Abstract
In the last decade, major advances in venous thromboembolism (VTE) prophylaxis in orthopaedic surgery have included the development of new anticoagulants that are poised to replace low molecular weight heparins (LMWHs) and improvements in operative and perioperative care that have likely led to a decline in the rates of symptomatic VTE and mortality independent of anticoagulant use. A systematic review of the literature was performed to identify phase III randomized controlled trials of VTE prevention that compared new anticoagulants (fondaparinux, rivaroxaban, dabigatran, apixaban) with LMWH (enoxaparin) in major elective orthopaedic surgery. Our aims were to obtain best estimates of the rates of patient important events (symptomatic VTE, mortality, and bleeding) in contemporary trials of VTE prevention, and to consider the implications of these contemporary rates for clinical practice and future research. Fourteen studies, which enrolled 40,285 patients, were included in the analyses. The combined median rates (ranges) for all five anticoagulants for symptomatic VTE and mortality to the end of follow-up were 0.99 % (0.15–2.58 %) and 0.26 % (0–0.92 %) respectively, whereas the median rate (range) of clinically important bleeding was 3.44 % (2.25–7.74 %). In contemporary trials of anticoagulants, the rates of symptomatic VTE and mortality are low, but the rates of clinically important post-operative bleeding remain relatively high. Based on these results, we propose that approaches that minimize bleeding without substantially reducing efficacy merit investigation, particularly if improvement in surgical and perioperative care have also resulted in falling baseline patient important VTE rates independent of anticoagulant use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fatal and non-fatal pulmonary embolism (PE), symptomatic deep vein thrombosis (DVT) of the leg and bleeding are patient important complications of major hip and knee surgery [1]. It is widely accepted that patients undergoing such surgery should receive prophylactic anticoagulant therapy. Although the number of studies examining the effect of prophylaxis on the most important complication, fatal PE, is limited, the results are consistent. Studies performed between 1959 and 2009, comparing no prophylaxis with anticoagulants such as vitamin K antagonists, low dose unfractionated heparin and low molecular weight heparins (LMWHs) have shown that anticoagulants produce a relative risk reduction in fatal PE of up to 70 % [2, 3]. The corresponding reductions in the risk of symptomatic non-fatal symptomatic PE and DVT were similar [3]. In many of these trials, anticoagulants were associated with a modest increase in risk of major bleeding but rates of other types of bleeding were not recorded [3]. Bleeding associated with anticoagulant use can vary in severity, and though some bleeds do not meet criteria for major bleeding, “clinically relevant non-major” (CRNM) bleeding, a recently defined outcome, can have important adverse consequences for patients [4, 5].
Since the approval and widespread clinical use of LMWHs for prevention of PE and DVT, collectively known as venous thromboembolism (VTE), two parallel developments, that could influence future approach to VTE prevention in orthopaedic surgery, have occurred. First, improvements in operative and perioperative care have reduced postoperative complications, and likely led to a reduction in baseline incidence of VTE and mortality. Second, four additional anticoagulants (fondaparinux, apixaban, rivaroxaban and dabigatran) have been evaluated in large, similarly designed phase III studies; all have been approved by regulatory agencies for the prevention of VTE in total hip or knee replacement surgery because they were found to be at least as effective as LMWH, and in the case of new oral anticoagulants, have the convenience of easy administration. These new oral anticoagulants are now poised to replace LMWH.
Because of the large combined sample size and similarity in design, the trials comparing the new oral anticoagulants with LMWH (enoxaparin) provide a unique opportunity to re-examine the contemporary risk of VTE, mortality and bleeding following orthopaedic surgery. All used mandatory venography performed at the end of drug treatment, typically between days 5 and 15 (for standard thromboprophylaxis), and between days 28 and 35 (for extended thromboprophylaxis) after surgery [6–19]. The primary efficacy outcome included DVT detected by mandatory venography, symptomatic DVT, and fatal and non-fatal PE. The importance for patients of asymptomatic DVT diagnosed by venography, included as a component of the primary composite outcome to increase the event rates and make the studies feasible, is unknown since most such thrombi remain asymptomatic and only an uncertain (but likely small) proportion become symptomatic [20, 21]. We therefore limit our primary analyses to patient important outcomes, namely symptomatic VTE confirmed by objective testing, mortality, including fatal PE during the period of surveillance, and bleeding.
