Abstract
Isolated-subsegmental-pulmonary-embolism (SSPE) is increasingly diagnosed with the use of computed-tomography-pulmonary-angiogram (CTPA). There remains clinical equipoise for management of SSPE with previous studies not accounting for frailty while determining clinical outcomes. Clinical outcomes among patients with isolated SSPE were compared with those with a more proximal PE after accounting for frailty and other risk-factors. This study included all patients with a positive CTPA for pulmonary embolism (PE) admitted between 2017 and 2021 to two Australian-tertiary-hospitals. Frailty was determined by use of the hospital-frailty-risk-score (HFRS). Competing-risk-analysis and Cox-proportional hazard models determined the cumulative-risk of VTE and mortality within 3 months and 1 year of index PE event after adjustment for frailty and other variables. Of 334 patients with positive CTPA for PE, 111 (33.2%) had isolated-SSPE. The mean (SD) age was 64.3 (17.7) years, 50.9% were males and 9.6% were frail. The risk of recurrent VTE within 3-months (0.9% vs. 1.8%, P = 0.458) and within 1-year of follow-up (2.7% vs. 6.3%, P = 0.126) did not differ significantly between patients with isolated SSPE and those with more proximal PE. After adjusted analyses, the cumulative-incidence of recurrent VTE was not different among patients with isolated SSPE within 1 year of index event [subdistribution-hazard-ratio (HR) 0.84, 95% CI 0.19 to 3.60]. Similarly, mortality within 1 year of index event was also not different between the two groups (aHR 1.72, 95% CI 0.92–3.23). The prevalence of SSPE was 33.2% and even after adjustment for frailty these patients had no different clinical outcomes than those with proximal PE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Clinical equipoise remains regarding the management of subsegmental pulmonary embolism (PE), and no previous study has accounted for frailty.
-
Observational study involving two tertiary hospitals in Australia.
-
Prevalence of SSPE was 33.1% and 9.6% of patients were frail.
-
Clinical outcomes of patients with SSPE were no different from those with proximal PE after adjustment for frailty and other risk factors.
Introduction
Each year 30,000 Australians develop venous thromboembolism (VTE) (including deep vein thrombosis, DVT or PE), at a cost of $1.72 billion to the Australian Health Care System [1, 2]. In 2020, PE accounted for 368 deaths (standardised death rate 1.1%) with 3080 years of potential life lost in Australia [3]. PE can be classified as acute isolated subsegmental PE (SSPE) when it is localised only to one or more subsegmental pulmonary arteries but not in larger order vessels [4]. Previous evidence suggest that SSPE may account for 7–36% of PE diagnosed on computed tomography pulmonary angiogram (CTPA) and majority of these patients receive anticoagulation [5, 6]. The diagnosis and management of SSPE remains challenging because there is only a fair interobserver agreement between radiologists (K = 0.38, 95% CI 0.0–0.89) in interpreting CTPAs for SSPE [7]. A study [8] reported that 11% of CTPAs positive for SSPE, when reinterpreted by a thoracic radiologist were found to have no evidence of PE. Thus, many SSPEs detected on CTPA may represent false-positive results (artefacts) rather than true PE [9]. Many experts also feel that because lungs act as a natural filter for the systemic circulation, therefore, SSPE may represent a normal finding [10]. This hypothesis is further supported by studies suggesting that patients with SSPE have a less severe clinical presentation with a lower risk of VTE recurrence and PE related mortality compared to more proximal PE [6, 11]. This is in contrast to other studies which have indicated that SSPE poses similar risk in terms of VTE recurrence and PE-related mortality as more proximal PE [4, 12].
