated cells, respectively. The combined treatment of UV-C and MeJA highly promoted total phenolics content to the maximum at 36 h, which was 1.95-fold higher than in untreated cells. SA did not significantly influence total phenolics content. The combination of UV-C and SA increased total phenolics content to the maximum at 36 h, which was 1.72-fold higher than in untreated cells.
Total flavonoids content (Fig. 7b) was induced to the peak at 48 h in cells subjected to UV-C and MeJA individual treatments, which was 2.1- and 2-fold higher than in untreated cells, respectively. The combined treatment of UV-C and MeJA enhanced total flavonoids content to a 2.99-fold higher level than in untreated cells at 36 h. In cells treated with SA, total flavonoid content increased to the peak at 48 h (1.49-fold higher than in untreated cells), and then decreased to the control levels. Total flavonoids content in cells treated with the combination of UV-C and SA reached the peak at 36 h with a 2.56-fold increase.
Effect of UV-C, MeJA and SA, alone or in combination, on expression of genes involved in stilbene and flavonoid biosynthesis in V. vinifera cell cultures
The changes of the expression levels of five genes (PAL, C4H, 4CL, STS and CHS) encoding enzymes involved in stilbene and flavonoid biosynthesis in cells treated with UV-C (20 min), MeJA (100 μM) and SA (100 μM), alone or in combination, are shown in Fig. 8. In untreated cells, there was no significant transcript change of these genes during the experimental period. The addition of 0.1 % (v/v) ethanol or 0.2 % (v/v) methanol did not significantly affect the expression patterns of these genes.
Changes of relative expression of PAL (a), C4H (b), 4CL (c), STS (d) and CHS (e) in V. vinifera cell cultures treated with UV-C, MeJA and SA, alone or in combination, over a 96-h time course. Different letters indicate the statistically significant differences (Duncan’s multiple range test, p < 0.05) among different treatments at each time point. Values are mean ± SE (n = 3)
PAL, C4H and 4CL (Fig. 8a–c) are key genes encoding enzymes involved in the phenylpropanoid pathway. There were similar trends in the expression profiles of PAL, C4H and 4CL after elicitations. The expression levels of these three genes reached the maximum between 24 and 36 h in cells subjected to UV-C and MeJA individual elicitations. The expression levels of PAL, C4H and 4CL were induced to a peak within 24–36 h after the combined elicitation of UV-C and MeJA, which were 5.52-, 4.62- and 6.01-fold higher than in untreated cells, respectively. SA induced the expression levels of these three genes to an approximately 2-fold increase at 36 h. The expression levels of these genes were highly induced by the combined treatment of UV-C and SA to the maximum within 24–36 h, which was 4.46-, 4.08- and 4.63-fold higher than in untreated cells for PAL, C4H and 4CL, respectively.
STS (Fig. 8d) is the crucial gene encoding the enzyme responsible for stilbene synthesis. In cells treated with UV-C, the expression levels of STS were induced to a 17.38-fold accumulation at 24 h, and then declined gradually to control levels. MeJA induced the expression levels of STS to two peaks at 6 and 36 h (25.38- and 52.85-fold higher than in untreated cells, respectively). The expression levels of STS significantly increased after the combined elicitation of UV-C and MeJA, reaching an 80.15-fold accumulation at 24 h. SA slightly induced the expression levels of STS to a 4-fold increase at 36 h, although it was not significant. The combined treatment of UV-C and SA induced the expression levels of STS to the peak at 24 h, which was 40.63-fold higher than in untreated cells. The maximum of STS expression under the combined treatment of UV-C and MeJA was higher than that obtained for the sum of UV-C and MeJA individual treatments. Therefore, this result revealed a synergistic effect of UV-C and MeJA on STS expression. Also, there was a synergistic interaction between UV-C and SA on STS expression.
