Abstract
Trehalose, a non-reducing disaccharide, can effectively protect the biological structures of plants from damage under stress conditions (i.e., high or low temperature, drought, and dehydration) by forming a special protective membrane on the plant-cell surface. Transformation of maize with stably expressed trehalose-6-phosphate synthase (TPS), the key enzyme of trehalose biosynthesis, to improve drought-resistance has important theoretical and economic values. In this study, we constructed the TPS1 gene expression vector driven by the rd29a promoter, transformed immature embryos using Agrobacterium-mediated methods, and screened the transgenic maize plants. As a result, trehalose accumulated in rd29a::TPS1 transgenic maize plants even though it was not detected in wild-type (WT) maize. After determination under repeated drought conditions, we found that the survival rate of the rd29a::TPS1 maize was 70 % higher than that of the WT. Photosynthetic physiological indicators of transgenic maize under drought conditions were better than those of the WT. Microscopic observations of the detached newborn leaves showed that stomata density on the leaf surface of rd29a::TPS1 transgenic maize after drought treatment was reduced by 20 % rom that on the WT leaf, while there were fewer and shorter villi on the leaf surface of transgenic maize than on the WT leaf. In addition, real-time PCR analyses showed that stomata density and distribution 1 (SDD1) expression in the rd29a::TPS1 transgenic maize increased. We concluded that TPS1 improves drought-resistance in maize not only by increasing trehalose content but also by decreasing stomata density and reducing the transpiration rate in transgenic maize plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maize, or corn, is one of the three major crops and has the highest production of any other crop worldwide, although drought is an important limiting factor of its high yield (Wang et al. 2013). In different ecological zones, the yield will vary from 9.3 to 35.1 % depending on the severity and extent of the drought. According to the yield potential of corn in regional trials, the loss of yield per hectare will be 359.48–1,187.99 kg, which is particularly serious in Southwest China’s maize-producing areas, especially in Guizhou Province (Yun et al. 2012). Improving drought-resistance through genetic engineering is one of the most cost-effective ways by which to overcome drought stress. Traditional breeding techniques play an important role in the selection of drought-resistant maize varieties, but it is time consuming because it requires a longer breeding cycle (Li et al. 2005; Zhou et al. 2014). In recent years, the application of transgenic technology to transform plants accelerated the process of screening and fostered superior biological strains, which expanded the use of genetic resources (Basu et al. 2010).
Trehalose is a non-reducing disaccharide first extracted from rye ergot by Wiggers in 1832 (Almeida et al. 2007). Subsequent studies found that trehalose is widely distributed in nature in a variety of species, such as bacteria, fungi, yeast, plants, insects, and invertebrates (Elbein et al. 2003); however, trehalose in most higher plants is rare. It was found that the otsA-otsB pathway is common from prokaryotic to eukaryotic organisms, and is also the only trehalose-synthesis pathway in plants (Paul et al. 2008). In this pathway, otsA gene-encoded trehalose-6-phosphate synthase (TPS) catalyzes uridine diphosphate glucose and glucose 6-phosphate to form a 6-trehalose phosphate through the α-1,1-glycosidic linkage. Then, otsB gene-encoded trehalose-6-phosphatase (TPP) generates trehalose (Kaasen et al. 1992).
In bacteria and yeast, trehalose serves not only as a structural component and energy material, but also protects biomembranes and proteins against damage from stress conditions such as drought, cold, high salinity (because of its resistance to acidic and alkaline conditions), and high temperatures and has strong water absorption potential (Wingler et al. 2002). Thus, trehalose can improve the stress-resistant capability of plants, the direct correlation of which has been demonstrated in in vivo studies (Crowe et al. 1984; Hottiger et al. 1994). Consistent with endogenous effects, trehalose exogenously applied to the cell membrane also has a significant protective effect on active substances, such as proteins and enzymes (Yoshida et al. 1998; Zhao et al. 2009). In fact, the starch and chlorophyII content of the transgenic tomato containing yeast TPS1 are higher than those in the WT, and the transgenic tomato has an improved tolerance to drought (Cortina et al. 2005; Lyu et al. 2013). Holmstrom et al. (1996) and Han et al. (2005) also reported that transgenic tobacco containing TPS has stronger drought-tolerance than the WT tobacco. Romero et al. (1997) reported that yeast TPS1 gene expression driven by the CaMV35S promoter in tobacco endows more drought-tolerance and increases the trehalose content over that found in the WT tobacco. Garg et al. (2002) transferred the trehalose synthesis genes (otsA and otsB) of Escherichia coli into rice and found that the transgenic plants accumulated 3–10 times more trehalose than the WT plants. A trehalose content of 1.0 mg/g keeps the plants in good condition. Overexpressing TPS gene in Arabidopsis (Miranda et al. 2007), potato (Stiller et al. 2008; Yeo et al. 2000), bean (Suarez et al. 2008), and other plants can effectively improve the drought-resistance of these plants. Tao et al. (2008) reported the acquisition of a new trans-TPS1 maize germplasm but without the identification of drought-resistance. As a consequence, little information about the drought-resistant mechanism in the transgenic maize was provided.