The clinical significance of SSPE thus remains unclear and whether the use of anticoagulation therapy in such patients carries a favourable risk benefit ratio is still controversial [13,14,15]. Guidelines from the American College of Chest Physicians recommends surveillance over anticoagulation for patients with SSPE who have no evidence of proximal DVT and who are not at a higher risk for recurrent VTE [16]. On the other hand, a recent prospective study [17] suggests that patients with SSPE who were managed without anticoagulation had a higher risk of progression of their PE and were more likely to develop proximal DVT in the first 90 days of the index event than might be expected in patients without a history of VTE. Furthermore, that same study excluded patients who had active cancer and found that recurrent VTE within 90 days was much less frequent in younger patients when compared to older subjects. It is quite possible that apart from comorbidities such as cancer, frailty could have been associated with poorer clinical outcomes in patients who develop and receive treatment for SSPE. To our knowledge previous studies on SSPE have not taken into account the impact of frailty (and its attendant pro-thrombotic and mobility issues [18]) while determining risk of recurrent VTE or mortality. The aims of this study were, therefore, to determine the risk of recurrent VTE and mortality determined at different time points among patients with SSPE after taking into account their frailty status along with other risk factors. We also determined whether these clinical outcomes were any different among patients who were younger or older than 60 years of age. The hypothesis for this study was that the risk of recurrent VTE will be no different in patients with isolated SSPE when compared to those with a more proximal PE irrespective of their age and frailty status, thus supporting treatment rather than ongoing surveillance.
Methods
This study was designed as a retrospective observational study and included all patients who were diagnosed with PE at two tertiary hospitals Flinders Medical Centre (FMC) and Royal Adelaide Hospital (RAH) between 1 January 2017 and 31 December 2021. The study protocol was reviewed by the Southern Adelaide Human Clinical Research Ethics Committee (SAHREC) and was determined to be exempt.
Identification of SSPE
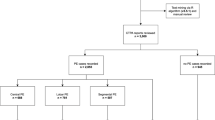
We identified all consecutive patients who were admitted with a primary diagnosis of PE during the study period using the I26, I26.0 and I26.9 International Classification of Diseases 10th Revision Australian Classification (ICD-AM) codes, as has been reported in a previous study [19]. The CTPAs of all patients with confirmed PE were examined to determine whether patients had isolated SSPE according to the radiologist’s final conclusion. “Isolated SSPE” was defined as single or multiple filling defects localised at the level of subsegmental pulmonary arteries with no filling defects identified at the more proximal pulmonary arteries level. Patients were therefore classified into two groups based on the location of PE as either isolated SSPE or proximal PE (the latter being diagnosed if filling defects were located at a more central location (segmental, pulmonary arteries, or pulmonary bifurcation). Patients were excluded from the study if they had inconclusive results, for example when the radiologist was uncertain about the diagnosis of PE due to it being a suboptimal study due to either patient related or technical factors such as incomplete visualisation of pulmonary arteries, were excluded from the study.
The frailty status of patients at the time of the index PE event was determined by use of the hospital frailty risk score, HFRS [20]. The HFRS is determined from the International Disease Classification codes (ICD-10) and correlates with frailty risk and has been validated for use in hospitalised patients [20, 21]. Higher HFRS scores are associated with a greater risk of frailty, and the scores can be categorised into three levels as per original study: low risk (< 5 points), intermediate risk (5–15 points) and high-risk (> 15 points) [20]. For this study we classified patients as frail if HFRS was greater than 5 points as been reported in a previous study [21].
We also identified the following variables: any previous history of cancer or active cancer (defined as new or ongoing cancer not in remission or receipt of any cancer related treatment in the previous 6 months of the diagnosis of PE), history of previous VTE, atrial fibrillation, the comorbidity burden as determined by the Charlson comorbidity index (CCI) [22], history of cardiovascular disease (CAD), heart failure, valvular heart disease, chronic lung disease, history of chronic kidney disease (CKD), use of anticoagulants prior to hospitalization—warfarin, direct oral anticoagulants (DOACs), heparin and enoxaparin, use of antithrombotic agents (aspirin, clopidogrel, ticagrelor), history of inherited thrombophilia, and presence of autoimmune diseases, and any previous hospitalisations or surgery within 3 months of index admission.