CHS (Fig. 8e) encodes the entrance enzyme of flavonoid synthesis. In cells treated with UV-C, the expression levels of CHS peaked at 48 h (4.64-fold higher than in untreated cells) and declined. The expression levels of CHS were induced to a 3.58-fold increase at 24 h after MeJA treatment. The combined treatment of UV-C and MeJA highly induced the expression levels of CHS to the peak at 24 h (11.15-fold higher than in untreated cells). The expression levels of CHS accumulated with a 2.25-fold increase at 48 h after SA treatment, and then declined to control levels. The expression levels of CHS reached a 6.12-fold increase at 36 h in cells treated with the combination of UV-C and SA. A synergistic effect was observed between UV-C and MeJA on CHS expression, since the peak of CHS expression under the combined treatment was higher than that obtained for the sum of the two individual treatments.
Discussion
In the present work, we observed that the combined elicitation of UV-C together with MeJA or SA, significantly stimulated the biosynthesis of stilbene and flavonoid in V. vinifera cell cultures.
The cell growth of V. vinifera cell cultures was not inhibited by UV-C irradiation at low dosages (10 or 20 min), but UV-C for 30 min triggered a significant biomass reduction. This result was in agreement with Liu et al. (2010), who observed a negative effect on growth index of V. labrusca × V. riparia calli after 30 min UV-C irradiation. MeJA or SA at lower concentration (50 or 100 μM) did not affect cell growth, but the biomass accumulation decreased when the concentration of elicitor was higher. The biomass reduction in MeJA-elicited cell cultures at high concentrations was in agreement with the previous studies on Fagopyrum esculentum (Hu et al. 2011) and Mentha × piperita (Krzyzanowska et al. 2012) cell cultures. In the previous studies, it was found that SA inhibited the biomass of Hypericum perforatum calli and cell cultures (Gadzovska et al. 2013), and increase of SA concentration strongly suppressed the growth of Rubia cordifolia calli (Bulgakov et al. 2002). In this study, the production of stilbene and flavonoid was enhanced by elicitations without loss of biomass and cell viability, and this could be regarded as a prerequisite for obtaining secondary metabolites in plant cell cultures.
Both intracellular stilbene and trans-resveratrol release into culture medium were promoted in V. vinifera cell cultures treated with UV-C, MeJA and SA, alone or in combination. Intracellular trans-resveratrol was induced to a transient accumulation in cells after treatments, but intracellular trans-piceid content maintained high and stable after reaching the maximum. The peak of intracellular ε-viniferin and extracellular trans-resveratrol appeared later than that of the increase of intracellular trans-resveratrol content. It has been reported that piceid is the stored and/or transported form of resveratrol (Morales et al. 1998), and resveratrol can be converted into more toxic derivatives, such as ε-viniferin (Pezet et al. 2004). The results suggested that resveratrol might be first synthesized in large amount in the cells, and then resveratrol might be excreted into the culture medium or utilized to form other derivatives in response to the biotic or abiotic stresses.
Postharvest UV-C irradiation has been widely used to improve fruit quality. UV-C irradiation stimulated the phenylpropanoid pathway with the accumulation of the corresponding phenolic compounds, such as stilbene in V. vinifera berry skins (Wang et al. 2013) and flavonoid in Vaccinium corymbosum berries (Wang et al. 2009). UV-C treatment enhanced both stilbene and anthocyanin contents in V. vinifera berry skins, and the induction capacity depended on the dosages of irradiation (Crupi et al. 2013) and grape varieties (Guerrero et al. 2010). In this study, UV-C strongly promoted stilbene accumulation in V. vinifera cell cultures, and the effect depended on UV-C dosages. These results were in agreement with the findings of Liu et al. (2010) on UV-C irradiated grape calli. To the best of our knowledge, we reported, for the first time, data on the release of trans-resveratrol in V. vinifera L. cv. Cabernet Sauvignon culture medium subjected to UV-C individual treatment. Previous studies showed that the expression levels of genes encoding enzymes involved in stilbene biosynthesis were increased in V. vinifera × V. amurensis leaves after UV-C irradiation (Wang et al. 2013), and CHS mRNA was strongly induced in UV-irradiated Petroselinum crispum cells (Logemann et al. 2000). In the present study, UV-C irradiation highly induced transcript accumulation of genes associated with stilbene and flavonoid biosynthesis. The results suggested that UV-C irradiation may enhance the production of corresponding stilbene and flavonoid by inducing the expression of the related genes.