Recent studies have shown that, in addition to sucrose, trehalose can act as a signal molecule. Trehalose was found to mediate stomata regulation by affecting abscisic acid (ABA) in Arabidopsis (Schluepmann et al. 2004). TPS1 transgenic potatoes had lower stomata density (reduced by 35 %) and were more resistant to drought than WT potatoes (Stiller et al. 2008); therefore, defining the relationship between trehalose and a stoma is crucial to be able to elucidate trehalose mechanisms and improve drought-resistance in maize. To make use of trehalose’s drought-tolerance characteristics, it is helpful to transfer TPS1 into maize and screen new drought-resistant strains using genetic engineering.
Materials and methods
Test materials
Maize Jiao51, plant expression vectors, and Agrobacterium tumefaciens strain LBA4404 were stored in our laboratory. Restriction endonuclease SalI, EcoRI, SacI, XbaI, Premix Taqver 2.0 plus dye, and DNA 2,000 marker Ladder were purchased from Takara biotechnology (Dalian) Co., Ltd; Plasmid Mini Kit I and plant RNA Kit were purchased from OMEGA Bio-tek Inc.; Kanamycin, Rifampicin, Glufosinate, Bialaphos, Timentin were purchased from Sigma; TPS1 detection primer and other Real-Time PCR primers were synthesized by Invitrogen biotechnology Co., Ltd.
Vector construction and genetic transformation
The poplar rd29a promoter-driven TPS1 expression cassette was inserted into the pCAMBIA1305 vector, the Ubiquitin promoter-driven Bar::GUS fusion gene as a selectable marker and reporter gene, to obtain the recombinant expression vector pGM626-Ubi-BG-rd29a-TPS1 (pTPS1). pTPS1 was transferred into Agrobacterium LBA4404 using the freeze–thaw transformation method. Maize was genetically transformed using the immature embryo transformation method as previously described (Frame et al. 2002). After artificial pollination by bagging, young ears of corn were harvested, stripped of their husks, disinfected on a clean bench with 75 % alcohol for 5 min and in 0.1 % mercuric chloride for 8 min, and then rinsed 5 times with sterile water. The grain endosperm was slashed and immature embryos were placed into a 2.0-mL centrifuge tube. Agrobacterium (OD600 = 0.3–0.4) suspended in infection medium (Murashige and Skoog Basal Salt Mixture [MS] and vitamins, 0.7 g/L l-proline [PRO], 0.1 g/L hydrolyzed casein, 1.5 mg/L 2, 4-D, 68 g/L sucrose, 36 g/L glucose, 100 μM Acetosyringone [AS], pH 5.2) was added to the tube, which was then allowed to stand for 5 min. A co-culture medium (MS salts and vitamins, 1.5 mg/L 2, 4-D, 0.7 g/L L-PRO, 0.1 g/L hydrolyzed casein, 0.1 g/L myoinositol, 30 g/L sucrose, 1.0 g/L filtered sterilized silver nitrate, 300 mg/L l-cysteine, 100 μM AS, 2.3 g/L Phytagel, pH 5.2) was added to the surface of the immature embryo’s excess broth using a pipette. The broth was then incubated in the dark at 23 °C for 3 d. The immature embryos were transferred to a recovery area after the conversion medium (MS salts and vitamins, 1.5 mg/L 2, 4-D, 0.7 g/L L-PRO, 100 mg/L hydrolyzed casein, 100 mg/L myoinositol, 30 g/L sucrose, 1.0 mg/L filtered sterilized silver nitrate, 100 mg/L Timentin, 0.5 g/L MES, 2.3 g/L Phytagel, pH 5.2) was added and cultured for 7 d. They were then transferred to selective medium (MS salts and vitamins, 1.5 mg/L 2, 4-D, 0.7 g/L L-PRO, 100 mg/L hydrolyzed casein, 100 mg/L myoinositol, 20 g/L sucrose, 1.0 mg/L filtered sterilized silver nitrate, 100 mg/L Timentin, 1.5 mg/L bialaphos, 2.3 g/L Phytagel, pH 5.8), cultured for 60 d and screened, and induced resistance callus subculture among 2–3 times. The resistant callus subculture was then transferred to differentiation medium (MS salts and vitamins, 700 mg/L L-PRO, 100 mg/L hydrolyzed casein, 100 mg/L myoinositol, 60 g/L sucrose, 100 mg/L Timentin, 4 mg/L glufosinate, 2.3 g/L Phytagel, pH 5.8) to produce differentiation resistant buds. The resistant buds were cut to 1–2 cm long and placed in rooting medium (MS salts and vitamins, 100 mg/L myoinositol, 0.7 g/L L-PRO, 100 mg/L hydrolyzed casein, 30 g/L sucrose, 100 mg/L Timentin, 2.3 g/L Phytagel, pH 5.8). Finally, the roots were transplanted to nutritive soil and generated healthy growth.