Our aims are twofold: the first is to document the rates of symptomatic VTE, postoperative mortality and bleeding with new anticoagulants and enoxaparin in patients undergoing major elective hip and knee surgery in contemporary practice; the second is to discuss the implications of the results of these studies in the light of evidence suggesting that baseline postoperative risks of symptomatic VTE and mortality have fallen independent of thromboprophylaxis use.
Methods
We included all phase III randomized trials that compared fondaparinux, apixaban, rivaroxaban, or dabigatran with a LMWH (enoxaparin) in patients undergoing elective total hip or knee arthroplasty.
Search criteria, study selection, and quality assessment
We searched Medline via PubMed for articles published between Jan 1990 and July 2013 with the following search terms: “fondaparinux or apixaban or BMS-562247 or rivaroxaban or BAY 59-7939 or dabigatran or BIBR-1048 and prophylaxis” and limited the search to clinical trials. This was supplemented by a manual review of reference lists of review articles and eligible papers. A trial was eligible for inclusion if it was a phase III randomized study that compared a new anticoagulant to a LMWH (enoxaparin) in patients undergoing elective total hip or knee arthroplasty and reported incidence of symptomatic VTE. Studies were selected independently by two authors (NC and DS) and disagreements were resolved by consensus. We assessed study quality using the Jadad scale [22] (reported in Table 1).
Data extraction
Two investigators (NC and DS) independently extracted data for type of surgery, dose and duration of prophylaxis, timing of prophylaxis relative to surgery, median time of venography following surgery, site of adjudication committee, venographic DVT, symptomatic VTE, fatal PE (when reported), major bleeding, CRNM bleeding and mortality during the intended treatment period and to end of follow-up. Disagreements regarding data extraction were resolved by consensus.
Outcome measures
The primary efficacy outcome measures were the rates of symptomatic VTE and mortality at end of follow-up, calculated as the number of subjects with the event of interest divided by the size of the population at the start of the study period. The primary safety outcome measures were the rates of the composite of major bleeding or CRNM bleeding, and major bleeding within 2 days of anticoagulant cessation. Secondary outcomes included the rates of venographic DVT, fatal PE at follow-up.
Statistical analysis
We report the medians and ranges of the primary and secondary outcomes. The median rates (ranges) of symptomatic VTE, postoperative mortality, venographic DVT and major bleeding (and ranges) were based on all 14 trials while median rates (ranges) of CRNM bleeding and fatal PE were based on the 11 and 12 trials, respectively, that reported on these outcomes.
Results
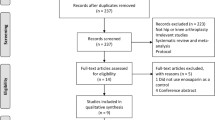
Our search strategy identified 220 potentially eligible citations. The flow diagram (Fig. 1) shows an overview of the study selection process. After screening titles, and abstracts, we retained 15 citations. One additional study, PENTHIFRA [23], performed in non-elective hip fracture patients was excluded, leaving 14 randomized trials at low risk of bias in the full review.
Seven trials were conducted in knee arthroplasty patients and seven trials in hip arthroplasty patients (Table 1). Five trials in hip arthroplasty evaluated extended duration of thromboprophylaxis following elective hip surgery. The earliest start times of the anticoagulants postoperatively were: 4 h for dabigatran, 6 h for both rivaroxaban and fondaparinux, 12 h for apixaban, and 12 h for enoxaparin.
The 14 studies randomized a total of 40,285 patients and documented 260 (0.7 %) symptomatic VTE events, 56 (0.1 %) fatal events, 9 (<0.1 %) fatal PE events, 2,988 (10.5 %) venographic DVT events, 383 (1.0 %) major bleeds and 1,371 (4.0 %) major or CRNM bleeds during the intended treatment period. An additional 120 symptomatic episodes of VTE occurred and 39 patients died in the post-treatment follow-up period. The follow-up duration varied among trials (see Table 1) with a median of 30–40 days after cessation of study drug.