MeJA has been used as an important elicitor on the production of secondary metabolites. The phenylpropanoid pathway can be induced by MeJA elicitation in cell cultures of various plant species, such as Arnebia euchroma (Wang et al. 2014) and Artemisia absinthium (Ali et al. 2015). In this study, the results showed that MeJA induced two peaks of STS expression at 6 and 36 h, and intracellular trans-resveratrol content was also stimulated to two peaks at 12 and 48 h. The peaks of STS expression occurred earlier than those of the increase of intracellular trans-resveratrol content. Therefore, the results suggested that the enhancement of stilbene production in V. vinifera cell cultures subjected to MeJA may be obtained by the up-regulation of STS expression. Stilbene production in V. vinifera cell cultures could be significantly enhanced by the combined treatments of MeJA together with physical elicitors, such as MeJA plus red light (Tassoni et al. 2012) and MeJA in combination with low energy ultrasound (Santamaria et al. 2012). In the present work, the combined treatment of UV-C and MeJA induced a higher stilbene production in V. vinifera cell cultures, more than each individual treatment. This result was in agreement with the findings of Fernández-Marín et al. (2014), who reported that wine achieved by V. vinifera berries under combination of MeJA and UV-C treatments contained significantly higher stilbene concentration. Additionally, a positive synergic effect was observed on extracellular trans-resveratrol accumulation and STS expression under the combined elicitation of UV-C and MeJA. It seemed that the synergistic interaction of UV-C and MeJA on extracellular trans-resveratrol accumulation could be the result of the synergistic effect on STS expression. Besides, the maximum of intracellular stilbenes in cells subjected to the combined treatment appeared earlier than UV-C and MeJA individual treatments. The results suggested that the addition of MeJA may accelerate the induction of stilbene production by UV-C irradiation.
SA has long been recognized as a signal molecule which enhances plant defense mechanism and modulates plant secondary metabolites production. SA elicited the production of phenylpropanoids in H. perforatum shoots, calli and cell cultures (Gadzovska et al. 2013). SA in vivo incubation promoted flavonol production in V. vinifera berry tissues (Fang and Huang 2013). Our present results showed that SA was less efficient in stilbene production than UV-C or MeJA. However, the combined treatment of UV-C and SA highly enhanced stilbene production, more than each individual treatment, and there was also a synergistic interaction between UV-C and SA on STS expression. The results suggested that the presence of SA may reinforce the effect of UV-C irradiation on stilbene production.
4CL, STS and CHS constitute the branching point of stilbene and flavonoid biosynthesis. In the present study, elicitations of UV-C, MeJA and SA, alone or in combination, highly promoted the entire phenylpropanoid pathway, and no preferential orientation was found at this branching point. UV-C in presence of MeJA displayed a positive synergistic effect on up-regulation of CHS transcript with a higher production of total flavonoids; and the combined treatment of UV-C and SA also led to more flavonoid accumulation than each individual treatment. The enhancement of stilbene production did not affect the accumulation of flavonoid, which is an important parameter of wine-making. The results suggested that the combined treatment of UV-C together with MeJA or SA can be a good way to produce stilbene-enriched grapes and wines without negative effects on wine quality.