Detection of transgenic maize plants
A histochemical GUS assay was performed using the method as described by Jefferson et al. (1987). The assay working solution contained 0.5 mM potassium ferrocyanide, 0.1 % (v/v) TritonX-100, and 1.0 mg/mL 5-bromo-4-chloro-3-indolyl-D-glucuronide (X-gluc) in 50 mM phosphate buffer at a final neutral solution of pH 7.0. The 3- to 5-leaf stage of the herbicide phosphinothricin (PPT)-resistant maize plants was used for histochemical GUS staining. The staining results were observed using a stereoscopic microscope and a negative control was set. Total genomic DNA of the positive maize plants was extracted using a Plant DNA Kit (Tiangen Biotech Co., Ltd, Beijing, China) for PCR detection of transgenic maize plants. The specific primers for detecting these transgenic maize plants were as follows:(5′-CGTGTGAGAGGCAAGAAGTTAG-3′ and 5′-CAGACGAAGGGAATGGTGTATG-3′). The PCR procedure was as follows: 94 °C, 5 min; 94 °C, 1.0 min; 50 °C, 30 s, 72 °C, 30 s; 35 cycles; 72 °C extension 10 min. The 5-µL PCR product was added to 1.0 % agarose gel containing red stain for gel electrophoresis at 90 V and the results were detected under UV-light.
Detection of trehalose in maize
Transgenic and WT maize leaves in the 5- to 8-leaf stage were taken before and after drought treatment and the trehalose content was detected using the K-TREH trehalose assay kit protocol (Megazyme International Ireland, Wicklow, Ireland). Each test was repeated 3 times.
Measurement of drought resistance and physiological parameters
The maize drought-resistance parameters were determined by repeating the drought experiment (Cai et al. 2014). The experiment was carried out in 3 batches, each batch containing 20 genetically modified and 20 WT maize strains in the 5- to 8-leaf stage. The leaves were transplanted into pots containing nutritional soil in the greenhouse, and cultured under normal growth conditions (16 h light/8 h dark, 29 °C) for 2 weeks. The plants were not watered for 2 weeks and were rehydrated only once. This cycle was repeated 3 times for total of 45 d. Resilient maize plant phenotypes were observed and the survival rate of the transgenic and WT maize plants was calculated and photographed. Plants with wilted leaves depletion were identified as dead plants.
Leaves were then placed into vials filled with distilled water for 24 h, blotted to remove excess water, and weighed to obtain the leaf turgid weight (TW). Leaves were then dried at 65 °C and reweighed to obtain leaf dry weight (DW) (Yoo et al. 2010).
Leaf relative water content (RWC) = (fresh weight [FW] − DW)/(TW − DW) × 100. To determine the rate of water loss, 4- to 6-leaf stage leaves (approximately 1.0 g) were harvested from transgenic and WT maize plants and placed on dry filter paper. FWs were measured every hour for 12 h to determine the rate of water loss (Yan et al. 2014).
Determination of biochemical parameters
The malondialdehyde (MDA) and PRO content, and the superoxide dismutase (SOD) and peroxidase (POD) enzyme activity of the inverted fourth leaves of the genetically modified and WT maize were measured before and after the drought as per the manufacturer’s protocols for the MDA, PRO, SOD, and POD test kits (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China,). Each test was repeated 3 times.
Determination of photosynthetic parameters
Photosynthetic physiological parameters, including net photosynthetic rate (Pn), transpiration rate (Tr), stomata conductance (Gs), and intercellular carbon dioxide (CO2) concentration (Ci), of the inverted fourth leaves of genetically modified and WT maize were measured using the LI-6400 portable photosynthesis system before and after drought treatment. Measurements were taken from 10:00 a.m. to 12:00 p.m. with the artificial light intensity at 1,500 μmol m−2 s−1 (Purohit et al. 2002). Each test was repeated 3 times.