We determined the modified Wells score [23] to assess the pretest probability of PE and the short pulmonary embolism severity index (sPESI) [24] was used to estimate the 30-day mortality risk. Patients’ risk of bleeding was assessed by use of the VTE-bleed score [25]. We assessed patients’ treatment during the index PE event [unfractionated heparin, low molecular weight heparin (LMWH), warfarin, direct acting oral anticoagulants (DOACs), thrombolysis, inferior vena cava filter] and the duration of anticoagulation was recorded.
We recorded routine investigations performed during index hospitalisation such as haemoglobin, white cell count (WBC), D-dimer, C-reactive protein (CRP), creatinine, albumin, N terminal pro brain natriuretic peptide (NT-proBNP) and cardiac troponin T(TnT).
Patient follow-up
We followed patients for any recurrence of VTE (DVT or PE) in the 12 months period following the index PE event. This was done through evaluation of our radiological data base for follow-up investigations such as venous ultrasound of legs, CTPA or ventilation perfusion scan as well as any future admissions due to VTE symptoms. All hospital visits and admissions for the next 12 months were reviewed for possible symptoms related to VTE and any new imaging studies relating to the diagnosis of DVT or PE. Medical records of patients who died were evaluated to determine the cause of death and whether VTE was a contributing factor to death.
Outcomes
The primary outcome was the cumulative incidence of recurrent VTE (defined as an objective evidence of new or recurrent, fatal or non-fatal, symptomatic PE or DVT on imaging, as previously described [26]) within 3 months and 1 year following the date of the index event. Secondary outcomes included overall mortality and PE-related mortality within 3 months and 1 year, length of hospital stay (LOS), number of medical emergency response team (MET) calls during index hospitalisation, intensive care unit (ICU) admission, and the 30-day readmission rates following hospital discharge from the index PE event. We also determined whether patients developed any major bleeding event during their index PE event. Major bleeding was defined as any bleeding which was fatal or resulted in ≥ 20 g/L drop in haemoglobin levels or requiring ≥ 2 units of blood transfusion or bleeding into any of the following critical anatomic areas (retroperitoneal, intraocular, intraarticular, intracranial or intraspinal). Two blinded clinical experts adjudicated all outcomes. They classified the cause of all deaths as definitely due to PE (if confirmed on autopsy or death following clinically severe PE) or possibly due to PE (when death was sudden and without other explanation or due to other causes). We classified PE-related death as death definitely or possibly related to new/recurrent PE.
Statistics
We compared the baseline characteristics, Wells score, sPESI score and VTE-Bleed score according to the location of PE using t-tests/Wilcoxon’s rank sum test for continuous variables and chi-squared tests for categorical variables, as appropriate. Recurrent VTE, overall mortality and PE-related mortality were compared among patients with SSPE vs. those with more proximal PE within 3 months and 1 year following the index event. In addition, we determined and compared the PE-related deaths during index hospitalisation (case fatality rate). As death can be a competing risk for recurrent VTE, so we used Fine and Gray model [27], and explored associations between SSPE and recurrent VTE using competing risk regression. This method yields sub-distribution hazard ratios (SHR) with corresponding 95% confidence intervals (CI) and cumulative incidence function (CIF) curve was plotted. We adjusted the models for risk factors known to be associated with recurrent VTE (age, sex, active cancer, prior history of VTE, HFRS, CCI, whether on anticoagulation, type of anticoagulant used and the duration of anticoagulation as a time-varying covariate. Mortality was compared using the Kaplan–Meier estimator and use of the log-rank test. In addition, Cox regression with robust standard errors determined hazard ratios after adjustment for the above-mentioned co-variates. All tests were 2-sided and a P-value of < 0.05 was considered statistically significant. All analyses were performed using Stata software vs. 15.0 (Stata Corporation, College Station, Texas).
Sensitivity analysis
Sensitivity analysis was performed by limiting analysis to patients who were older or younger than 60 years of age as well as those who were identified as frail according to the HFRS, to determine whether recurrent VTE or mortality within 1 year was significantly different in these groups.
Results
Of 340 patients who had a positive CTPA for PE between 1 January 2017 and 31 December 2021, we excluded 6 patients due to various reasons (Fig. 1). One hundred and eleven patients (33.2%) patients were found to have isolated SSPE, of whom, 60 (54.1%) had a solitary SSPE. The mean (SD) age was 64.3 (17.7) years, 50.9% were males and 9.6% were classified as frail according to the HFRS.