In conclusion, the present study demonstrated that elicitations of UV-C, MeJA and SA, alone or in combination, had a significant effect on the enhancement of stilbene production, as well as total phenolics and total flavonoids contents, in V. vinifera cell suspension cultures. Elicitations at the optimal dosage or concentration did not exert a negative effect on cell growth. Relative expression of genes involved in stilbene and flavonoid synthesis was also highly up-regulated, and there was a synergistic effect of UV-C together with MeJA or SA on STS expression. The combined application of UV-C together with MeJA or SA can be an efficient way to enhance stilbene production in V. vinifera cell cultures. Further experiments will be required to confirm the effect of the combined use of UV-C and SA in grape berries in order to produce stilbene-enriched grapes, which can be a good resource for healthy-winemaking. The molecular mechanism and the signal transduction pathway underlying the synergistic effect of these elicitors should be investigated further.
Abbreviations
- 4CL:
-
4-Coumarate:CoA ligase
- C4H:
-
Cinnamate 4-hydroxylase
- CHS:
-
Chalcone synthase
- DW:
-
Dry weight
- FW:
-
Fresh weight
- JA:
-
Jasmonic acid
- MeJA:
-
Methyl jasmonate
- PAL:
-
Phenylalanine ammonia lyase
- qPCR:
-
Quantitative real-time polymerase chain reaction
- SA:
-
Salicylic acid
- STS:
-
Stilbene synthase
- UV-C:
-
Ultraviolet C
References
Ali M, Abbasi BH, Ali GS (2015) Elicitation of antioxidant secondary metabolites with jasmonates and gibberellic acid in cell suspension cultures of Artemisia absinthium L. Plant Cell Tissue Organ Cult 120:1099–1106. doi:10.1007/s11240-014-0666-2
Almagro L, Gutierrez J, Pedreño MA, Sottomayor M (2014) Synergistic and additive influence of cyclodextrins and methyl jasmonate on the expression of the terpenoid indole alkaloid pathway genes and metabolites in Catharanthus roseus cell cultures. Plant Cell Tissue Organ Cult 119:543–551. doi:10.1007/s11240-014-0554-9
Belchí-Navarro S, Almagro L, Lijavetzky D, Bru R, Pedreño MA (2012) Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and methyljasmonate. Plant Cell Rep 31:81–99. doi:10.1007/s00299-011-1141-8
Belhadj A, Saigne C, Telef N, Cluzet S, Bouscaut J, Corio-Costet MF, Mérillon JM (2006) Methyl jasmonate induces defense responses in grapevine and triggers protection against Erysiphe necator. J Agric Food Chem 54:9119–9125. doi:10.1021/jf0618022
Belhadj A, Telef N, Saigne C, Cluzet S, Barrieu F, Hamdi S, Mérillon JM (2008) Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiol Biochem 46:493–499. doi:10.1016/j.plaphy.2007.12.001
Bulgakov VP, Tchernoded GK, Mischenko NP, Khodakovskaya MV, Glazunov VP, Radchenko SV, Zvereva EV, Fedoreyev SA, Zhuravlev YN (2002) Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with the rolB and rolC genes. J Biotechnol 97:213–221. doi:10.1016/S0168-1656(02)00067-6
Cai ZZ, Kastell A, Mewis I, Knorr D, Smetanska I (2012) Polysaccharide elicitors enhance anthocyanin and phenolic acid accumulation in cell suspension cultures of Vitis vinifera. Plant Cell Tissue Organ Cult 108:401–409. doi:10.1007/s11240-011-0051-3
Chen JY, Wen PF, Kong WF, Pan QH, Zhan JC, Li JM, Wan SB, Huang WD (2006) Effect of salicylic acid on phenylpropanoids and phenylalanine ammonia-lyase in harvested grape berries. Postharvest Biol Technol 40:64–72. doi:10.1016/j.postharvbio.2005.12.017
