Abstract
This meta-analysis was performed to investigate the efficacy and safety of tranexamic acid (TXA) in the elderly patients undergoing intertrochanteric fracture surgery from the current literatures. The electronic literature database of PubMed, Embase and Cochrane library were searched in October 2019. The intraoperative blood loss, hidden blood loss, postoperative drainage and total blood loss, postoperative hemoglobin, length of stay, transfusion rate, mortality rate, thromboembolic events and wound complications were extracted. Stata 14.0 software was used for our meta-analysis. A total of 11 RCTs (3 new RCTs in 2019) with 1202 patients met our inclusion criteria. This meta-analysis showed that administration of TXA can reduce intraoperative blood loss (P = 0.009), hidden blood loss (P = 0.000), total blood loss (P = 0.000), length of stay (P = 0.003), transfusion rate (P = 0.000) and the occurrence of wound complications (P = 0.006). Furthermore, administration of TXA was associated with an increase in the postoperative Hb level at day 1, 2 and 3 (P = 0.000, P = 0.000 and P = 0.000, respectively) after surgery. However, no significant difference was found between the TXA group and control group regarding the occurrence of thromboembolic events (P = 0.978, including deep vein thrombosis, P = 0.850; pulmonary embolism, P = 0.788; cerebrovascular accident, P = 0.549; myocardial infarction, P = 0.395) and mortality rate (P = 0. 338). Our meta-analysis suggested that administration of TXA is effective in reducing intraoperative blood loss, hidden blood loss, total blood loss, length of stay, transfusion rate, wound complications and enhancing postoperative Hb without increasing the risk of thromboembolic events and mortality rate in intertrochanteric fracture surgery. More large multi-center and high-quality RCTs are required for further research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
This meta-analysis was to investigate the efficacy and safety of tranexamic acid (TXA) in the elderly patients undergoing intertrochanteric fracture surgery.
-
TXA can reduce intraoperative blood loss, hidden blood loss, total blood loss, length of stay, transfusion rate and the occurrence of wound complications.
-
TXA was associated with an increase in the postoperative Hb level at day 1, 2 and 3 after surgery.
-
No significant difference was found between the TXA group and control group regarding the occurrence of thromboembolic events and mortality rate.
Introduction
Hip fractures are common in the elderly and have become a major burden for health care systems [1]. Their annual incidence is mpounded by the problems of aging, hip fractures are a increasing rapidly and is projected to surpass 6.3 million by 2050 [2, 3]. More than 250 000 hip fractures occur annually and mostly in the elderly, with the 1-year mortality rates ranging from 14 to 36%, due to the frequent association with osteoporosis [4, 5]. As the main type, intertrochanteric fracture accounts for half of hip fractures, nearly 30% of intertrochanteric fracture patients die in the first 12 months, especially the elderly with limited activity [6, 7]. This common type of fractures frequently results in considerable blood loss, which exposes patients to postoperative anaemia and reduced functional recovery [8, 9]. Furthermore, large amounts of blood loss usually lead to blood transfusion and a high risk of perioperative morbidity and mortality [10]. Blood transfusion increases the incidence of adverse reactions related to allogeneic blood transfusion, such as infectious diseases, haemolytic reaction, cardiovascular dysfunction, postoperative infection and elevated hospitalisation costs [11, 12]. Therefore, reducing perioperative blood loss concomitant to intertrochanteric fractures in elderly patients would help to decrease the rate of complications and improve functional outcomes.
A variety of methods are used in orthopaedic surgery to reduce blood loss, including the application of antifibrinolytic agent, autologous blood transfusion, controlled hypotension, intraoperative blood salvage, navigation, and minimally invasive surgery [13, 14]. Despite the effectiveness, these methods are still faced by many shortcomings.
Tranexamic acid (TXA), a synthetic derivative of the amino acid lysine, is an antifbrinolytic drug that competitively blocks the plasminogen-binding site, inhibits plasminogen activation, and interferes with fibrinolysis [15]. In recent years, many studies have demonstrated that TXA can effectively reduce blood loss and transfusion rate in hip and knee replacement surgery, without increasing the incidence of thrombotic events [16, 17]. Also, clinical trials have proved that TXA is effective in decreasing blood loss in spine surgery without incremental risk or complications [18]. Despite the extensive study of TXA in spine and arthroplasty surgery, a paucity of studies regarding its use in orthopaedic trauma surgery has limited its integration into the field, although it is these patients that may benefit most from TXA therapy. Recently, a number of studies have reported on the use of TXA in intertrochanteric fractures, but due to varying methods of administration and dosage, no consistent conclusions can be drawn. The efficacy and safety of TXA in the treatment of intertrochanteric fractures remains controversial.