Symptomatic VTE with individual anticoagulants
Figure 2 shows the median symptomatic VTE rates (and ranges) at end of follow-up with all five anticoagulants. The rates were all low and similar among anticoagulants: 1.06 % (0.97–2.58 %) for fondaparinux, 0.79 % (0.15–1.44 %) for apixaban, 0.62 % (0.31–1.09 %) for rivaroxaban, 0.87 % (0.30–1.56 %) for dabigatran, and 1.20 % (0.44–2.22 %) for enoxaparin (see Table 1 for individual trial results).
Mortality with individual anticoagulants
Figure 2 also shows the mortality rates at the end of the follow-up with the five anticoagulants. The rates were very low with all anticoagulants used: 0.39 % (0.18–0.53 %) with fondaparinux, 0.19 % (0.18–0.20 %) with apixaban, 0.27 % (0–0.39 %) with rivaroxaban, 0.20 % (0–0.40 %) with dabigatran and 0.33 % (0.07–0.92 %) with enoxaparin.
Bleeding with individual anticoagulants (within 2 days of cessation)
Data addressing the risks of major or CRNM bleeding were available for 11 studies. The three fondaparinux studies did not report rates of CRNM bleeding.
Figure 3 shows the median rates (and ranges) for composite outcome of major or CRNM bleeding and for major bleeding for each anticoagulant. The major or CRNM bleeding rates were: 3.53 % (2.88–4.90) with apixaban, 3.25 % (3.01–3.34 %) with rivaroxaban, 4.88 % (3.18–7.74 %) with dabigatran, and 3.80 % (2.25–6.65 %) with enoxaparin.
Safety outcomes of new anticoagulants and enoxaparin. Box-plots of major bleeding or CRNM bleeding and major bleeding rates of individual anticoagulants showing median, interquartile range, and range. CRNM bleeding rates were not available for the three fondaparinux trials. The major bleeding rates in the fondaparinux trials were primarily driven by bleeding with a bleeding index ≥ 2. CRNM clinically relevant non-major
The major bleeding rates with the five anticoagulants were: 2.13 % (1.77–4.12 %) with fondaparinux, 0.69 % (0.60–0.82 %) with apixaban, 0.42 % (0.08–0.66 %) with rivaroxaban, 1.38 % (0.58–1.65 %) with dabigatran, and 0.89 % (0.19–2.82 %) with enoxaparin.
Combined rates of patient important events
Figure 4 shows median rates (ranges) of symptomatic VTE, mortality and major or CRNM bleeding for all anticoagulants combined. The rates of symptomatic VTE and mortality to end of follow-up were 0.99 % (0.15–2.58 %) and 0.26 % (0–0.92 %) respectively while the median rate (range) of major or CRNM bleeding was 3.44 % (2.25–7.74 %).
Very few fatal PE (n = 21) were observed in the 12 studies that reported on this outcome; this total included fatal events in which PE could not be excluded as cause of death. The median rate of fatal PE (and range) by end of follow-up with all anticoagulants was 0.08 % (0–0.19 %).
Venographic DVT rates
All 14 studies used mandatory venography, which was performed shortly after cessation of study drug, and reported a composite primary efficacy outcome made up of a combination of asymptomatic DVT and symptomatic VTE (including fatal PE). The median rates (and ranges) of venographic DVT for the new anticoagulants and enoxaparin were 6.95 % (0.75–38.27 %) and 8.58 % (3.40–37.57 %), respectively.
Discussion
The main reason to use prophylactic anticoagulants in high risk surgical patients is to reduce mortality, particularly fatal PE. Prevention of other patient important events is also relevant, but if there is no reduction in mortality, any observed benefit must be weighed against harm from postoperative bleeding. Our analysis, which included over 40,000 patients enrolled in clinical trials and randomized to either one of the new anticoagulants or LMWH, demonstrates that contemporary risks of symptomatic VTE and mortality are low. The median rate of symptomatic VTE was 0.99 % (range: 0.15–2.58 %) and the median postoperative mortality rate was 0.26 % (range: 0–0.92 %) by the end of follow-up.
Our interest here is not to estimate the relative or absolute effects of LMWH versus the other four anticoagulants; others have dealt with this issue [24, 25]. Rather, our interest is to document that the current rates of patient-important adverse thrombotic events and mortality are low, irrespective of what antithrombotic agent is used, and that these low rates are consistent across studies. Because of statistical heterogeneity, the consistent low incidence is best captured by the median and range, which our summaries present, rather than meta-analytic estimates.