The baseline characteristics of patients who had an isolated SSPE were compared with the proximal PE group and are presented in Table 1. The characteristics of patients were similar between the two groups apart from a history of hypertension, which was more common among patients who had proximal PE when compared to those with isolated SSPE. There were no differences between the two groups in the mean frailty scores or the proportion of patients identified as frail according to the HFRS (P < 0.05). Patients who had isolated SSPE had a lesser clot burden [28], as was reflected by significantly lower d-Dimer levels (P = 0.018), and they were less likely to have pulmonary infarction and had lower TnT levels than patients who had a more proximal PE (P < 0.05). The clinical presentation of PE was similar between the two groups, apart from history of dyspnoea, which was less common among patients who had isolated SSPE when compared to those who had a more proximal PE (P = 0.026). However, the proportion of isolated SSPE patients who presented with dyspnoea was not significantly different from those with a more proximal PE, if they were frail (77.8% vs. 56.5%, P = 0.264). Fifteen (4.5%) patients with PE were asymptomatic, and the proportion of patients who had an asymptomatic PE was not significantly different between isolated SSPE and more proximal PE group (5.4% vs. 4.0%, P = 0.569). The mean (SD) Wells score and the severity of PE as determined by the sPESI were similar between the two groups (P > 0.05). Similarly, the risk of bleeding as determined by the VTE-bleed score was also similar between the two groups (P > 0.05, Table 1).
All but 10 patients (4 with isolated SSPE, 6 with more proximal PE) were treated with anticoagulation and there was no difference in receipt of this treatment between the SSPE and the more proximal PE group [107 (96.4%) vs. 217 (98.6%), P = 0.181]. The mean (SD) duration of anticoagulation treatment was 257.8 (119.7) days and patients with SSPE were more likely to receive a shorter duration of treatment than those with more proximal PE (P < 0.001, Table 2). Most patients (61.3%) received initial anticoagulation treatment with enoxaparin overlapping with oral agents (DOACs or warfarin) and this initial treatment was more likely to be prescribed to patients who had proximal PE when compared to those who had isolated SSPE (P = 0.03). However, there was no difference in the prescription of individual oral anticoagulant agents upon discharge from hospital between the two groups (P > 0.05, Table 2).
Outcomes
Recurrent VTE
Overall, 5 (1.5%) patients developed recurrent VTE within 3 months of the index event. Of these 3 (0.9%) developed DVT while 2 (0.6%) patients were diagnosed with PE. Within 12 months following the index event and including VTE events within 3 months, 17 (5.1%) of patients were diagnosed with recurrent VTE. Of these, 3 (0.9%) had DVT and 14 (4.2%) had PE. The risk of recurrent VTE within three months and within 1 year of follow-up did not differ significantly between patients with isolated SSPE and those with more proximal PE (Table 3).
The cumulative incidence of recurrent VTE within 1 year was not significantly different between isolated SSPE and more proximal PE (SHR 0.84, 95% CI 0.22 to 3.19, P = 0.785, Fig. 2). After adjusted analysis, there was no statistically significant difference in recurrent VTE during follow-up between the two groups (SHR 0.84, 95% CI 0.19 to 3.60, P = 0.864, Table 4).
Mortality
Of the 24 patients with isolated SSPE who died within the 12 months of follow-up period, 2 died of recurrent confirmed PE during index hospitalisation while 1 patient died of possible PE on day 21 of follow-up. Of the 33 patients with proximal PE who died during follow-up, 6 patients died from definite PE (n = 2) or possible PE (n = 4). Of these patients, 1 patient died from PE during the hospitalisation for index PE. Overall mortality and PE related mortality during the index hospitalisation, within 3 months, and over 1 year of follow-up period (Table 3; Fig. 3) did not differ significantly between patients with isolated SSPE and those with a more proximal PE. After adjusted analysis, the risk of overall mortality did not differ between the two groups within 1 year of follow-up period. (HR 1.72, 95% CI 0.92–3.23, P = 0.115, Table 4).