Therefore, we conducted a meta-analysis of some published randomized controlled trials (RCTs) to explore the efficacy and safety of TXA in intertrochanteric fracture surgery by comparing their clinical results. The outcomes including intraoperative blood loss, hidden blood loss, postoperative drainage and total blood loss, postoperative hemoglobin, length of stay, transfusion rate, mortality rate, thromboembolic events (deep vein thrombosis, pulmonary embolism, cerebrovascular accident and myocardial infarction) and wound complications (infection and hematoma).
Methods
This systematic review and meta-analysis was conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statement [19]. No primary personal data will be collected; therefore no additional ethical approval needs to be obtained.
Search strategy
The electronic databases of PubMed, Embase and Cochrane library were searched from the inception of the database to October 2019, only English studies were included. Two independent researchers conducted literature searches using the search strategy of (“intertrochanteric fracture” or “hip fracture” or “trochanteric fracture” or “peritrochanteric fracture”) and (“tranexamic acid”). In addition, the reference lists of previously published randomized trials, review articles, and meta-analyses were manually searched for additional eligible studies. Related articles and reference lists were searched to avoid original miss.
Inclusion and exclusion criteria
We identified literature that met the following inclusion criteria: (1) RCTs; (2) skeletally mature patients (older than 18 years); (3) evaluation of the efficacy and safety of TXA in the treatment of intertrochanteric fracture. The exclusion criteria were: (1) non-randomized controlled studies, abstracts, case reports, letters, editorials, conference articles; (2) repeated studies and data.
Selection of literature
We used the PRISMA flow diagram to select the included studies. The results of literature search were imported into the software Endnote X7. Two authors independently assessed the potentially eligible studies. Firstly, the titles and abstracts were screened to exclude the duplicated and apparently irrelevant ones or those that do not meet our inclusion criteria. After then, the remaining potential studies were full-text downloaded and reviewed. Any disagreement between two above authors was sent and discussed with the third independent author.
Data extraction
Two reviewers independently extracted data, and the third reviewer checked the consistency between them. A standard form was used; the extracted items included the following: (1) the general study information, for example, the authors, publishing date, study design, case number, age, gender, fixation method, intervention method of tranexamic acid, thromboprophylaxis method, transfusion trigger and follow-up term. (2) clinical outcomes, including blood loss (intraoperative blood loss, hidden blood loss, postoperative drainage and total blood loss), postoperative hemoglobin (Hb), length of stay. (3) transfusion rate and mortality rate. (4) complications: thromboembolic events (deep vein thrombosis, pulmonary embolism, cerebrovascular accident and myocardial infarction) and wound complications (infection and hematoma). For continuous outcomes, we extracted the mean and standard deviation (SD) and participant number will be extracted. For dichotomous outcomes, we extracted the total numbers and the numbers of events of both groups. The data in other forms was recalculated when possible to enable pooled analysis. Disagreements between two researchers were resolved by discussion. Whenever necessary, we contacted the authors of the studies for the missing data and additional information.
Quality assessment of included studies
Two authors independently performed methodological quality and risk of bias assessment of the included RCTs using Cochrane collaboration's tool [20]. The Cochrane tool assesses following items: randomization, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other bias, for each individual item, classifies studies into low, unclear, and high risk of bias.
Statistical analysis
The data was collected and input into the STATA software (version 12.0; StataCorp, College Station, TX) for meta-analysis. A random-effects model was applied when heterogeneity was detected or the statistical heterogeneity was high (P < 0.05 or I2 > 50%) and then further subgroup study and meta-regression analysis were performed to detect the origin of heterogeneity. Otherwise, a fixed-effects model was used (P ≥ 0.05 or I2 ≤ 50%). To test the strength and stability of the pooled results, we performed a sensitivity analysis by omitting the individual studies one by one. Moreover, the effect of publication bias was investigated by Begg’s test and Egger’s test. Relative risk (RR) was calculated for dichotomous outcomes, standard mean difference (SMD) was calculated for continuous outcomes.