Chung IM, Park MR, Chun JC, Yun SJ (2003) Resveratrol accumulation and resveratrol synthase gene expression in response to abiotic stresses and hormones in peanut plants. Plant Sci 164:103–109. doi:10.1016/S0168-9452(02)00341-2
Crupi P, Pichierri A, Basile T, Antonacci D (2013) Postharvest stilbenes and flavonoids enrichment of table grape cv Redglobe (Vitis vinifera L.) as affected by interactive UV-C exposure and storage conditions. Food Chem 141:802–808. doi:10.1016/j.foodchem.2013.03.055
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097. doi:10.1105/tpc.7.7.1085
Durner J, Shah J, Klessig DF (1997) Salicylic acid and disease resistance in plants. Trends Plant Sci 2:266–274. doi:10.1016/S1360-1385(97)86349-2
Fang F, Huang WD (2013) Salicylic acid modulated flavonol biosynthesis in three key phases during grape berry development. Eur Food Res Technol 237:441–448. doi:10.1007/s00217-013-2008-8
Fernández-Marín MI, Puertas B, Guerrero RF, García-Parrilla MC, Cantos-Villar E (2014) Preharvest methyl jasmonate and postharvest UVC treatments: increasing stilbenes in wine. J Food Sci 79:310–317. doi:10.1111/1750-3841.12368
Gadzovska S, Maury S, Delaunay A, Spasenoski M, Hagège D, Courtois D, Joseph C (2013) The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tissue Organ Cult 113:25–39. doi:10.1007/s11240-012-0248-0
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158. doi:10.1016/0014-4827(68)90403-5
Guerrero RF, Puertas B, Fernández MI, Palma M, Cantos-Villar E (2010) Induction of stilbenes in grapes by UV-C Comparison of different subspecies of Vitis. Innov Food Sci Emerg Technol 11:231–238. doi:10.1016/j.ifset.2009.10.005
Hu YH, Yu YT, Piao CH, Liu JM, Yu HS (2011) Methyl jasmonate- and salicylic acid-induced D-chiro-inositol production in suspension cultures of buckwheat (Fagopyrum esculentum). Plant Cell Tissue Organ Cult 106:419–424. doi:10.1007/s11240-011-9938-2
Jeandet P, Douillt-Breuil AC, Bessis R, Debord S, Sbaghi M, Adrian M (2002) Phytoalexins from the Vitaceae: biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J Agric Food Chem 50:2731–2741. doi:10.1021/jf011429s
Krzyzanowska J, Czubacka A, Pecio L, Przybys M, Doroszewska T, Stochmal A, Oleszek W (2012) The effects of jasmonic acid and methyl jasmonate on rosmarinic acid production in Mentha × piperita cell suspension cultures. Plant Cell Tissue Organ Cult 108:73–81. doi:10.1007/s11240-011-0014-8
Langcake P, Pryce RJ (1977) The production of resveratrol and viniferins by grapevines in response to ultraviolet irradiation. Phytochemistry 16:1193–1196. doi:10.1016/S0031-9422(00)94358-9
Larkin PJ (1976) Purification and viability determinations of plant protoplasts. Planta 128:213–216. doi:10.1007/BF00393231
Larronde F, Krisa S, Decendit A, Cheze C, Merillon JM (1998) Regulation of polyphenol production in Vitis vinifera cell suspension cultures by sugars. Plant Cell Rep 17:946–950. doi:10.1007/s002990050515
Liu W, Liu CY, Yang CX, Wang LJ, Li SH (2010) Effect of grape genotype and tissue type on callus growth and production of resveratrols and their piceids after UV-C irradiation. Food Chem 122:475–481. doi:10.1016/j.foodchem.2010.03.055
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi:10.1006/meth.2001.1262
Logemann E, Tavernaro A, Schulz WG, Somssich IE, Hahlbrock K (2000) UV light selectively coinduces supply pathways from primary metabolism and flavonoid secondary product formation in parsley. Proc Natl Acad Sci USA 97:1903–1907. doi:10.1073/pnas.97.4.1903
