Abstract
An efficient propagation system of pitaya with diverse genetic background was established by using tender stem as explants. Various types of plant growth regulators (PGRs) were used to determine the most effective hormone combination for shoot proliferation. Zeatin (ZT), thidiazuron (TDZ), 2, 4-dichlorophenoxyacetic acid (2, 4-D) and 6-benzylaminopurine (6-BA) alone could induce multiple shoots. In that case, the most shoots per explant i.e. 4.6 (13.68 µM ZT), 4.8 (0.11 µM TDZ), 4.3 (17.76 µM 6-BA) and 3.2 (0.23 µM 2, 4-D) were obtained. The best PGRs combination was MS medium with 3.0 µM ZT and 0.5 µM IBA producing the most shoots per explant and the most vigorous shoots. Of the two varieties and six superior selections cultured on the best PGRs combination, more than 6.0 shoots per explant were obtained. No polymorphism was detected among in vitro-derived plantlets selected at random after 11 sub-cultures. Those results showed that MS media with 13.68 µM ZT and 2.46 µM IBA was suitable for shoot propagation of diverse pitaya varieties and selections. The protocol can be used for large-scale propagation of pitaya to meet the demand of increasing commercial cultivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pitaya (dragon fruit) is the fruit produced by the plant with the same name belonging to the genus Hylocereus of the Cactaceae. It contains glucose, betalains, vitamins, organic acids, soluble dietary fiber, phyto albumins and minerals (Stintzing and Carle 2007; Nurliyana et al. 2010; Wichienchot et al. 2010; Liaotrakoon, 2013a). Pitaya is not only utilized as fruit, but also can be taken as flower, vegetable, health products and medicine. The fruit is delicious and can be processed into juice, jam, ice cream, pastries, vinegar and wine. Dragon fruit seed oils are a good source of essential fatty acids and tocopherols, with a high oxidative stability (Liaotrakoon et al. 2013b). Pitaya flowers and vines can be used as vegetables and herbs while peels are used to extract color ingredient for food and cosmetic industries (Esquivel et al. 2007; Harivaindaran et al. 2008). Pitaya is also considered a potential economic crop for harsh conditions such as drought, heat, and poor soil (Mizrahi and Nerd 1999). It has drawn much attention and growing areas of pitaya are increasing rapidly in many countries due to its economic potential and nutritional benefits (Le Bellec et al. 2006; Esquivel et al. 2007; Ortiz-Hernández and Carrillo-Salazar 2012).
Pitaya is mainly propagated by cuttings (Elobeidy 2006; Cavalcante and Martins 2008). However, the method is inefficient, time-consuming and susceptible to damping-off. Tissue culture is an efficient method for rapid propagation of plants, and to obtain healthy and pathogen-free plants in a relatively short time and minimal space using very few starting materials. Currently, several researchers have established protocols for propagation of pitaya (Infante 1992; Drew and Azimi 2002; Mohamed-Yasseen 2002; Pelah et al. 2002; Viñas et al. 2012; Fan et al. 2013). However, pitaya with diverse genetic backgrounds respond differently to the same culture medium, probably due to genetic conditioning (Drew and Azimi 2002; Mohamed-Yasseen 2002; Fan et al. 2013). Improved protocols are needed to better suit the genetic diversity of this crop. Therefore, it is essential to develop an efficient propagation procedure for pitaya with genetic diversity. In this study, an efficient protocol for in vitro propagation and acclimatization was developed for eight genetically diverse pitaya. Genetic fidelity of in vitro-derived plantlets was assessed by Start Codon Targeted (SCoT) marker.
Two varieties and six superior selections with four flesh colors and excellent quality were used as materials (Supplementary Fig. 1). Young vines, without removing thorns, were washed in running tap water for 30 min and cut into segments (4.0–5.0 cm). Segments were surface disinfected in 75 % (v/v) ethanol for 1 min, then 0.1 % (w/v) HgCl2 for 10 min followed by rinsing five times with sterile water. After discarding both ends, segments were vertically inserted in MS medium (Murashige and Skoog 1962) without plant growth regulators (PGRs). New shoots were subcultured on the same medium through a few cycles to produce materials for experiments. Explants (0.5 cm) were placed vertically on MS media containing 3 % sucrose, 0.8 % agar, and 0–31.93 µM ZT (Genview, USA), 0–13.62 µM TDZ (Sigma, USA); 0–13.57 µM 2, 4-D (Beijing Dingguo Changsheng Biotechnology Co. Ltd, China); 0–31.08 µM 6-BA (Beijing Dingguo Changsheng Biotechnology Co. Ltd, China). Unless otherwise specified, superior selection ‘10–2’ was used as a model system to screen for the optimum PGRs combination. Responses of two varieties and six superior pitaya selections were subsequently characterized on the optimum PGRs combination. All cultures were maintained at 25 ± 2 °C under a light–dark cycle of 16:8 with a light intensity of 50 μE m−2 s−1. The elongated shoots (approximately 2.5 cm) were rooted in MS media having 1.23–2.46 µM IBA (Sigma, USA) or 0.54–1.34 µM NAA (Beijing Dingguo Changsheng Biotechnology Co. Ltd, China) (data not published yet). Rooted plantlets were removed after 30 days and kept in large Petri dishes with 10 ml for 7–10 days. Thereafter they were transferred to peat moss and kept in room temperature for 90 days and finally transferred to soil for acclimatization.