Results
Included studies
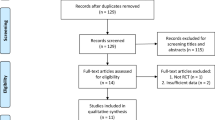
A total 152 potentially relevant articles were identified from the databases. After removal of duplicates, 53 articles were screened for relevance on the basis of the title and abstract. Of the 20 articles that were possibly eligible for inclusion, nine were excluded for reasons of “the papers were review or without available data” and some other reasons (details were showed in Fig. 1). The remaining 11 RCTs [21,22,23,24,25,26,27,28,29,30,31] were included in this meta-analysis.
Characteristics and quality assessment of the eligible studies
The characteristics of all the 11 included studies were summarized and shown in Table 1. All of them were published between 2015 and 2019. A total of 596 participants in tranexamic acid group and 606 in control group were included in this meta-analysis. In this meta-analysis, we included three new high quality RCTs [29,30,31] (all published in 2019) compared to the previous meta-analysis. Risk of bias assessment of RCTs was presented in Table 2.
Results of meta-analysis
Blood loss
Intraoperative blood loss
Six studies reported intraoperative blood loss [22, 24, 26, 27, 29, 30]. Heterogeneity was found in the pooled outcomes, so a random-effects model was utilized in our study (χ2 = 45.56, df = 5, I2 = 89.0%, P = 0.000). As shown in Fig. 2a, the pooled results showed that intraoperative blood loss in TXA group was significantly less than that in control group (SMD = − 0.754; 95% CI − 1.318 to − 0.190; P = 0.009).
Hidden blood loss
There were four studies reporting hidden blood loss [26, 27, 30, 31]. The pooled results indicated that TXA group showed significantly less hidden blood loss than control group (SMD = − 0.775; 95% CI − 1.055 to − 0.494; P = 0.000, Fig. 2b).
Postoperative drainage
The results of a pooled statistical analysis of five studies [22, 25,26,27, 30] are shown in Fig. 2c and indicate that there was significantly heterogeneity (χ2 = 66.06, df = 4, I2 = 93.9%, P = 0.000). The results revealed that postoperative drainage was not significantly different between the two groups (SMD = − 0.787; 95% CI − 1.580 to 0.005; P = 0.051).
Total blood loss
Seven studies stated total blood loss [22, 24, 26, 27, 29,30,31]. Based on the seven studies providing available data, the pooled results showed that significant heterogeneity was found (χ2 = 63.91, df = 6, I2 = 90.6%, P = 0.000), and therefore, a random-effects model was used. The available data demonstrated that total blood loss in TXA group was significantly less compared with that in control group (SMD = − 1.109; 95% CI − 1.656 to − 0.562; P = 0.000, Fig. 2d). The causes of heterogeneity in the results were explored by subgroup analysis and meta-regression. Subgroup analyses stratified by age, transfusion trigger, country and control group were conducted to investigate the difference between TXA and control group (Table 3). The subgroup analysis of age showed that total blood loss in TXA group was significantly less than that in control group in either low age group (< 70, SMD = − 2.744; 95% CI − 3.455 to − 2.033; P = 0.000) or high age group (≥ 70, SMD = − 0.876; 95% CI − 1.364 to − 0.389; P = 0.000). Meanwhile, the subgroup analysis of transfusion trigger indicated that TXA group had significantly less total blood loss in either low transfusion trigger group (SMD = − 1.166; 95% CI − 1.997 to − 0.336; P = 0.006) or high transfusion trigger group (SMD = − 1.071; 95% CI − 1.870 to − 0.273; P = 0.009). As for country subgroup, total blood loss was obviously less in TXA group than that in control group in China group (SMD = − 0.944; 95% CI − 1.501 to − 0.387; P = 0.001). However, there was no statistically significant difference between the two groups in non-China group (SMD = − 1.617; 95% CI − 3.793 to 0.559; P = 0.145). Besides, the subgroup analysis of the type of control group showed that no significant difference was found in placebo group (SMD = − 1.226; 95% CI − 2.585 to 0.134; P = 0.077). Whereas, TXA group had evidently less total blood loss in both normal saline group (SMD = − 1.226; 95% CI − 2.400 to − 0.053; P = 0.041) and blank group (SMD = − 0.855; 95% CI − 1.146 to − 0.565; P = 0.000). In addition, the meta-regression analysis for publication year, age of patients and sample size were performed to analyze the potential sources of inter-study heterogeneity (Fig. 3). Overall, the publication year (β = 0.096; P = 0.742; R2 = 21.52%) and sample size (β = − 0.004; P = 0.67; R2 = − 16.31%) were not the the major sources of heterogeneity for total blood loss, but the age of patients (β = 0.089; P = 0.04; R2 = 56.63%) may be the dominant factor.