Morales M, Bru R, García-Carmona F, Barceló AR, Pedreño MA (1998) Effect of dimethyl-β-cyclodextrins on resveratrol metabolism in Gamay grapevine cell cultures before and after inoculation with Xylophilus ampelinus. Plant Cell Tissue Organ Cult 53:179–187. doi:10.1023/A:1006027410575
Nopo-Olazabal C, Condori J, Nopo-Olazabal L, Medina-Bolivar F (2014) Differential induction of antioxidant stilbenoids in hairy roots of Vitis rotundifolia treated with methyl jasmonate and hydrogen peroxide. Plant Physiol Biochem 74:50–69. doi:10.1016/j.plaphy.2013.10.035
Palomer X, Capdevila-Busquets E, Álvarez-Guardia D, Barroso E, Pallàs M, Camins A, Davidson MM, Planavila A, Villarroya F, Vázquez-Carrera M (2013) Resveratrol induces nuclear factor-κB activity in human cardiac cells. Int J Cardiol 167:2507–2516. doi:10.1016/j.ijcard.2012.06.006
Pastrana-Bonilla E, Akoh CC, Sellappan S, Krewer G (2003) Phenolic content and antioxidant capacity of muscadine grapes. J Agric Food Chem 51:5497–5503. doi:10.1021/jf030113c
Pezet R, Perret C, Jean-Denis JB, Tabacchi R, Gindro K, Viret O (2003) δ-Viniferin, a resveratrol dehydrodimer: one of the major stilbenes synthesized by stressed grapevine leaves. J Agric Food Chem 51:5488–5492. doi:10.1021/jf030227o
Pezet R, Gindro K, Viret O, Spring JL (2004) Glycosylation and oxidative dimerization of resveratrol are respectively associated to sensitivity and resistance of grapevine cultivars to downy mildew. Physiol Mol Plant Pathol 65:297–303. doi:10.1016/j.pmpp.2005.03.002
Qu JG, Zhang W, Yu XJ (2011) A combination of elicitation and precursor feeding leads to increased anthocyanin synthesis in cell suspension cultures of Vitis vinifera. Plant Cell Tissue Organ Cult 107:261–269. doi:10.1007/s11240-011-9977-8
Riha J, Brenner S, Böhmdorfer M, Giessrigl B, Pignitter M, Schueller K, Thalhammer T, Stieger B, Somoza V, Szekeres T, Jäger W (2014) Resveratrol and its major sulfated conjugates are substrates of organic anion transporting polypeptides (OATPs): impact on growth of ZR-75-1 breast cancer cells. Mol Nutr Food Res 58:1830–1842. doi:10.1002/mnfr.201400095
Sansanelli S, Zanichelli D, Filippini A, Ferri M, Tassoni A (2014) Production of free and glycosylated isoflavones in in vitro soybean (Glycine max L.) hypocotyl cell suspensions and comparison with industrial seed extracts. Plant Cell Tissue Organ Cult 119:301–311. doi:10.1007/s11240-014-0534-0
Santamaria AR, Innocenti M, Mulinacci N, Melani F, Valletta A, Sciandra I, Pasqua G (2012) Enhancement of viniferin production in Vitis vinifera L. cv. Alphonse Lavallée cell suspensions by low-energy ultrasound alone and in combination with methyl jasmonate. J Agric Food Chem 60:11135–11142. doi:10.1021/jf301936u
Shumakova OA, Manyakhin AY, Kiselev KV (2011) Resveratrol content and expression of phenylalanine ammonia-lyase and stilbene synthase genes in cell cultures of Vitis amurensis treated with coumaric acid. Appl Biochem Biotechnol 165:1427–1436. doi:10.1007/s12010-011-9361-5
Silja PK, Gisha GP, Satheeshkumar K (2014) Enhanced plumbagin accumulation in embryogenic cell suspension cultures of Plumbago rosea L. following elicitation. Plant Cell Tissue Organ Cult 119:469–477. doi:10.1007/s11240-014-0547-8
Soleas GJ, Diamandis EP, Goldberg DM (1997) Resveratrol: a molecule whose time has come and gone. Clin Biochem 30:91–113. doi:10.1016/S0009-9120(96)00155-5