Our estimates of symptomatic VTE based on trials using mandatory venography are susceptible to two biases, but as discussed elsewhere, the biases work in opposite directions, so their overall effect on our estimate of symptomatic VTE is likely minimal [4]. Large observational studies in major orthopaedic surgery performed over the last decade that showed similarly low incidence of symptomatic VTE, ranging from 1.1 to 3.7 %, support for the validity of our estimates [26–32]. The most recent of these observational studies reflecting real life practice, the XAMOS study [33] (n = 17,000 patients), a phase IV study of rivaroxaban with LMWH control in an unselected population, showed that the incidence of symptomatic VTE (about 1 %), and mortality (about 0.07 %) were both low.
The low post-operative incidences of symptomatic VTE and death with the new oral anticoagulants, together with their convenience represent an important advance for patients and their physicians. We may, however, have reached the end of the line with orthopaedic thromboprophylaxis. Previous developments in anticoagulant prophylaxis focused on efficacy and convenience. In the light of the falling baseline rates of symptomatic and fatal VTE, other approaches that might be less effective but that are less expensive, as convenient, and/or produce less bleeding might be worth considering.
Considering such approaches is justifiable on the following grounds: First, the current low symptomatic VTE, and very low mortality rates are not solely due to effective thromboprophylaxis. Since the first thromboprophylaxis trial, surgical techniques and postoperative care have improved. A report by Murray and associates [34] also indicates that mortality following elective joint replacement surgery has fallen over time. In their meta-analysis (n > 100,000 patients), 30 day mortality risk following total hip replacement were reported to be 1.10 % (95 % CI 0.96–1.24 %) in 1960s, 1.11 % (95 % CI 1.00–1.21 %) in 1970s and 0.38 % (95 % CI 0.29–0.47 %) in the 1980s and 1990s. A secular trend for a reduction in mortality was also reported in a recent retrospective study, which showed that in an 8 year period (from 2003 to 2011), the 90 day mortality following total hip replacement in the United Kingdom’s National Joint registry (>400,000 patients) fell from 0.56 % in 2003 to 0.29 % in 2011 [35]. In the latter study, improvements in surgical approach and anesthetic procedure as well as the use of thromboprophylaxis were all associated with a reduction in mortality. Although there are limitations in estimating baseline symptomatic VTE incidence from indirect comparisons, others have shown a similar secular trend for a reduction [3]. It is therefore likely, as suggested by others, that improvement in operative technique, methods of anesthesia and postoperative care have contributed to the reduction in postoperative mortality and symptomatic VTE over time. Although the true baseline mortality and symptomatic VTE incidences (without prophylaxis) are unknown, both are likely to be low and much lower than previously reported. Therefore, a substantial number of patients are being exposed to anticoagulant without the expectation of a clinical benefit in contemporary practice.
Second, in contrast to the low VTE and mortality rates with anticoagulant prophylaxis, bleeding rates remain substantial (Fig. 3). Although the comparative bleeding rates without prophylaxis, using contemporary surgical techniques and similar definitions for clinically important bleeding, are unknown, the evidence indicates that anticoagulants used in effective anti-thrombotic doses increase the risk of bleeding. If indeed contemporary baseline patient important efficacy outcomes are lower than previously thought, then any harm induced by anticoagulant use assumes greater importance in any trade-off. Postoperative bleeding with anticoagulants is important because it can lead to wound infection and in the longer term, to joint dysfunction [36]. Postoperative bleeding continues to concern orthopaedic surgeons [37–41]. Consequently, an approach to VTE prophylaxis that has the potential to lower bleeding without substantially reducing efficacy, would constitute an important advance in health care, particularly if such an approach is inexpensive and simple to use.
A number of plausible strategies have been proposed to reduce bleeding, namely, aspirin ± compression stockings, delay in the first postoperative anticoagulant dose, reduction in anticoagulant dose and confining prophylaxis to high risk subgroups (risk stratification) [41–44]. Of these, we consider aspirin the most appealing because it is effective, inexpensive, and simple to use. Aspirin proved effective in reducing the frequency of patient important outcomes in high risk orthopaedic patients [42] but, thus far, it has not been considered to be a first line agent for this indication. Even though aspirin might be less effective than anticoagulants, it is likely to cause less bleeding than new anticoagulants, and in the context of their low baseline incidence, be associated with acceptable risks of symptomatic VTE and mortality.