Other clinical outcomes
The index admission median (IQR) LOS was significantly prolonged among patients who had more proximal PE compared to those with SSPE [3.1 (1.7, 4.8) vs. 1.8 (0.8, 3.8) days, P = 0.0001]. Similarly, patients with proximal PE had a significantly higher number of MET calls during their index hospitalisation when compared to those with SSPE (P < 0.05), however, the risk of admission to ICU was similar between the two groups (P > 0.05). Risk of major bleeding episodes were also similar between the two groups (P > 0.05, Table 3).
Sensitivity analysis
There was no significant difference in the risk of recurrent VTE within 1 year of follow-up whether the analysis was limited to patients who were 60 years of age or over and patients who had isolated SSPE when compared to those with proximal PE (SHR 0.36, 95% CI 0.07–1.79, P = 0.465) or whether we compared patients with differing PEs who were younger than 60 years (SHR 0.57, 95% CI 0.05–5.95, P = 0.644, Table 4). Similarly, overall mortality was not significantly different between the two PE groups within 1 year of follow up when the analysis was limited to patients ≥ 60 years (HR 1.41, 95% CI 0.71–2.82, P = 0.196) nor when limited to patients who were < 60 years of age (HR 8.12, 95% CI 0.52–125.26, P = 0.133, Table 4). The risk of recurrent VTE and mortality within 1 year was also found to be not significantly higher among SSPE patients who were identified as frail when compared to frail patients with a more proximal PE (Table 4).
Discussion
The results of this study suggest that isolated SSPE accounted for 33.2% of all CTPAs which were positive for PE over the 5-year study period. Almost all patients (96.2%) received anticoagulation and there was no difference in receipt of treatment between isolated SSPE and the more proximal PE group. The risk of recurrent VTE, overall mortality and PE-related mortality was not significantly different within 3 months and 1 year of follow up between the two groups and was also not different in patients who were younger or older than 60 years of age, irrespective of their frailty status.
The clinical characteristics of patients with isolated SSPE were similar to those with more proximal PE in terms of VTE risk factors, CCI and frailty status. These results are similar to previous studies [4, 29] which have found similar prevalence of VTE risk factors and comorbid conditions between isolated SSPE and more proximal PE, although, no previous study has previously determined and compared frailty status of patients according to the location of PE. Our study also found that there was no difference in the prevalence of cancer between the two groups, although, a previous study which included 54 patients with SSPE found a significantly higher risk of active cancer among patients with isolated SSPE when compared to proximal PE. This could be related to the higher median age (76 vs. 63 years) of patients with SSPE in that study when compared to our study. Whether clinical presentation of patients who have isolated SSPE is more benign as compared to more proximal PE is controversial. Similar to other studies [5, 13], our study indicate that patients with isolated SSPE were less likely to present with dyspnoea, had lower levels of biomarkers (D-dimer and TnT), and were less likely to have a CT evidence of RV dysfunction than patients with more proximal PE.
Our study suggests that the vast majority of patients with isolated SSPE are managed with anticoagulation treatment which is consistent with the results of previous studies [30, 31]. We, however, found that patients with isolated SSPE received a significantly shorter duration of anticoagulation treatment than those with a more proximal PE. This might indicate that physicians also consider the localisation of PE, in addition to other factors while determining the duration of anticoagulation. Our study also indicates that patients with isolated SSPE are significantly less likely to receive initial overlapping treatment with LMWH (53.2% vs. 65.5%) when compared to those with more proximal PE. This might indicate that physicians perceive the risk of adverse outcomes related to isolated SSPE to be lower than those with proximal PE with a focus on outpatient management of such patients, as has been found in a previous study [4].