Microstructure of the leaf surface
Two leaves of the same size were taken from genetically modified and WT maize in the 6- to 8-leaf stage (including leaves at near and distal ends of the shaft). The area measured was placed between 2 slides and observed using an optical microscope. Five different leaf parts were selected for observing the number of stomata per leaf. Stomata density was determined as the number of stomata/mm2 (de Carvalho et al. 2005). Each test was repeated 3 times. Another sample was fixed with glutaraldehyde and examined using a scanning electron microscopy. Each test was repeated 3 times.
Relative expression analysis of key genes in stomata development
Primers for each gene were designed according to maize TPS1, SDD1, too many mouths (TMM), mitogen-activated protein kinase 3 (MAPK3), and mitogen-activated protein kinase 6 (MAPK6) gene sequences in the National Center for Biotechnology Information database. Actin served as an internal reference. The primer sequences are shown in Table 1. Total RNA was extracted from a 0.1-g leaf sample (the fifth leaf from the bottom of the plant) of transgenic and WT maize in the 6- to 8-leaf stage, and cDNAs were obtained by reverse transcription. Real-time (RT)-PCR was performed according to the instructions for the SYBR Green I Dye Kit (Applied Biosystems Inc., CA, USA).
Results
Obtaining TPS1 transfer maize
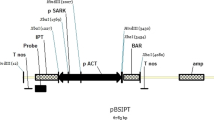
Poplar rd29a promoter-driven TPS1 gene expression cassettes and Ubiquitin promoter-driven Bar::GUS fusion gene expression vector pTPS1 were constructed in the laboratory (Fig. 1a). Immature embryos were chosen, infection medium was added to a suspension of A. bacterium, and embryos were placed directly on a culture medium together with the bacteria (Fig. 1bA) and incubated in the dark for 3 d. Immature embryos were then transferred to recovery media after 7 d of culture medium, and then to screening medium to culture for 60 d (Fig. 1bB) to induce resistance callus subcultures, intermediate 2–3 times, The resistant callus subculture was transferred to differentiation medium to generate resistant shoots (Fig. 1bC), and the resistant transformed shoots were transferred to rooting medium (Fig. 1bD). Finally, the healthy regenerated plants (Fig. 1bE) were transplanted to nutrient soil after practicing (Fig. 1bF) until flowering and seeding. After growing and seeding the healthy regenerated plants, as described in the methods section, the T0 generation was self-hybridized to get the T1 generation. IHC staining of the GUS gene in the leaves of the selected 3- to 5-leaf stage transgenic and WT maize plants illustrated GUS gene expression (Fig. 1c) in the transformed plants. Plant DNA was extracted from WT maize plants and 5 independent transgenic lines. The TPS1 gene expression was checked by RT-PCR using specific primers. No specific band was amplified in the-WT plants but it was amplified with the expected 564-bp target band in the transgenic maize plant (Fig. 1d). The above results suggested that the TPS1 transgenic maize plant was obtained.
Drought-induced trehalose content in transgenic TPS1 maize plants
The trehalose content assay was performed for 10 lines of WT and TPS1 transgenic maize and only TPS content signals from three lines of the transgenic plants were detected, while the remains were undetectable before drought treatment. We proposed that the three lines might suffer from biotic or abiotic stress. The trehalose mean content in 3 TPS1 transgenic maize strains was 0.3 mg/g before drought treatment, while the value was 1.51 mg/g in 10 transgenic lines after drought treatment (Fig. 2a). The determined results of trehalose content indicated there was a significant difference in trehalose content in plants before and after drought treatment. Meanwhile, we found that the relative expression of TPS1 gene was 9.8fold higher in transgenic plants after drought treatment than before drought treatment (Fig. 2b).
The detection of trehalose content and the identification of drought-resistance in transgenic maize plants. a Detection of trehalose content in transgenic plants before and after drought. b Relative expression level of TPS1 gene in drought-treated plants. c Identification of drought resistance in transgenic maize plants after drought treatment. d Statistical survival of wild-type maize and transgenic maize after drought treatment. e Leaf relative water content (RWC) of wild-type maize and transgenic maize. f Rate of water loss from detached leaves of WT and transgenic plants. In all cases, data represent mean values ± SD from three independent experiments. Asterisks indicate statistical significance (*P<0.05, **P<0.01, Student’s t test) of differences between transgenic and WT plants. (WT wild type, TP transgenic plant)
TPS1 gene transfer enhanced drought resistance in maize
After repeating the 15-d drought experiment 3 times, the statistical analysis on the survival of 20 WT strains and 20 TPS1 transgenic strains showed that 19 TPS1 transgenic maize strains had a 95 % survival rate and 5 WT strains had a 25 % survival rate. The survival rate of TPS1 transgenic strains was 70 % higher than that of the WT strains (Fig. 2d). After a 30-d drought treatment, the leaf-wilting symptoms of the transgenic maize strains was lower than that of the WT maize (Fig. 2c). Leaf RWC of the WT was 15 % and that of the transgenic maize strain was 48 %, indicating that the transgenic maize strain had 33 % higher RWC than the WT (Fig. 2e). Measurement of the rate of water loss from the leaves of both transgenic and WT plants at various time points following leaf detachment demonstrated that the transgenic lines displayed less water loss than WT lines at every time point (Fig. 2f).