Postoperative Hb
Hb at postoperative day 1
Four studies reported this information [21, 25, 30, 31]. Significant heterogeneity was found in the pooled outcomes, so a random-effects model was utilized in our study (χ2 = 12.84, df = 3, I2 = 76.6%, P = 0.005). As shown in Fig. 4a, the pooled results showed that Hb at postoperative day 1 was significantly higher in TXA group than that in the control group (SMD = 0.579; 95% CI 0.401 to 0.757; P = 0.000, Fig. 4).
Hb at postoperative day 2
Two studies [22, 31] described Hb at postoperative day 2. The meta-analysis showed that TXA group showed distinctly higher Hb at postoperative day 2 than control group (SMD = 0.736; 95% CI 0.472 to 1.000; P = 0.000, Fig. 4b). While, the heterogeneity among studies was very low (χ2 = 0.72, df = 1, I2 = 0%, P = 0.397).
Hb at postoperative day 3
Four articles [26, 29,30,31] provided the relevant data. The summarized estimate of effect size showed Hb at postoperative day 3 in TXA group was clearly higher than control group (SMD = 0.497; 95% CI 0.307 to 0.686; P = 0.000, Fig. 4c). At the same time, no significant statistical heterogeneity was present (χ2 = 4.85, df = 3, I2 = 38.2%, P = 0.183).
Length of stay
Four studies [23, 26, 29, 30] described length of stay. The pooled results showed no significant heterogeneity (χ2 = 3.56, df = 3, I2 = 15.8%, P = 0.313), and therefore, a fixed-effects model was used. The available data demonstrated that length of stay was significantly shorter in TXA group in contrast with control group (SMD = − 0.281; 95% CI − 0.463 to − 0.098; P = 0.003, Fig. 5).
Transfusion rate
Nine studies reported transfusion rate [21, 23, 25,26,27,28,29,30,31]. No significant heterogeneity was found in the pooled outcomes, so a fixed-effects model was utilized in our study (χ2 = 13.35, df = 8, I2 = 40.1%, P = 0.100). As shown in Fig. 6, the pooled results showed that transfusion rate was apparently lower in TXA group than that in control group (RR = 0.559; 95% CI 0.469 to 0.667; P = 0.000, Fig. 6).
Mortality rate
There were five studies reporting mortality rate [23, 24, 28, 29, 31]. A fixed-effects model was applied because no significant heterogeneity was found between the studies (χ2 = 3.34, df = 4, I2 = 0%, P = 0.503). We found that there was no significant difference between the two groups (RR = 1.270; 95% CI 0.779 to 2.069; P = 0. 338, Fig. 7).
Complications
Thromboembolic events (deep vein thrombosis, pulmonary embolism, cerebrovascular accident and myocardial infarction)
The meta-analysis showed that there was no significant difference between the two groups regarding the total rate of thromboembolic events. (RR = 1.006, 95% CI 0.669 to 1.511, P = 0.978, Fig. 8). While, the heterogeneity among studies was very low (χ2 = 9.27, df = 20, I2 = 0%, P = 0.980).
Deep vein thrombosis
Eight studies [23, 24, 26,27,28,29,30,31] described deep vein thrombosis. The meta-analysis showed that there was no significant difference between the two groups (RR = 1.057, 95% CI 0.594 to 1.882, P = 0.850, Fig. 8a). While, the heterogeneity among studies was very low (χ2 = 2.20 df = 7, I2 = 0%, P = 0.948).
Pulmonary embolism
Four articles [23, 26, 30, 31] provided the relevant data. The summarized estimate of effect size showed there was no significant difference regarding the pulmonary embolism between the two groups (RR = 0.857, 95% CI 0.279 to 2.636, P = 0.788, Fig. 8b). At the same time, no significant statistical heterogeneity was present (χ2 = 0.93, df = 3, I2 = 0%, P = 0.818).
Cerebrovascular accident
Data extracted from six studies [23, 24, 28,29,30,31] substantiated that no statistically significant difference was found between the two groups (RR = 0.779, 95% CI 0.344 to 1.765 P = 0.549, Fig. 8c), with an absence of statistical heterogeneity (χ2 = 3.60, df = 5, I2 = 0%, P = 0.609).