We now have evidence that aspirin used post-discharge (after 10 days of prophylactic dalteparin), is at least as effective and safe as extended dalteparin in the primary prevention of VTE following total hip replacement [45]. With these positive results and the real possibility that the baseline rates of patient important events have been lowered by improvements in operative and postoperative care, the ethical barrier to evaluating aspirin as a first line treatment no longer exists. If successful, a randomized controlled trial comparing aspirin directly with one of the new oral anticoagulants using patient important outcomes could have an enormous impact on clinical practice and health care costs.
Conclusions
In contemporary trials of new anticoagulants in major elective hip and knee surgery, the rates of patient important events are low (symptomatic VTE) to very low (fatal PE and mortality) with the use of any of the four new anticoagulants or enoxaparin. However, the risk of postoperative bleeding remains high. Based on the strong possibility that owing to improvements in surgical and perioperative care, the incidence of patient important thrombotic events without prophylaxis is now low, prophylactic approaches that minimize bleeding without substantially reducing efficacy merit investigation, particularly if such approaches are inexpensive and simple to use.
References
MacLean S, Mulla S, Akl EA, Jankowski M, Vandvik PO, Ebrahim S, McLeod S, Bhatnagar N, Guyatt GH (2012) Patient values and preferences in decision making for antithrombotic therapy: a systematic review: antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl):e1S–e23S. doi:10.1378/chest.11-2290
Collins R, Scrimgeour A, Yusuf S, Peto R (1988) Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med 318(18):1162–1173. doi:10.1056/nejm198805053181805
Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell JCW (2012) Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. CHEST J 141 (2_suppl):e278S–e325S. doi:10.1378/chest.11-2404
Guyatt GH, Eikelboom JW, Gould MK, Garcia DA, Crowther M, Murad MH, Kahn SR, Falck-Ytter Y, Francis CW, Lansberg MG, Akl EA, Hirsh J (2012) Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. CHEST J 141 (2_suppl):e185S–e194S. doi:10.1378/chest.11-2289
Guideline on clinical investigation of medicinal products for the prevention of venous thromboembolism (VTE) in patients undergoing high VTE-risk surgery. (2013) European Medicines Agency. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/05/WC500143764.pdf. Accessed 7 Oct 2014
Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ (2009) Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med 361(6):594–604. doi:10.1056/NEJMoa0810773
Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P (2010) Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet 375(9717):807–815. doi:10.1016/s0140-6736(09)62125-5
Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM (2010) Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 363(26):2487–2498. doi:10.1056/NEJMoa1006885
Lassen MR, Bauer KA, Eriksson BI, Turpie AG (2002) Postoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hip-replacement surgery: a randomised double-blind comparison. Lancet 359(9319):1715–1720. doi:10.1016/s0140-6736(02)08652-x
Bauer KA, Eriksson BI, Lassen MR, Turpie AG (2001) Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med 345(18):1305–1310. doi:10.1056/NEJMoa011099
Turpie AG, Bauer KA, Eriksson BI, Lassen MR (2002) Postoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip-replacement surgery: a randomised double-blind trial. Lancet 359(9319):1721–1726. doi:10.1016/s0140-6736(02)08648-8
Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhofer E, Misselwitz F, Geerts W (2008) Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 358(26):2765–2775. doi:10.1056/NEJMoa0800374
Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, Soglian AG, Pap AF, Misselwitz F, Haas S (2008) Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 372(9632):31–39. doi:10.1016/s0140-6736(08)60880-6
Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, Misselwitz F, Turpie AG (2008) Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 358(26):2776–2786. doi:10.1056/NEJMoa076016
Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, Cushner FD, Lotke PA, Berkowitz SD, Bandel TJ, Benson A, Misselwitz F, Fisher WD (2009) Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 373(9676):1673–1680. doi:10.1016/s0140-6736(09)60734-0
Ginsberg JS, Davidson BL, Comp PC, Francis CW, Friedman RJ, Huo MH, Lieberman JR, Muntz JE, Raskob GE, Clements ML, Hantel S, Schnee JM, Caprini JA (2009) Oral thrombin inhibitor dabigatran etexilate versus North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 24(1):1–9. doi:10.1016/j.arth.2008.01.132
Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Kalebo P, Christiansen AV, Hantel S, Hettiarachchi R, Schnee J, Buller HR (2007) Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 5(11):2178–2185. doi:10.1111/j.1538-7836.2007.02748.x
Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Prins MH, Hettiarachchi R, Hantel S, Schnee J, Buller HR (2007) Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 370(9591):949–956. doi:10.1016/s0140-6736(07)61445-7
Eriksson BI, Dahl OE, Huo MH, Kurth AA, Hantel S, Hermansson K, Schnee JM, Friedman RJ (2011) Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomised, double-blind, non-inferiority trial. Thromb Haemost 105(4):721–729. doi:10.1160/th10-10-0679
Berger J, Eikelboom JW, Quinlan DJ, Guyatt G, Büller HR, Sobieraj-Teague M, Harrington RA, Hirsh J (2013) Venous thromboembolism prophylaxis: do trial results enable clinicians and patients to evaluate whether the benefits justify the risk? Proceedings of an Ad Hoc Working Group Meeting. J Thromb Haemost 11 (4):778–782. doi:10.1111/jth.4900
Quinlan DJ, Eikelboom JW, Dahl OE, Eriksson BI, Sidhu PS, Hirsh J (2007) Association between asymptomatic deep vein thrombosis detected by venography and symptomatic venous thromboembolism in patients undergoing elective hip or knee surgery. J Thromb Haemost 5(7):1438–1443. doi:10.1111/j.1538-7836.2007.02571.x
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12
Eriksson BI, Bauer KA, Lassen MR, Turpie AG (2001) Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip-fracture surgery. N Engl J Med 345(18):1298–1304. doi:10.1056/NEJMoa011100
Adam SS, McDuffie JR, Lachiewicz PF, Ortel TL, Williams JJW (2013) Comparative effectiveness of new oral anticoagulants and standard thromboprophylaxis in patients having total hip or knee replacement: a systematic review. Ann Intern Med 159(4):275–284. doi:10.7326/0003-4819-159-4-201308200-00008
Turpie AG, Bauer KA, Eriksson BI, Lassen MR (2002) Fondaparinux versus enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of four randomized double-blind studies. Arch Intern Med 162(16):1833–1840
Anderson F, Hirsh J, White K, Fitzgerald R (2003) Temporal trends in prevention of venous thromboembolism following primary total hip or knee arthroplasty 1996–2001: findings from the Hip and Knee Registry. Chest 124(Suppl 6):349–356
Bjornara B, Gudmundsen T, Dahl O (2006) Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br 88:386–391
Lapidus L, Ponzer S, Pettersson H, de Bri E (2013) Symptomatic venous thromboembolism and mortality in orthopaedic surgery—an observational study of 45,968 consecutive procedures. BMC Musculoskelet Disord 14(1):177
Leclerc J, Gent M, Hirsh J, Geerts W, Ginsberg J (1998) The incidence of symptomatic venous thromboembolism during and after prophylaxis with enoxaparin: a multi-institutional cohort study of patients who underwent hip or knee arthroplasty. Canadian collaborative group. Arch Intern Med 158:873–878
Samama C, Ravaud P, Parent F, Barre J, Mertl P, Mismetti P (2007) Epidemiology of venous thromboembolism after lower limb arthroplasty: the FOTO study. J Thromb Haemost 5:2360–2367
White R, Romano P, Zhou H, Rodrigo J, Bargar W (1998) Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med 158:1525–1531
White R, Zhou H, Romano P (2003) Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost 90:446–455