Changes in biochemical parameters in TPS1 transgenic plants
There was no significant difference in biochemical parameters between WT and TPS1 transgenic maize plants before drought treatment (Fig. 3a–d), but PRO content, POD, and SOD activity was higher than in WT plants (Fig. 3a, c, d) after drought treatment, although the MDA content in TPS1 transgenic plants was lower than in WT plants (Fig. 3b), The above results suggested that better physiological and biochemical properties improved drought-resistance in transgenic maize plants.
Determination of drought-related physiological indices and detection of photosynthetic characteristics in transgenic maize plants. a Proline (PRO) content in the leaves of wild-type (WT) and transgenic (TP) plants. b Malondialdehyde (MDA) content. c Peroxidase (POD) activity in the leaves of WT and TP plants. d Superoxide dismutase (SOD) activity. e Photosynthetic rate (Pn). f Stomatal conductance (Gs). g Intercellular CO2 concentration. h Transpiration rate (Tr) respectively. In all cases, data represent mean values ± SD from 3 independent experiments. Asterisks indicate statistical significance (*P < 0.05, **P < 0.01, Student’s t test) of differences between TP and WT plants
Changes in photosynthetic characteristics in TPS1 transgenic plants
There was no significant difference in photosynthetic characteristics between WT maize and TPS1 transgenic plants before drought treatment (Fig. 3e–h), but after drought treatment, the net photosynthetic rate decreased in the WT plants (4.1 μmol CO2 m−2 S−1) more significantly than in the transgenic plants (12.2 μmol CO2 m−2 S−1; Fig. 3e). Meanwhile, stomata conductance decreased significantly in the TPS1 transgenic strains (0.038 mol m−2 S−1) compared to that in the WT plants (0.052 mol m−2 S−1; Fig. 3f). The transpiration rate also decreased significantly in the TPS1 transgenic strains (1.46 mmol m−2 S−1) compared to that in the WT plants (1.87 mmol m−2 S−1; Fig. 3h). Intercellular CO2 concentration in the transgenic plants was lower than that in WT plants after drought treatment (Fig. 3g). The above results suggested that the TPS1 in transgenic maize enhances drought-resistance because by lowering stomata conductance, thereby reducing transpiration, water use efficiency increases.
TPS1 gene reduced the stomata density in transgenic plants
Optical microscopy results showed that there was no significant difference in the number of stomata between WT maize and TPS1 transgenic plants before drought treatment; however, after drought treatment, the number of stomata (28) in the leaf adxial and abxial epidermis of the transgenic plants was lower than that (36) in the leaf adxial and abxial epidermis of WT maize (Fig. 4aA, B, E, F). Under 200X magnification, the WT maize had 10 stomata at the leaf adxial epidermis (Fig. 4aC) but the transgenic maize had 7 stomata (Fig. 4aD). Scanning electron microscopy of the same visual field showed that WT plants had 25 stomata (Fig. 4bG) and transgenic plants had 20 stomata (Fig. 4bH). The stomata density of the WT maize was 90/mm2, while that of the transgenic maize was 72/mm2. The stomata density on the leaf surface of the transgenic maize was 20 % lower than that of the WT plants (Fig. 4c). Scanning electron microscopy showed that the number of villi on the leaf surface of WT maize was 4/5 mm2 (Fig. 4bI), while it was 2/5 mm2 on transgenic maize (Fig. 4bJ). Villi were also shorter on the leaf surface of the transgenic maize (Fig. 4bM) compared to those on the WT maize (Fig. 4bK). The above results suggested that the TPS1 transgenic maize transpiration rate decreased because of reduced stomata density.
The microstructural change of stoma and surface structure in TP maize leaves. a Observation of leaf surface by microscope. b Observation of leaf surface by scanning electron microscope. c Comparison of stomatal density between WT and TP maize. Data represent mean values ± SD from 3 independent experiments. Asterisks indicate statistical significance (**P < 0.01, Student’s t test) of differences between TP and WT plants (WT wild type, TP transgenic plant)
TPS1 enhanced the gene expression of proteins for stomata development
We found no significant changes in SDD1, TMM, MAPK3, and MAPK6 expression in transgenic strains before drought treatment, but SDD1 was 18 times higher than that in WT plants after drought treatment (Fig. 5a), the stomata development signaling pathway gene TMM expression levels rose 15-fold (Fig. 5b), and MAPK3 (Fig. 5c) and MAPK6 (Fig. 5d) increased 12- and 26-fold, respectively, after drought treatment; therefore, TPS1 regulates the genes associated with stomata development. The TPS1 in transgenic maize reduces stomata density by increasing the expression of SDD1.