Myocardial infarction
Three studies [23, 28, 30] provided data regarding myocardial infarction. The results revealed that the rate of myocardial infarction was not significantly different between the two groups (RR = 1.703, 95% CI 0.500 to 5.799, P = 0.395, Fig. 8d), and statistical heterogeneity was not present (χ2 = 1.82, df = 2, I2 = 0%, P = 0.403).
Wound complications
Four studies reported wound complications [23, 26, 30, 31]. No significant heterogeneity was found in the pooled outcomes, so a fixed-effects model was utilized in our study (χ2 = 2.09, df = 3, I2 = 0%, P = 0.555). As shown in Fig. 9, the pooled results showed that rate of wound complications was apparently lower in TXA group than that in control group (RR = 0.370; 95% CI 0.183 to 0.750; P = 0.006, Fig. 9).
Publication bias and sensitivity analysis
Begg’s funnel plot and Egger’s test (Fig. 10a–d) were used to assess the potential publication bias of the transfusion rate and deep vein thrombosis rate studies included in this meta-analysis. The symmetrical shape of the funnel plots and the P values from Begg’s and Egger’s tests indicated that there was no significant publication bias for transfusion rate and deep vein thrombosis rate (P = 0.144 and P = 0.092, P = 0.383 and P = 0.265, respectively).
To determine the influence of each study on the pooled SMDs for intraoperative blood loss, postoperative drainage and total blood loss to verify the robustness of our results, sensitivity analysis was performed by omitting one study at a time and calculating the pooled SMDs for the remaining studies. The results of the sensitivity analysis indicated that no significant effect on pooled SMDs was observed after excluding any single study, suggesting that the results of this meta-analysis were relatively robust (Fig. 11a–c).
Discussion
Intertrochanteric fractures caused by osteoporosis have become common. They account for a large number of hospital days, much blood loss and high mortality [32]. With a mortality rate of up to 30% in the year after injury, these patients are among the most frail that orthopaedic surgeons treat [33]. Intertrochanteric fracture surgery may result in substantial blood loss in elderly patients, therefore, it is important to identify the optimal blood management to control blood loss during surgery. TXA is an antifibrinolytic agent that has been widely used to reduce bleeding following trauma and surgery, including cardiac surgery, total hip and knee replacement [16, 34]. However, there are few studies investigating its safety and effectiveness in intertrochanteric fracture surgery, with no consistent conclusion reached [35]. To our knowledge, few high-quality meta-analysis has been reported to analyse the high level clinical evidence in evaluating the efficacy and safety of TXA for intertrochanteric fracture surgery. The present study summed up high-quality studies after strict screening in order to find more reliable outcomes. In this meta-analysis, the pooled results indicated that administration of TXA can reduce intraoperative blood loss, hidden blood loss, total blood loss, length of stay, transfusion rate and the occurrence of wound complications. Furthermore, administration of TXA was associated with an increase in the postoperative Hb level at day 1, 2 and 3 after surgery. However, no significant difference was found between the TXA group and control group regarding the occurrence of thromboembolic events (including deep vein thrombosis, pulmonary embolism, cerebrovascular accident and myocardial infarction) and mortality rate.
Tranexamic acid acts by blocking the lysate binding sites of plasminogen and plasmin, thus inhibiting fibrinolytic and inflammatory effect [36, 37]. The administration of TXA has shown to reduce blood loss in patients with acute femoral neck fractures undergoing hip arthroplasty [36]. The results of the current meta-analysis indicated that application of TXA for intertrochanteric fractures surgery was effective in reducing intraoperative blood loss, hidden blood loss, total blood loss, need for transfusion and was associated with higher postoperative Hb level. Our results are supported by two previous meta-analyses [35, 38]. However, our meta-analysis include three newest high quality RCTs with large patients, therefore, the results are much more credible and robust. Nevertheless, there was a notable heterogeneity in the analysis of total blood loss, and subgroup analysis and meta-regression were used to find out the causes of heterogeneity. In assessment of potential sources of heterogeneity, subgroup analysis and meta-regression suggested that the type of control group and age of patients might be the sources of heterogeneity. Besides, we found that patients with hip fracture were usually frail and prone to anemia, which additionally suggested that health status might be a possible source of the variance.
We also compared the length of hospital stay between TXA and control groups, and the results indicated that administration of TXA can shorten the length of hospital stay in intertrochanteric fracture surgery. A shorter length of hospital stay typically results in lower patient costs and higher patient satisfaction. However, pre-existing comorbidities as well as the fracture pattern, quality of reduction achieved, stability of fixation, and quality of bone all contribute to postoperative mobility and the possibility of an early discharge. Thus, all of these factors may affect the length of hospital stay [38].