Thirty randomly-selected plantlets from 11 sub-cultures cycles, as well as the parental plants (control), were used to assess their genetic fidelity by SCoT marker. The PCR parameters were performed according to the procedure of Xia et al. (2014). PCR products were examined by ethidium bromide staining 2.0 % agarose gels.
All experiments were carried out in a completely randomized design thrice. Data analyses were performed using DPS 7.05 software. Differences of compared sets were considered significant using LSD test at P ≤ 0.05.
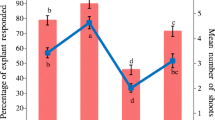
TDZ, ZT, 6-BA and 2, 4-D are commonly used for shoot regeneration in many plant species (Ellis et al. 1991; Huetteman and Preece 1993). Micropropagation of pitaya has been achieved using TDZ or 6-BA alone (Mohamed-Yasseen 2002; Viñas et al. 2012; Fan et al. 2013), however, the role of ZT and 2, 4-D in pitaya propagation is not reported yet. In this study, we found that pitaya explants were more sensitive to TDZ than ZT, 6-BA and 2, 4-D (Supplementary Tables 1–4 and Supplementary Fig. 2). TDZ at as low as 0.11 µM resulted in formation of green compact callus at the base of the proximal part of explants which significantly inhibited shoot growth already formed (Supplementary Table 2 and Supplementary Fig. 2b). By increasing 6-BA, TDZ or ZT concentrations, shoot number per explant increased significantly while shoot heights were reduced significantly. Dual effects of 2, 4-D on pitaya propagation were detected. Lower concentrations of 2, 4-D (<0.45 µM) is beneficial to shoot propagation while higher concentration (≥ 0.45 µM) induced friable callus and hairy roots (Supplementary Table 4 and Supplementary Fig. 2d). The most shoot number per explant i.e. 4.6 (13.68 µM ZT), 4.8 (0.11 µM TDZ), 4.3 (17.76 µM 6-BA) and 3.2 (0.23 µM 2, 4-D) were obtained. However, the highest shoot production is not always the best and might lead to somaclonal variation. Higher concentrations of 6-BA, TDZ, ZT and 2, 4-D had negative effects on pitaya shoot propagation (Supplementary Tables 1–4 and Supplementary Fig. 2). ZT had a greater effect than that TDZ, 6-BA and 2, 4-D for the activation of axillary buds. Explants cultured on MS medium containing ZT gave better shoot production and elongation without callus induction, reducing the time and labor involved.
The formation of adventitious shoots is regulated by interactions between cytokinins and auxins. Mohamed-Yasseen (2002) reported that the best shoot proliferation of H. undatus was 0.5 mM TDZ and 0.5 mM NAA. However, Fan et al. found that the best medium for micropropagation of H. undatus was MS with 2.0 mM BA and 0.5 mM NAA. In this study, the highest shoot number per explant was obtained with 13.68 µM ZT and 2.46 µM IBA (Table 1 and Supplementary Fig. 3). Areoles began to swell after 5 days of culture (Supplementary Fig. 4b) and multiple buds emerged directly from areoles after 10 days of culture on the best PGRs combination, without an intermediate callus phase (Supplementary Fig. 4 c). Average shoots elongated to 2.0 cm and shoot number per explant was 7.1 after 60 days of culture (Supplementary Fig. 4 d–f). Of the two varieties and six superior pitaya selections tested, more than 6.0 vigorous shoots per explant were obtained in 60 days on the best PGRs combination (Table 2 and Supplementary Fig. 5). Rooted plantlets were initially maintained in normal conditions then transferred to the field with 100 % survival rate. Figure 1 is a schematic representation of the pitaya in vitro propagation procedure, including the approximate time required for each step.
SCoT, a simple and novel DNA marker, has been used for genetic diversity analysis of micropropagated plants due to its high reproducibility and sensitivity (Agarwal et al. 2014; Rathore et al. 2014). Results from SCoT analyses showed that there was no polymorphic bands among propagated plants and parental plants (Fig. 2 and Supplementary Table 5), implying that high genetic fidelity can be guaranteed using the in vitro protocol.
This is the first report on the effects of ZT and 2, 4-D in pitaya propagation. The protocol of pitaya propagation in our experiments is to avoid shoot production from callus, and production of multiple shoots directly from areoles. The protocol has been applied in large-scale propagation of pitaya (Supplementary Fig. 6).