The relative expression level of the gene-related stomatal ontogeny between TP and WT maize plants before and after drought treatment. a SDD1 gene. b TMM gene. c MAPK3 gene. d MAPK6 gene. In all cases, data represent mean values ± SD from 3 independent experiments. Asterisks indicate statistical significance (**P < 0.01, Student’s t test) of differences between TP and WT plants (WT wild type, TP transgenic plant)
Discussion
Most transgenic maize plants derived from model inbred lines (i.e., lines A188, line B73, Hi-II, and H99) could not satisfy production needs because of poor agronomic traits; however, line Jiao51 is considered the most important inbred line in the Guizhou Province and drew much attention because of its many merits, such as high genetic inheritance, good yield, and good quality (Zhang et al. 2008). In Guizhou, special topography and climatic conditions will inevitably lead to drought, and drought is an important restriction in crop yield; therefore, we transferred the trehalose synthesis gene TPS1 into the Jiao51 line to improve its drought-resistance.
In this study, the use of stress-induced promoter rd29a can increase TPS1 expression, especially under drought conditions. In genetically modified maize, the trehalose content of the plants under drought conditions was 1.39–2.8 mg/g, and no detectable trehalose levels were found before drought treatment. Drought stress can activate transgenic drought defense mechanisms to improve drought-resistance in transgenic plants. Similarly, Pellegrineschi et al. (2004) used an inducible promoter rd29a drive DREBA/CBF3 expression to improve drought-tolerance in wheat. In addition, the application of stress promoter rd29a could avoid the adverse effects of the expression of exogenous genes on transgenic strains under non-stress conditions. For example, Zhu et al. (2010) reported that a constitutive CaMV35S promoter-driven BADH overexpression will cause growth delay in transgenic tobacco, and that transgenic potatoes with yeast TPS1 driven by promoter CaMV35S leads to dwarfing, yellowing, willow-leaf shaping, unusual root development, and deformity.
Stiller et al. (2008) reported that the stomata density of TPS1 transgenic potato plants decreased by 35 % compared to that in the WT potato. We first found this phenomenon when TPS1 transgenic maize also decreased stomata density, but only by 20 %. Stomatal development in the A. thaliana model includes a signaling pathway that negatively regulates the basal pathway of stomata lineage, which is necessary to achieve a balance between the pavement and guard cells in the leaf epidermis (Casson and Hetherington 2010). Determinants of the negative signal regulatory pathway included the leucine-rich repeat receptor-like protein TMM that is presumed to interact with the ERECTA (ER) family members of leucine-rich repeat receptor-like kinases (Shpak et al. 2005). SDD1 most likely processes propeptides into ligands that activate the TMM-ER complex (Von Groll et al. 2002). Ligand interaction with the receptor is presumed to activate the MPAK3/6 cascade, which leads to its inactivation and repression of the basal pathway to reduce stomata density (Lampard et al. 2008). Simply put, stomata development showed that several genes, including TMM (Bhave et al. 2009), MAPK3 (Bergmann et al. 2004), MAPK6 (Wang et al. 2007), and SDD1 (Berger and Altmann 2000; Von Groll et al. 2002; Yoo et al. 2010), negatively regulate stomata development in plants. In this paper, we performed RT-PCR analysis and found that TMM, MAPK3, MAPK6, and SDD1 obviously increased in TPS1 transgenic maize plants, indicating that TPS1 can negatively regulate stomata development and reduce the number of stomata in plants to improve drought-resistance. We believe that stomata development in maize and Arabidopsis uses the same signaling pathway. The precise regulation of stomata development by TPS1 is worthy of more in-depth study.
In summary, transgenic plants launch a drought response mechanism under drought conditions, boost trehalose content, effectively protect cells from dehydration damage, and enhance drought-resistance. In addition, TPS1 increases water utility efficiency because of reduced stomata density on the leaf surface and decreases transpiration rate by increasing the expression of SDD1.