Despite the evident benefits from the administration of TXA in intertrochanteric fracture surgery, there are still some concerns about the incidence of thrombotic events and mortality after TXA use. The main thrombotic events including deep vein thrombosis (DVT), pulmonary embolism (PE), cerebrovascular accident and myocardial infarction. Zufferey et al. [39] and Schiavone et al. [28] reported a three-fold increase in vascular events with intravenous TXA administration in hip fracture surgery, but this was not statistically significant. A recent population-based study conducted by Poeran et al. [40] involving 872,416 patients showed no increase in thromboembolic events. Consistent with the previous studies [35, 38], no statistically significant increase in thrombotic events and mortality rate was observed in patients treated with TXA in our meta-analysis, which suggested that the administration of TXA is relatively safe in intertrochanteric fracture surgery. Another interesting finding of our meta-analysis is that administration of TXA can decrease the occurrence of wound complications (infection and hematoma), which may attribute to the reduction of blood loss caused by TXA.
The strengths of this meta-analysis include the clear definition of the research question to reduce bias in the selection of the studies, adherence to an explicit research protocol that was developed prior to the analysis, the comprehensive literature search, consensus between the two reviewers with the entry data elements, and a quality control review of all results. All of our included studies in this meta-analysis were RCTs (three of them were newest published in 2019), most of them were high quality, which therefore overcomes the shortcomings of recall or selection bias in non-randomized studies. Besides, the total sample size was relatively large (1202 patients). No publication bias was found in our meta-analysis and sensitivity analysis indicated that the results of this meta-analysis were relatively robust.
Nonetheless, some limitations in the present meta-analysis should be recognized. (1) Only 11 articles were included in the study, which might lower the evidence level. (2) The duration of the follow-up of the included studies was variable. (3) The methods and dosages of TXA used in various studies are inconsistent, which may be the sources of heterogeneity. Therefore, we suggest that larger sample sizes and multicentric high-quality randomized controlled trials could be carried out to evaluate the efficacy and safety of TXA in intertrochanteric fracture surgery in the future. Despite these limitations, this meta-analysis provides evidence that use of TXA in intertrochanteric fracture surgery could significantly reduce intraoperative blood loss, hidden blood loss, total blood loss, length of stay, transfusion rate and the occurrence of wound complications without increasing the risk of thromboembolic events and mortality rate. Furthermore, administration of TXA could remain significantly higher postoperative Hb in intertrochanteric fracture surgery.
Conclusion
In conclusion, administration of TXA is effective in reducing intraoperative blood loss, hidden blood loss, total blood loss, length of stay, transfusion rate, wound complications and enhancing postoperative Hb without increasing the risk of thromboembolic events and mortality rate in intertrochanteric fracture surgery. However, taking the heterogenicity and small sample size into consideration, more large multi-center and high-quality RCTs are required to go a step further in demonstrating the efficacy and safety of TXA before its use is recommended in intertrochanteric fracture surgery.