This study did not find any statistically significant differences in recurrent VTE, overall mortality and PE related mortality between patients with isolated SSPE and proximal PE irrespective of the age and frailty status, while acknowledging that the number of recurrent VTE events was low in our study (1.5%; five VTE events within 3 months). Our results are consistent with a previous study which found that the risk of recurrent VTE at 3 months follow up among patients with isolated SSPE who were older than 65 years was 2% and was not significantly different (HR 0.64, 95% CI 0.25–1.62) from patients with a more proximal PE. In our study, overall mortality within 1 year of follow up was, if anything, higher among patients with isolated SSPE when compared to patients with proximal PE (21.6% vs. 14.8%). After adjustment for potential confounders including frailty, isolated SSPE was associated with an equivalent risk of death at 1 year (HR 1.64, 95% 0.88–3.05) to the risk for those with more proximal PEs. This presents a startling contrast to earlier cited studies [6, 11], where isolated SSPE was associated with a lower risk of adverse clinical outcomes than proximal PE. Our results match a study by den Exter et al. [12], which also found that overall mortality between anticoagulated patients with isolated SSPE and proximal PE was not significantly different (HR 1.5, 95% CI 0.8–2.8). It is however important to note that because almost all patients in our study and the above-mentioned studies received anticoagulation, so outcome comparisons between isolated SSPE and proximal PE may have been blurred. Interestingly, in our study the case fatality rate during the index PE event was 1.8% for isolated SSPE compared to 0.4% for more proximal PE and all patients who died were receiving anticoagulation treatment at the time of death. However, isolated SSPE patients who died during their index admission were more likely to have a history of active cancer [2 (5.6%) vs. 0, P = 0.04] than patients who died during their index admission with a more proximal PE. Our data indicate that isolated SSPE is not necessarily a benign event, especially among patients with active cancer.
This study has several limitations. This study was limited to hospitalised patients with PE, and thus relatively healthier patients who were diagnosed with a clinically more benign PE and managed in the outpatient service may have been underrepresented. We cannot exclude the possibility that some patients without a PE were misclassified as having an isolated SSPE because interobserver agreement for detection of SSPE based upon CTPA is only fair (K = 0.38). There were only a small number of patients who developed recurrent VTE which could potentially limit the power to detect potential outcome differences between SSPE and proximal PE.
In conclusion, isolated SSPE represent a significant proportion of positive CTPAs and not many clinical characteristics differentiate the hospitalised patient with SSPE from one with more proximal PE. Multimorbidity and frailty risk were similar between patients with these two types of PE and the incidences of adverse outcomes in terms of VTE recurrence and death were not different between patients with SSPE and those with more proximal PE irrespective of frailty status and age. The patient with an SSPE may derive the same benefits from anticoagulation as are enjoyed by those with a more proximal PE. There is a need for a future RCT to determine whether withholding anticoagulation is safe in all or in certain select low-risk patients with SSPE.
Data availability
Data used for this research are available from the authors on reasonable request and if permitted by the ethics committee.
References
Gallerani M, Imberti D, Ageno W, Dentali F, Manfredini R (2011) Higher mortality rate in patients hospitalised for acute pulmonary embolism during weekends. Thromb Haemost 106:83–89. https://doi.org/10.1160/th11-02-0068