References
Almeida AM, Cardoso LA, Santos DM, Torné JM, Fevereiro PS (2007) Trehalose and its applications in plant biotechnology. In Vitro Cell Dev Biol-Plant 43:167–177
Basu SK, Dutta M, Goyal A, Bhowmik PK, Kumar J, Nandy S, Scagliusi SM, Prasad R (2010) Is genetically modified crop the answer for the next green revolution? GM crops 1:68–79
Berger D, Altmann T (2000) A subtilisin-like serine protease involved in the regulation of stomatal density and distribution in Arabidopsis thaliana. Genes Dev 14:1119–1131
Bergmann DC, Lukowitz W, Somerville CR (2004) Stomatal development and pattern controlled by a MAPKK kinase. Science 304:1494–1497
Bhave NS, Veley KM, Nadeau JA, Lucas JR, Bhave SL, Sack FD (2009) TOO MANY MOUTHS promotes cell fate progression in stomatal development of Arabidopsis stems. Planta 229:357–367
Cai R, Zhao Y, Wang Y, Lin Y, Peng X, Li Q, Chang Y, Jiang H, Xiang Y, Cheng B (2014) Overexpression of a maize WRKY58 gene enhances drought and salt tolerance in transgenic rice. Plant Cell Tissue Org Cult 1–13
Casson SA, Hetherington AM (2010) Environmental regulation of stomatal development. Curr Opin Plant Biol 13:90–95
Cortina C, Culiáñez-Macià FA (2005) Tomato abiotic stress enhanced tolerance by trehalose biosynthesis. Plant Sci 169:75–82
Crowe JH, Crowe LM, Chapman D (1984) Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science 223:701–703
de Carvalho JFRP, de Carvalho CRdP, Otoni WC (2005) In vitro induction of polyploidy in annatto (Bixa orellana). Plant Cell Tissue Org Cult 80:69–75
Elbein AD, Pan YT, Pastuszak I, Carroll D (2003) New insights on trehalose: a multifunctional molecule. Glycobiology 13:17R–27R
Frame BR, Shou H, Chikwamba RK, Zhang Z, Xiang C, Fonger TM, Pegg SE, Li B, Nettleton DS, Pei D, Wang K (2002) Agrobacterium tumefaciens-mediated transformation of maize embryos using a standard binary vector system. Plant Physiol 129:13–22
Garg AK, Kim JK, Owens TG, Ranwala AP, Choi YD, Kochian LV, Wu RJ (2002) Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc Natl Acad Sci USA 99:15898–15903
Han S-E, Park S-R, Kwon H-B, Yi B-Y, Lee G-B, Byun M-O (2005) Genetic engineering of drought-resistant tobacco plants by introducing the trehalose phosphorylase (TP) gene from Pleurotus sajor-caju. Plant Cell Tissue Org Cult 82:151–158
Holmstrom K-O, Mantyla E, Welin B, Mandal A, Palva ET, Tunnela OE, Londesborough J (1996) Drought tolerance in tobacco. Nature 379:683–684
Hottiger T, De Virgilio C, Hall MN, Boller T, Wiemken A (1994) The role of trehalose synthesis for the acquisition of thermotolerance in yeast. II. Physiological concentrations of trehalose increase the thermal stability of proteins in vitro. Eur J Biochem 219:187–193
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Kaasen I, Falkenberg P, Styrvold OB, Strom AR (1992) Molecular cloning and physical mapping of the otsBA genes, which encode the osmoregulatory trehalose pathway of Escherichia coli: evidence that transcription is activated by katF (AppR). J Bacteriol 174:889–898
Lampard GR, Macalister CA, Bergmann DC (2008) Arabidopsis stomatal initiation is controlled by MAPK-mediated regulation of the bHLH SPEECHLESS. Science 322:1113–1116
Li S-H, Kuoh C-S, Chen Y-H, Chen H-H, Chen W-H (2005) Osmotic sucrose enhancement of single-cell embryogenesis and transformation efficiency in Oncidium. Plant Cell Tissue Org Cult 81:183–192
Lyu JI, Min SR, Lee JH, Lim YH, Kim JK, Bae CH, Liu JR (2013) Overexpression of a trehalose-6-phosphate synthase/phosphatase fusion gene enhances tolerance and photosynthesis during drought and salt stress without growth aberrations in tomato. Plant Cell Tissue Org 112:257–262
Miranda JA, Avonce N, Suarez R, Thevelein JM, Van Dijck P, Iturriaga G (2007) A bifunctional TPS-TPP enzyme from yeast confers tolerance to multiple and extreme abiotic-stress conditions in transgenic Arabidopsis. Planta 226:1411–1421
Paul MJ, Primavesi LF, Jhurreea D, Zhang Y (2008) Trehalose metabolism and signaling. Annu Rev Plant Biol 59:417–441
Pellegrineschi A, Reynolds M, Pacheco M, Brito RM, Almeraya R, Yamaguchi-Shinozaki K, Hoisington D (2004) Stress-induced expression in wheat of the Arabidopsis thaliana DREB1A gene delays water stress symptoms under greenhouse conditions. Genome 47:493–500
