Magnesium oxide in a ZnO-CuO-MgO/Al 2 O 3 /cordierite catalyst contributes to an enhanced yield of hydrogen in steam and steam-oxygen reforming of methanol and to a decreased yield of carbon monoxide in a broad temperature range (230–550 °C). Promotion by magnesium oxide and excess water in the reaction mixture (CH3OH/H2O from 1/5 to 1/10) levels out the sharp decrease in the hydrogen yield at 320–400 °C as a result of a decrease in the reducibility of cupric oxide, which is an active catalyst component.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The ever increasing interest in methanol as a source of hydrogen fuel [1] has led to a search for efficient catalysts for methanol reforming, which, depending on the practical application, is carried out by decomposition (Eq. (1)), steam conversion (Eq. (2)), partial oxidation (Eq. (3)), as well as a combination of steam conversion and partial methanol oxidation known as steam-oxygen or combined reforming under conditions close to an autothermal mode (Eq. 4)):
The decomposition of methanol holds interest in a number of uses of hydrogen fuel including high-temperature solid oxide fuel cells [2]. Oxidative reforming is carried out for low-temperature fuel cells with polymer-electrolyte proton exchange membranes since the carbon monoxide formed in the decomposition of methanol in addition to hydrogen deactivates the platinum electrodes of this type of fuel cell [3].
Among the variety of catalytic compositions used for reforming, Cu-ZnO-containing systems with various additives, which are employed in the industrial manufacture of methanol, have attracted considerable attention [3–6]. In previous work [7, 8], we studied the generation of hydrogen in the decomposition and partial oxidation of methanol in the presence of Cu-ZnO-M x O y /Al2O3 catalysts (M = Ce, La, Ni) supported on honeycomb cordierite structures and elucidated the effect of the components of such compositions on their catalytic properties.
In the present work, we studied the steam and combined steam-oxygen reforming of methanol in order to clarify the role of magnesium oxide as a dopant in ZnO-CuO-MgO/Al2O3/cordierite catalysts during these processes, especially the hydrogen yield and selectivity of CO formation.
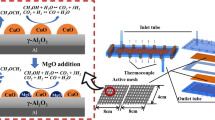
Synthetic honeycomb cordierite elements were used as the catalyst supports (2MgO·2Al2O3·5SiO2, S sp = 0.4 m2/g) with an alumina coating (Al2O3/cordierite, S sp = 6.0 m2/g). The deposition procedure was described in our previous work [9]. The active components were deposited from aqueous solutions of ZnSO4·7H2O, Cu(NO3)2·3H2O, and Mg(NO3)2·6H2O with subsequent drying and roasting (ZnSO4·7H2O at 800 °C, Mg(NO3)2·6H2O at 400 °C, and Cu(NO3)2·3H2Oat 350 °C). We used catalyst samples containing (mass %): 9% ZnO-7% CuO-0.5% MgO/Al2O3/cordierite and 6% ZnO-8% CuO/Al2O3/cordierite.
The X-ray diffraction was carried out using a Bruker AXS D8 Advance diffractometer. The phase composition was determined using the PDF-2 2006 data base.
The temperature-programmed hydrogen reduction (TPHR) of the catalyst samples was carried out in an argon stream containing 10 vol.% hydrogen at 20–800 °C at a heating rate of 17 °C/min.
The reactions of the steam and steam-oxygen conversion of methanol were studied in a flow reactor at atmospheric pressure with gas chromatographic monitoring. The CH3OH-H2O(O2)-Ar gas stream (30 cm3/min, 1250 h–1) was passed through a Pyrex glass reactor with a honeycomb element as the catalyst (~1 g). The methanol–water mixture was introduced using an MS-CA 4/820 peristaltic pump and injector at 150–180 °C. The following CH3OH/H2O/O2 working mixtures were used (vol.%): 4.5/45/0, 14.5/29/0, 20/20/0, 8/40/2, 14.5/29/2.2, and 26/19.5/2.2.
The reforming processes were characterized by the following indices: methanol conversion (%), hydrogen yield (moles H2/mole CH3OHin, in = initial), and selectivity of the formation of the carbon monoxide by-product (%). In some cases, the selectivity of methane formation was also determined.
where \( {C}_{{\mathrm{CH}}_3\mathrm{O}\mathrm{H}}^{\mathrm{in}} \) is the initial methanol concentration (vol.%),\( {C}_{{\mathrm{H}}_2} \), C CO, \( {C}_{{\mathrm{CO}}_2} \), and \( {C}_{{\mathrm{CH}}_4} \) are the concentrations of the reforming products at the reactor outlet at temperature T, °C, and n is the coefficient for taking account of the increase in volume during these processes depending on the reagent concentration.