Tassoni A, Fornale S, Franceschetti M, Musiani F, Michael AJ, Perry B, Bagni N (2005) Jasmonates and Na-orthovanadate promote resveratrol production in Vitis vinifera cv. Barbera cell cultures. New Phytol 166:895–905. doi:10.1111/j.1469-8137.2005.01383.x
Tassoni A, Durante L, Ferri M (2012) Combined elicitation of methyl-jasmonate and red light on stilbene and anthocyanin biosynthesis. J Plant Physiol 169:775–781. doi:10.1016/j.jplph.2012.01.017
Vang O, Ahmad N, Baile CA, Baur JA, Brown K, Csiszar A, Das DK, Delmas D, Gottfried C, Lin HY, Ma QY, Mukhopadhyay P, Nalini N, Pezzuto JM, Richard T, Shukla Y, Surh YJ, Szekeres T, Szkudelski T, Walle T, Wu JM (2011) What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One 6:e19881. doi:10.1371/journal.pone.0019881
Wang CY, Chen CT, Wang SY (2009) Changes of flavonoid content and antioxidant capacity in blueberries after illumination with UV-C. Food Chem 117:426–431. doi:10.1016/j.foodchem.2009.04.037
Wang HL, Wang W, Zhang P, Pan QH, Zhan JC, Huang WD (2010a) Gene transcript accumulation, tissue and subcellular localization of anthocyanidin synthase (ANS) in developing grape berries. Plant Sci 179:103–113. doi:10.1016/j.plantsci.2010.04.002
Wang W, Tang K, Yang HR, Wen PF, Zhang P, Wang HL, Huang WD (2010b) Distribution of resveratrol and stilbene synthase in young grape plants (Vitis vinifera L. cv. Cabernet Sauvignon) and the effect of UV-C on its accumulation. Plant Physiol Biochem 48:142–152. doi:10.1016/j.plaphy.2009.12.002
Wang LJ, Ma L, Xi HF, Duan W, Wang JF, Li SH (2013) Individual and combined effects of CaCl2 and UV-C on the biosynthesis of resveratrols in grape leaves and berry skins. J Agric Food Chem 61:7135–7141. doi:10.1021/jf401220m
Wang S, Guo LP, Xie T, Yang J, Tang JF, Li X, Wang X, Huang LQ (2014) Different secondary metabolic responses to MeJA treatment in shikonin-proficient and shikonin-deficient cell lines from Arnebia euchroma (Royle) Johnst. Plant Cell Tissue Organ Cult 119:587–598. doi:10.1007/s11240-014-0558-5
Wasternack C, Parthier B (1997) Jasmonate-signalled plant gene expression. Trends Plant Sci 2:302–307. doi:10.1016/S1360-1385(97)89952-9
Waterhouse AL, Lamuela-Raventós RM (1994) The occurrence of piceid, a stilbene glucoside in grape berries. Phytochemistry 37:571–573. doi:10.1016/0031-9422(94)85102-6
Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493. doi:10.1104/pp.126.2.485
Wolfe K, Wu XZ, Liu RH (2003) Antioxidant activity of apple peels. J Agric Food Chem 51:609–614. doi:10.1021/jf020782a
Zhao J, Zhu WH, Hu Q (2001) Enhanced catharanthine production in Catharanthus roseus cell cultures by combined elicitor treatment in shake flasks and bioreactors. Enzyme Microb Technol 28:673–681. doi:10.1016/S0141-0229(01)00306-4
Acknowledgments
This study was funded by the National Natural Science Foundation of China (No. 31471835) and the National “Twelfth Five-Year” Plan for Science and Technology Support (2012BAD31B07).
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xu, A., Zhan, JC. & Huang, WD. Effects of ultraviolet C, methyl jasmonate and salicylic acid, alone or in combination, on stilbene biosynthesis in cell suspension cultures of Vitis vinifera L. cv. Cabernet Sauvignon. Plant Cell Tiss Organ Cult 122, 197–211 (2015). https://doi.org/10.1007/s11240-015-0761-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0761-z