References
Ronga M, Bonzini D, Valoroso M, La Barbera G, Tamini J, Cherubino M et al (2017) Blood loss in trochanteric fractures: multivariate analysis comparing dynamic hip screw and Gamma nail. Injury 48(Suppl 3):S44–S47. https://doi.org/10.1016/S0020-1383(17)30657-5
Gullberg B, Johnell O, Kanis JA (1997) World-wide projections for hip fracture. Osteoporos Int 7:407–413
Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD (2008) Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg 16:596–607
Zuckerman JD (1996) Hip fracture. N Engl J Med 334:1519–1525. https://doi.org/10.1056/NEJM199606063342307
Sheehan SE, Shyu JY, Weaver MJ, Sodickson AD, Khurana B (2015) Proximal femoral fractures: what the orthopedic surgeon wants to know. Radiographics 35:1563–1584. https://doi.org/10.1148/rg.2015140301
Panula J, Pihlajamaki H, Mattila VM, Jaatinen P, Vahlberg T, Aarnio P et al (2011) Mortality and cause of death in hip fracture patients aged 65 or older: a population-based study. BMC Musculoskelet Disord 12:105. https://doi.org/10.1186/1471-2474-12-105
Blomfeldt R, Tornkvist H, Eriksson K, Soderqvist A, Ponzer S, Tidermark J (2007) A randomised controlled trial comparing bipolar hemiarthroplasty with total hip replacement for displaced intracapsular fractures of the femoral neck in elderly patients. J Bone Joint Surg Br 89:160–165. https://doi.org/10.1302/0301-620X.89B2.18576
Foss NB, Kehlet H (2006) Hidden blood loss after surgery for hip fracture. J Bone Joint Surg Br 88:1053–1059. https://doi.org/10.1302/0301-620X.88B8.17534
Lawrence VA, Silverstein JH, Cornell JE, Pederson T, Noveck H, Carson JL (2003) Higher Hb level is associated with better early functional recovery after hip fracture repair. Transfusion 43:1717–1722. https://doi.org/10.1046/j.0041-1132.2003.00581.x
Boddaert J, Raux M, Khiami F, Riou B (2014) Perioperative management of elderly patients with hip fracture. Anesthesiology 121:1336–1341. https://doi.org/10.1097/ALN.0000000000000478
Klein HG, Spahn DR, Carson JL (2007) Red blood cell transfusion in clinical practice. Lancet 370:415–426. https://doi.org/10.1016/S0140-6736(07)61197-0
Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG et al (2011) Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 365:2453–2462. https://doi.org/10.1056/NEJMoa1012452
Hughes NT, Burd RS, Teach SJ (2014) Damage control resuscitation: permissive hypotension and massive transfusion protocols. Pediatr Emerg Care 30:651–656. https://doi.org/10.1097/PEC.0000000000000217. quiz 657–658
Scully C, Robinson NA (2015) Anti-thrombotic agents. Br Dent J 219:515. https://doi.org/10.1038/sj.bdj.2015.904
Astedt B, Liedholm P, Wingerup L (1978) The effect of tranexamic acid on the fibrinolytic activity of vein walls. Ann Chir Gynaecol 67:203–205
Zhou XD, Tao LJ, Li J, Wu LD (2013) Do we really need tranexamic acid in total hip arthroplasty? A meta-analysis of nineteen randomized controlled trials. Arch Orthop Trauma Surg 133:1017–1027. https://doi.org/10.1007/s00402-013-1761-2
Wu Q, Zhang HA, Liu SL, Meng T, Zhou X, Wang P (2015) Is tranexamic acid clinically effective and safe to prevent blood loss in total knee arthroplasty? A meta-analysis of 34 randomized controlled trials. Eur J Orthop Surg Traumatol 25:525–541. https://doi.org/10.1007/s00590-014-1568-z
Cheriyan T, Maier SP 2nd, Bianco K, Slobodyanyuk K, Rattenni RN, Lafage V et al (2015) Efficacy of tranexamic acid on surgical bleeding in spine surgery: a meta-analysis. Spine J 15:752–761. https://doi.org/10.1016/j.spinee.2015.01.013
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. https://doi.org/10.1371/journal.pmed.1000097
Higgins J (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. In: The Cochrane Collaboration (ed) Naunyn-Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie 5:S38
Mohib Y, Rashid RH, Ali M, Zubairi AJ, Umer M (2015) Does tranexamic acid reduce blood transfusion following surgery for inter-trochanteric fracture? A randomized control trial. J Pak Med Assoc 65:S17–20
Baruah RK, Borah PJ, Haque R (2016) Use of tranexamic acid in dynamic hip screw plate fixation for trochanteric fractures. J Orthop Surg (Hong Kong) 24:379–382. https://doi.org/10.1177/1602400322
Drakos A, Raoulis V, Karatzios K, Doxariotis N, Kontogeorgakos V, Malizos K et al (2016) Efficacy of local administration of tranexamic acid for blood salvage in patients undergoing intertrochanteric fracture surgery. J Orthop Trauma 30:409–414. https://doi.org/10.1097/BOT.0000000000000577