Turpie AGG, Haas S, Kreutz R, Mantovani LG, Pattanayak CW, Holberg G, Jamal W, Schmidt A, van Eickels M, Lassen MR (2014) A non-interventional comparison of rivaroxaban with standard of care for thromboprophylaxis after major orthopaedic surgery in 17,701 patients with propensity score adjustment. Thromb Haemost 111(1):94–102. doi:10.1160/TH13-08-0666
Murray DW, Britton AR, Bulstrode CJK (1996) Thromboprophylaxis and death after total hip replacement. J Bone Joint Surg, Br 78-B(6):863–870
Hunt LP, Ben-Shlomo Y, Clark EM, Dieppe P, Judge A, MacGregor AJ, Tobias JH, Vernon K, Blom AW (2013) 90-day mortality after 409096 total hip replacements for osteoarthritis, from the National Joint Registry for England and Wales: a retrospective analysis. Lancet 382(9898):1097–1104
Kwong LM, Kistler KD, Mills R, Wildgoose P, Klaskala W (2012) Thromboprophylaxis, bleeding and post-operative prosthetic joint infection in total hip and knee arthroplasty: a comprehensive literature review. Expert Opin Pharmacother 13(3):333–344. doi:10.1517/14656566.2012.652087
Muntz J, Scott DA, Lloyd A, Egger M (2004) Major bleeding rates after prophylaxis against venous thromboembolism: systematic review, meta-analysis, and cost implications. Int J Technol Assess Health Care 20(4):405–414
Vera-Llonch M, Hagiwara M, Oster G (2006) Clinical and economic consequences of bleeding following major orthopedic surgery. Thromb Res 117(5):569–577. doi:10.1016/j.thromres.2005.04.018
Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH (2007) Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplast 22(6 Suppl 2):24–28. doi:10.1016/j.arth.2007.03.007
Sanchez-Ballester J, Smith M, Hassan K, Kershaw S, Elsworth CS, Jacobs L (2005) Wound infection in the management of hip fractures: a comparison between low-molecular weight heparin and mechanical prophylaxis. Acta Orthop Belg 71(1):55–59
Kulshrestha V, Kumar S (2013) DVT prophylaxis after TKA: routine anticoagulation Vs risk screening approach—A randomized study. J Arthroplast. doi:10.1016/j.arth.2013.05.025
Pulmonary Embolism Prevention (PEP) Trial Collaborative Group (2000) Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 355(9212):1295–1302
Raskob GE, Hirsh J (2003) Controversies in timing of the first dose of anticoagulant prophylaxis against venous thromboembolism after major orthopedic surgery. Chest 124(6 Suppl):379S–385S
Neumann I, Rada G, Claro JC, Carrasco-Labra A, Thorlund K, Akl EA, Bates SM, Guyatt GH (2012) Oral direct Factor Xa inhibitors versus low-molecular-weight heparin to prevent venous thromboembolism in patients undergoing total hip or knee replacement: a systematic review and meta-analysis. Ann Intern Med 156(10):710–719. doi:10.7326/0003-4819-156-10-201205150-00421
Anderson DR, Dunbar MJ, Bohm ER, Belzile E, Kahn SR, Zukor D, Fisher W, Gofton W, Gross P, Pelet S, Crowther M, MacDonald S, Kim P, Pleasance S, Davis N, Andreou P, Wells P, Kovacs M, Rodger MA, Ramsay T, Carrier M, Vendittoli PA (2013) Aspirin versus low-molecular-weight heparin for extended venous thromboembolism prophylaxis after total hip arthroplasty: a randomized trial. Ann Intern Med 158(11):800–806
Acknowledgment
NCC received an educational grant from the Haematology Society of Australia and New Zealand.
Conflicts of interest
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf. JWE has received consulting fees and/or honoraria from Astra-Zeneca, Bayer, Boehringer-Ingelheim, Bristol-Myer-Squibb, Daiichi-Sankyo, Eli-Lilly, Glaxo-Smith-Kline, Pfizer, Janssen, Sanofi-Aventis, and has received grants and/or in-kind support from Astra-Zeneca, Bayer, Boehringer-Ingelheim, Bristol-Myer-Squibb, Glaxo-Smith-Kline, Pfizer, Janssen, Sanofi-Aventis. All other authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chan, N.C., Siegal, D., Lauw, M.N. et al. A systematic review of contemporary trials of anticoagulants in orthopaedic thromboprophylaxis: suggestions for a radical reappraisal. J Thromb Thrombolysis 40, 231–239 (2015). https://doi.org/10.1007/s11239-014-1153-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-014-1153-7