References
Agarwal T, Gupta AK, Patel AK, Shekhawat NS. (2014) Micropropagation and validation of genetic homogeneity of Alhagi maurorum using SCoT, ISSR and RAPD markers. Plant Cell Tissue Org Cult doi:10.1007/s11240-014-0608-z
Cavalcante ÍHL, Martins ABG (2008) Effect of juvenility on cutting propagation of red pitaya. Fruits 63(5):277–283
Drew RA, Azimi M (2002) Micropropagation of red pitaya (Hylocereus undatus). Acta Hort 575:93–98
Ellis DD, Barczynska H, McCown BH, Nelson N (1991) A comparison of BA, zeatin and thidiazuron for adventitious bud formation from Picea glauca embryos and epicotyl explants. Plant Cell Tissue Org Cult 27:281–287
Elobeidy AA (2006) Mass propagation of pitaya (dragon fruit). Fruits 61(5):313–319
Esquivel P, Stintzing FC, Carle R (2007) Comparison of morphological and chemical fruit traits from different pitaya genotypes (Hylocereus sp.) grown in Costa Rica. J Appl Bot Food Qual 81(1):7–14
Fan QJ, Zheng SC, Yan FX, Zhang BX, Qiao G, Wen XP (2013) Efficient regeneration of dragon fruit (Hylocereus undatus) and an assessment of the genetic fidelity of in vitro: derived plants using ISSR markers. J Hortic Sci Biotechnol 88(5):631–637
Harivaindaran KV, Rebecca OPS, Chandran S (2008) Study of optimal temperature, pH and stability of dragon fruit (Hylocereus polyrhizus) peel for use as potential natural colorant. Pak J Biol Sci 11(18):2259–2263
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Org Cult 33(2):105–119
Infante R (1992) In vitro axillary shoot proliferation and somatic embryogenesis of yellow pitaya Mediocactus coccineus (Salm-Dyck). Plant Cell Tissue Org Cult 31:155–159
Le Bellec F, Vaillant F, Imbert E (2006) Pitahaya (Hylocereus spp.): a new fruit crop, a market with a future. Fruits 61(4):237–250
Liaotrakoon W (2013a) Characterization of dragon fruit (Hylocereus spp.) components with valorization potential. Dissertation for Ph.D. Faculty of Bioscience Engineering, Ghent University
Liaotrakoon W, De Clercq N, Van Hoed V, Dewettinck K (2013b) Dragon fruit (Hylocereus spp.) seed oils: their characterization and stability under storage conditions. J Am Oil Chem Soc 90(2): 207–215
Mizrahi Y, Nerd A (1999) Climbing and columnar cacti: new arid land fruit crops. In: Janick J (ed) Perspectives on new crops and new uses. ASHS Press, Alexandria, pp 358–366
Mohamed-Yasseen Y (2002) Micropropagation of pitaya (Hylocereus undatus Britton et rose). Vitro Cell Dev Biol-Plant 38(5):427–429
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 15:473–497
Nurliyana R, Syed Zahir I, Mustapha Suleiman K, Aisyah MR, Kamarul Rahim K (2010) Antioxidant study of pulps and peels of dragon fruits: a comparative study. Int Food Res J 17:367–375
Ortiz-Hernández YD, Carrillo-Salazar JA (2012) Pitahaya (Hylocereus spp.): a short review. Comunicata Scientiae 3(4):220–237
Pelah D, Kaushik RA, Mizrahi Y, Sitrit Y (2002) Organogenesis in the vine cactus Selenicereus megalanthus using thidiazuron. Plant Cell Tissue Org Cult 71:81–84
Rathore NS, Rai MK, Phulwaria M, Rathore N, Shekhawat NS (2014) Genetic stability in micropropagated Cleome gynandra revealed by SCoT analysis. Acta Physiol Plant 36(2):555–559
Stintzing FC, Carle R (2007) Betalains–emerging prospects for food scientists. Trends Food Sci Technol 18(10):514–525
Viñas M, Fernández-Brenes M, Azofeifa A, Jiménez VM (2012) In vitro propagation of purple pitahaya (Hylocereus costaricensis [F.A.C. Weber] Britton & Rose) cv. Cebra. In Vitro Cell Dev Biol Plant 48:469–477
Wichienchot S, Jatupornpipat M, Rastell RA (2010) Oligosaccharides of pitaya (dragon fruit) flesh and their prebiotic properties. Food Chem 120(3):850–857
Xia L, Qin YH, Liu CM, Hu GB (2014) Establishment and application of SCoT-PCR system for litchi. Chinese Agric Sci Bull 30(13):147–156
Acknowledgments
This work was supported by Science and Technology Planning Project of Guangzhou (2014Y2-00164); Agricultural Technology Extension Project of Guangdong Province (5300-F13085); Key Laboratory of Innovation and Utilization for Germplasm Resources in Horticultural Crops in South China of Guangdong Higher Education Institutes, South China Agricultural University (SCAU) (KBL11008); Key Laboratory of Biology and Genetic Improvement of Horticultural Crops, South China of Ministry of Agriculture, College of Horticulture, SCAU.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Qing-zhu Hua, Pengkun Chen and Wanqing Liu have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hua, Q., Chen, P., Liu, W. et al. A protocol for rapid in vitro propagation of genetically diverse pitaya. Plant Cell Tiss Organ Cult 120, 741–745 (2015). https://doi.org/10.1007/s11240-014-0643-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0643-9