Tengberg PT, Foss NB, Palm H, Kallemose T, Troelsen A (2016) Tranexamic acid reduces blood loss in patients with extracapsular fractures of the hip: results of a randomised controlled trial. Bone Joint J. https://doi.org/10.1302/0301-620X.98B6.36645
Virani SR, Dahapute AA, Panda I, Bava SS (2016) Role of local infiltration of tranexamic acid in reducing blood loss in peritrochanteric fracture surgery in the elderly population. Malays Orthop J 10:26–30. https://doi.org/10.5704/MOJ.1611.013
Lei J, Zhang B, Cong Y, Zhuang Y, Wei X, Fu Y et al (2017) Tranexamic acid reduces hidden blood loss in the treatment of intertrochanteric fractures with PFNA: a single-center randomized controlled trial. J Orthop Surg Res 12:124. https://doi.org/10.1186/s13018-017-0625-9
Tian S, Shen Z, Liu Y, Zhang Y, Peng A (2018) The effect of tranexamic acid on hidden bleeding in older intertrochanteric fracture patients treated with PFNA. Injury 49:680–684. https://doi.org/10.1016/j.injury.2018.01.026
Schiavone A, Bisaccia M, Inkov I, Rinonapoli G, Manni M, Rollo G et al (2018) Tranexamic acid in pertrochanteric femoral fracture: is it a safe drug or not? Folia Med (Plovdiv) 60:67–78. https://doi.org/10.1515/folmed-2017-0070
Luo X, He S, Lin Z, Li Z, Huang C, Li Q (2019) Efficacy and safety of tranexamic acid for controlling bleeding during surgical treatment of intertrochanteric fragility fracture with proximal femoral nail anti-rotation: a randomized controlled trial. Indian J Orthop 53:263–269. https://doi.org/10.4103/ortho.IJOrtho_401_17
Zhou XD, Zhang Y, Jiang LF, Zhang JJ, Zhou D, Wu LD et al (2019) Efficacy and safety of tranexamic acid in intertrochanteric fractures: a single-blind randomized controlled trial. Orthop Surg 11:635–642. https://doi.org/10.1111/os.12511
Chen F, Jiang Z, Li M, Zhu X (2019) Efficacy and safety of perioperative tranexamic acid in elderly patients undergoing trochanteric fracture surgery: a randomised controlled trial. Hong Kong Med J 25:120–126. https://doi.org/10.12809/hkmj187570
Dhanwal DK, Dennison EM, Harvey NC, Cooper C (2011) Epidemiology of hip fracture: worldwide geographic variation. Indian J Orthop 45:15–22. https://doi.org/10.4103/0019-5413.73656
Moran CG, Wenn RT, Sikand M, Taylor AM (2005) Early mortality after hip fracture: is delay before surgery important? J Bone Joint Surg Am 87:483–489. https://doi.org/10.2106/JBJS.D.01796
Yang ZG, Chen WP, Wu LD (2012) Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am 94:1153–1159. https://doi.org/10.2106/JBJS.K.00873
Zhou XD, Li J, Fan GM, Huang Y, Xu NW (2019) Efficacy and safety of tranexamic acid in elderly patients with intertrochanteric fracture: an updated meta-analysis. World J Clin Cases 7:1302–1314. https://doi.org/10.12998/wjcc.v7.i11.1302
Watts CD, Houdek MT, Sems SA, Cross WW, Pagnano MW (2017) Tranexamic acid safely reduced blood loss in hemi- and total hip arthroplasty for acute femoral neck fracture: a randomized clinical trial. J Orthop Trauma 31:345–351. https://doi.org/10.1097/BOT.0000000000000837
Gao F, Sun W, Guo W, Li Z, Wang W, Cheng L (2015) Topical administration of tranexamic acid plus diluted-epinephrine in primary total knee arthroplasty: a randomized double-blinded controlled trial. J Arthroplasty 30:1354–1358. https://doi.org/10.1016/j.arth.2015.03.003
Jiang W, Shang L (2019) Tranexamic acid can reduce blood loss in patients undergoing intertrochanteric fracture surgery: a meta-analysis. Medicine (Baltimore) 98:e14564. https://doi.org/10.1097/MD.0000000000014564
Zufferey PJ, Miquet M, Quenet S, Martin P, Adam P, Albaladejo P et al (2010) Tranexamic acid in hip fracture surgery: a randomized controlled trial. Br J Anaesth 104:23–30. https://doi.org/10.1093/bja/aep314
Poeran J, Rasul R, Suzuki S, Danninger T, Mazumdar M, Opperer M et al (2014) Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ 349:g4829. https://doi.org/10.1136/bmj.g4829
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no conflict of interest.
Ethical approval
All analyses were based on previous published studies; thus, no ethical approval is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, X., Wang, J., Wang, X. et al. The efficacy and safety of tranexamic acid in the treatment of intertrochanteric fracture: an updated meta-analysis of 11 randomized controlled trials. J Thromb Thrombolysis 50, 243–257 (2020). https://doi.org/10.1007/s11239-019-02034-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-019-02034-1