Ho WK, Hankey GJ, Eikelboom JW (2008) The incidence of venous thromboembolism: a prospective, community-based study in Perth, Western Australia. Med J Aust 189:144–147. https://doi.org/10.5694/j.1326-5377.2008.tb01947.x
Australian Bureau of Statistics (2020) Causes of death, Australia. ABS. https://www.abs.gov.au/statistics/health/causes-death/causes-death-australia/2020. Accessed 1 Feb 2023
Stoller N, Limacher A, Méan M, Baumgartner C, Tritschler T, Righini M, Beer JH, Rodondi N, Aujesky D (2019) Clinical presentation and outcomes in elderly patients with symptomatic isolated subsegmental pulmonary embolism. Thromb Res 184:24–30. https://doi.org/10.1016/j.thromres.2019.10.008
Lee YH, Cha SI, Shin KM, Lim JK, Lee WK, Park JE, Choi SH, Seo H, Yoo SS, Lee SY et al (2021) Clinical characteristics and outcomes of patients with isolated pulmonary embolism. Blood Coagul Fibrinolysis 32:387–393. https://doi.org/10.1097/mbc.0000000000001050
Donato AA, Khoche S, Santora J, Wagner B (2010) Clinical outcomes in patients with isolated subsegmental pulmonary emboli diagnosed by multidetector CT pulmonary angiography. Thromb Res 126:e266-270. https://doi.org/10.1016/j.thromres.2010.07.001
Stein PD, Hull RD (2007) Multidetector computed tomography for the diagnosis of acute pulmonary embolism. Curr Opin Pulm Med 13:384–388. https://doi.org/10.1097/MCP.0b013e32821acdbe
Pena E, Kimpton M, Dennie C, Peterson R, Gal GLE, Carrier M (2012) Difference in interpretation of computed tomography pulmonary angiography diagnosis of subsegmental thrombosis in patients with suspected pulmonary embolism. J Thromb Haemost 10:496–498. https://doi.org/10.1111/j.1538-7836.2011.04612.x
Moores LK (2018) Are we overtreating isolated subsegmental pulmonary embolism? First do no harm. JAMA Intern Med 178:1274–1275. https://doi.org/10.1001/jamainternmed.2018.2970
Baumgartner C, Klok FA, Carrier M, Limacher A, Moor J, Righini M, Beer JH, Peluso M, Rakovic D, Huisman MV, Aujesky D (2020) Clinical Surveillance vs. Anticoagulation For low-risk patiEnts with isolated SubSegmental Pulmonary Embolism: protocol for a multicentre randomised placebo-controlled non-inferiority trial (SAFE-SSPE). BMJ Open 10:e040151. https://doi.org/10.1136/bmjopen-2020-040151
Bariteau A, Stewart LK, Emmett TW, Kline JA (2018) Systematic review and meta-analysis of outcomes of patients with subsegmental pulmonary embolism with and without anticoagulation treatment. Acad Emerg Med 25:828–835. https://doi.org/10.1111/acem.13399
den Exter PL, van Es J, Klok FA, Kroft LJ, Kruip MJ, Kamphuisen PW, Büller HR, Huisman MV (2013) Risk profile and clinical outcome of symptomatic subsegmental acute pulmonary embolism. Blood 122:1144–1149 (quiz 1329). https://doi.org/10.1182/blood-2013-04-497545
Le Gal G, Righini M, Parent F, van Strijen M, Couturaud F (2006) Diagnosis and management of subsegmental pulmonary embolism. J Thromb Haemost 4:724–731. https://doi.org/10.1111/j.1538-7836.2006.01819.x
Eyer BA, Goodman LR, Washington L (2005) Clinicians’ response to radiologists’ reports of isolated subsegmental pulmonary embolism or inconclusive interpretation of pulmonary embolism using MDCT. Am J Roentgenol 184:623–628. https://doi.org/10.2214/ajr.184.2.01840623
Goodman LR (2005) Small pulmonary emboli: what do we know? Radiology 234:654–658. https://doi.org/10.1148/radiol.2343041326
Stevens SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing GJ, Huisman MV, Kearon C, King CS, Knighton AJ et al (2021) Antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest 160:e545–e608. https://doi.org/10.1016/j.chest.2021.07.055
Le Gal G, Kovacs MJ, Bertoletti L, Couturaud F, Dennie C, Hirsch AM, Huisman MV, Klok FA, Kraaijpoel N, Mallick R et al (2022) Risk for recurrent venous thromboembolism in patients with subsegmental pulmonary embolism managed without anticoagulation: a multicenter prospective cohort study. Ann Intern Med 175:29–35. https://doi.org/10.7326/m21-2981
Folsom AR, Boland LL, Cushman M, Heckbert SR, Rosamond WD, Walston JD (2007) Frailty and risk of venous thromboembolism in older adults. J Gerontol A 62:79–82. https://doi.org/10.1093/gerona/62.1.79