Purohit VK, Tamta S, Chandra S, Vyas P, Palni LMS, Nandi SK (2002) In vitro multiplication of Quercus leucotrichophora and Q.glauca: important Himalayan oaks. Plant Cell Tissue Org Cult 69:121–133
Romero C, Belles JM, Vaya JL, Serrano R, Culianez-Macia FA (1997) Expression of the yeast trehalose-6-phosphate synthase gene in transgenic tobacco plants: pleiotropic phenotypes include drought tolerance. Planta 201:293–297
Schluepmann H, van Dijken A, Aghdasi M, Wobbes B, Paul M, Smeekens S (2004) Trehalose mediated growth inhibition of Arabidopsis seedlings is due to trehalose-6-phosphate accumulation. Plant Physiol 135:879–890
Shpak ED, McAbee JM, Pillitteri LJ, Torii KU (2005) Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science 309:290–293
Stiller I, Dulai S, Kondrak M, Tarnai R, Szabo L, Toldi O, Banfalvi Z (2008) Effects of drought on water content and photosynthetic parameters in potato plants expressing the trehalose-6-phosphate synthase gene of Saccharomyces cerevisiae. Planta 227:299–308
Suarez R, Wong A, Ramirez M, Barraza A, del Orozco MC, Cevallos MA, Lara M, Hernandez G, Iturriaga G (2008) Improvement of drought tolerance and grain yield in common bean by overexpressing trehalose-6-phosphate synthase in rhizobia. Mol Plant Microbe Interact 21:958–966
Tao D, Mu Y, Fu F-L, Li W-C (2008) Transformation of maize with trehalose synthase gene cloned from Saccharomyces cerevisiae. Biotechnology 7:258–265
Von Groll U, Berger D, Altmann T (2002) The subtilisin-like serine protease SDD1 mediates cell-to-cell signaling during Arabidopsis stomatal development. Plant Cell 14:1527–1539
Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S (2007) Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 19:63–73
Wang XM, Li ZG, Yan F, Khalil R, Ren ZX, Yang CW, Yang YW, Deng W (2013) ZmSKIP, a homologue of SKIP in maize, is involved in response to abiotic stress in tobacco. Plant Cell Tissue Org 112:203–216
Wingler A (2002) The function of trehalose biosynthesis in plants. Phytochemistry 60:437–440
Yan Q, Hou H, Singer SD, Yan X, Guo R, Wang X (2014) The grape VvMBF1 gene improves drought stress tolerance in transgenic Arabidopsis thaliana. Plant Cell Tissue Org 118:571–582
Yeo ET, Kwon HB, Han SE, Lee JT, Ryu JC, Byu MO (2000) Genetic engineering of drought resistant potato plants by introduction of the trehalose-6-phosphate synthase (TPS1) gene from Saccharomyces cerevisiae. Mol Cells 10:263–268
Yoo CY, Pence HE, Jin JB, Miura K, Gosney MJ, Hasegawa PM, Mickelbart MV (2010) The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant Cell 22:4128–4141
Yoshida M, Nakamura N, Horikoshi K (1998) Production of trehalose by a dual enzyme system of immobilized maltose phosphorylase and trehalose phosphorylase. Enzyme Microb Technol 22:71–75
Yun S, Jun Y, Hong S (2012) Social perception and response to the drought process: a case study of the drought during 2009–2010 in the Qianxi’nan Prefecture of Guizhou Province. Nat Hazards 64:839–851
Zhang W, Zhao Z, Bai G, Fu F (2008) Study and evaluation of drought resistance of different genotype maize inbred lines. Front Agric China 2:428–434
Zhao X, Tan HJ, Liu YB, Li XR, Chen GX (2009) Effect of salt stress on growth and osmotic regulation in thellungiella and arabidopsis callus. Plant Cell Tissue Org 98:97–103
Zhou L, He H, Liu R, Han Q, Liu B, Shou H (2014) Overexpression of GmAKT2 potassium channel enhances resistance to soybean mosaic virus. BMC Plant Biol 14:154
Zhu L, Yu Z, Zou C, Li Q (2010) Plant stress-inducible promoters and their function. Hereditas 32:229–234
Acknowledgments
This work supported by the Genetically Breeding Major Project of the Ministry of Agriculture of China (2014ZX0801008B-002, 2014ZX08010-003) and Genetically Breeding Project of Science and Technology Agency of Guizhou Province (2004NZ004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Han, L., Qin, L. et al. Saccharomyces cerevisiae gene TPS1 improves drought tolerance in Zea mays L. by increasing the expression of SDD1 and reducing stomatal density. Plant Cell Tiss Organ Cult 120, 779–789 (2015). https://doi.org/10.1007/s11240-014-0647-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0647-5