Hoskin S, Brieger D, Chow V, Kritharides L, Ng ACC (2021) Trends in acute pulmonary embolism admission rates and mortality outcomes in Australia, 2002–2003 to 2017–2018: a retrospective cohort study. Thromb Haemost 121:1237–1245. https://doi.org/10.1055/s-0041-1725932
Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, Arora S, Street A, Parker S, Roberts HC et al (2018) Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 391:1775–1782. https://doi.org/10.1016/s0140-6736(18)30668-8
Sharma Y, Horwood C, Hakendorf P, Shahi R, Thompson C (2022) External validation of the hospital frailty-risk score in predicting clinical outcomes in older heart-failure patients in Australia. J Clin Med. https://doi.org/10.3390/jcm11082193
Shebeshi DS, Dolja-Gore X, Byles J (2021) Charlson Comorbidity Index as a predictor of repeated hospital admission and mortality among older women diagnosed with cardiovascular disease. Aging Clin Exp Res 33:2873–2878. https://doi.org/10.1007/s40520-021-01805-2
Angriman F, Ferreyro BL, Posadas-Martinez ML, Giunta D, Vazquez FJ, Vollmer WM (2015) Wells score and poor outcomes among adult patients with subsegmental pulmonary embolism: a cohort study. Clin Appl Thromb Hemost 21:539–545. https://doi.org/10.1177/1076029614559772
Wells P, Peacock WF, Fermann GJ, Coleman CI, Wang L, Baser O, Schein J, Crivera C (2019) The value of sPESI for risk stratification in patients with pulmonary embolism. J Thromb Thrombolysis 48:149–157. https://doi.org/10.1007/s11239-019-01814-z
Nishimoto Y, Yamashita Y, Morimoto T, Saga S, Amano H, Takase T, Hiramori S, Kim K, Oi M, Akao M et al (2020) Validation of the VTE-BLEED score’s long-term performance for major bleeding in patients with venous thromboembolisms: from the COMMAND VTE Registry. J Thromb Haemost 18:624–632. https://doi.org/10.1111/jth.14691
Méan M, Righini M, Jaeger K, Beer HJ, Frauchiger B, Osterwalder J, Kucher N, Lämmle B, Cornuz J, Angelillo-Scherrer A et al (2013) The Swiss cohort of elderly patients with venous thromboembolism (SWITCO65+): rationale and methodology. J Thromb Thrombolysis 36:475–483. https://doi.org/10.1007/s11239-013-0875-2
Austin PC, Fine JP (2017) Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat Med 36:4391–4400. https://doi.org/10.1002/sim.7501
Hochuli M, Duewell S, Frauchiger B (2007) Quantitative d-dimer levels and the extent of venous thromboembolism in CT angiography and lower limb ultrasonography. Vasa 36:267–274. https://doi.org/10.1024/0301-1526.36.4.267
Armitage MN, Mughal AZ, Huntley CC, Lasserson D, Newnham M (2022) A multicentre observational study of the prevalence, management, and outcomes of subsegmental pulmonary embolism. J Thromb Thrombolysis. https://doi.org/10.1007/s11239-022-02714-5
Raslan IA, Chong J, Gallix B, Lee TC, McDonald EG (2018) Rates of overtreatment and treatment-related adverse effects among patients with subsegmental pulmonary embolism. JAMA Intern Med 178:1272–1274. https://doi.org/10.1001/jamainternmed.2018.2971
Pesavento R, Casazza F, Filippi L, Milan M, Monreal M, Prandoni P (2013) An international survey on isolated subsegmental pulmonary embolism. Thromb Res 131:183–184. https://doi.org/10.1016/j.thromres.2012.11.017
Funding
The authors did not receive support from any organisation for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors have read and approved this manuscript for submission. YS conceived and designed this study. SS, RS and CH were involved in data collection. YS, SS, RS, CH, and CT contributed to analysis, interpretation, writing, and revising of the manuscript including final approval.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, Y., Sumanadasa, S., Shahi, R. et al. The value of distinguishing patients with isolated subsegmental pulmonary embolism presenting to two tertiary hospitals in Australia: an observational study. J Thromb Thrombolysis 56, 215–225 (2023). https://doi.org/10.1007/s11239-023-02845-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-023-02845-3