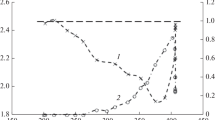
Figure 1a shows the diffraction pattern used to determine the structural and dimensional characteristics of the ZnO-CuO-MgO/Al2O3 composition listed in Table 1. The XRD data indicate that cupric oxide and magnesium oxide exist in the catalyst as monoclinic and cubic modifications, respectively, with mean particle diameter 32 nm (CuO) and 38 nm (MgO). The zinc-containing phase was identified as the cubic aluminate ZnAl2O4 (gahnite) with mean particle diameter 26 nm. Aluminum oxide was X-ray-amorphous.
The indices for the steam conversion and combined reforming of methanol in the presence of the ZnO-CuO-MgO/Al2O3/cordierite catalyst are given in Fig. 2 and summarized in Table 2 in comparison with the indices for ZnO-CuO/Al2O3/cordierite.
Temperature dependence curves for the hydrogen yield in the reforming of methanol on the 9% ZnO-7% CuO-0.5% MgO/Al2O3/cordierite catalyst: a, b) steam conversion before (a) and after reduction (b) of the catalyst sample in situ in an argon stream with 8 vol.%H2 at 400 °C and CH3OH/H2 Oratio 1/10 (1), 1/1 (2), and 1/2 (3); c) combined reforming for CH3OH/H2O/O2 1/5/0.25 (1), 1/2/0.15 (2), and 1/0.75/0.08 (3) \( \left({V}_{{\mathrm{CH}}_3\mathrm{O}\mathrm{H}-{\mathrm{H}}_2\mathrm{O}-\left({O}_2\right)-Ar}=1250{\mathrm{h}}^{-1}\right) \).
These results indicate that in the case of a multifold water excess in the reaction mixture (CH3OH/H2O = 1/10) favorable for preventing caking of the active phase particles and carbonization [10], a gradual increase in the hydrogen yield is observed in the steam conversion of methanol with increasing temperature up to 275 °C (Fig. 2a, curve 1). A further increase in temperature has hardly any effect on \( {Y}_{{\mathrm{H}}_2} \). On the other hand, under conditions of a stoichiometric reagent ratio (CH3OH/H2O= 1/1), after an initial sharp increase in the hydrogen yield with increasing temperature up to 275–300 °C, we find a sharp drop in \( {Y}_{{\mathrm{H}}_2} \) at 320 °C with a gradual increase upon a further increase in temperature (Fig. 2a, curve 2). We should note that a steady-state mode for the steam conversion of methanol at 320 °C is achieved at ~2 h after the decrease in \( {Y}_{{\mathrm{H}}_2} \). Such catalytic behavior might be attributed to caking of the copper-containing particles in the vicinity of ~300 °C [6]. However, taking account that the shape of curve 2 is reproduced during steam reforming when CH3OH/H2O = 1/1 after reoxidation of the ZnO-CuO-MgO/Al2O3/cordierite sample in a stream of 4 vol.% O2 in argon at 450 °C, the sharp decrease in activity at 320 °C is logically explained by consumption of the desired hydrogen product in the reduction of cupric oxide, which is the active component of the reforming catalyst. The subsequent increase in the hydrogen yield with increasing temperature after reaching a minimum on the curve for the temperature dependence of \( {Y}_{{\mathrm{H}}_2} \) may be caused by the catalytic activity of metallic copper.
An argument for this explanation of the temperature dependence is found in the markedly lower hydrogen yield in the presence of a previously reduced catalyst sample in the steam conversion of methanol in the temperature range up to 300 °C (Fig. 2b) than the corresponding values of \( {Y}_{{\mathrm{H}}_2} \) for the unreduced catalyst (Fig. 2a, curves 2 and 3).
The less sharp decrease in the hydrogen yield for reagent ratio CH3OH/H2O = 1/2 in the range 270–415 °C (Fig. 2a, descending branch of curve 3) in comparison with the decrease found when CH3OH/H2O = 1/1 (300–330 °C) may be attributed to the decrease in the reducibility of cupric oxide in the presence of excess water. Such a tendency is most clearly seen under conditions of a multifold excess of steam in the reaction mixture, which leads to elimination of the minimum on curve 1 (Fig. 2a).
The effect of the CH3OH/H2O ratio on the temperature dependence of the hydrogen yield in the combined reforming of methanol (Fig. 2c) is analogous to the effect described above for the steam conversion of the substrate.
We should note that the steam conversion of methanol on ZnO-CuO-MgO/Al2O3/cordierite in the broad temperature range from 230 to 550 °C proceeds virtually without formation of carbon monoxide as a side-product (Table 2). The selectivity relative to CO did not exceed 2% in the combine reforming of the substrate in the case of a five-fold excess of water (CH3OH/H2O/O2 = 1/5/0.25). On the other hand, with a higher CH3OH/H2O ratio (CH3OH/H2O/O2 = 1/2/0.15), including under conditions close to the autothermal mode (CH3OH/H2O/O2 = 1/0.75/0.08), the selectivity for CO formation reaches 11%-14%, which may be a consequence both of methanol decomposition and the reverse water–gas shift reaction: CO2 + H2→ CO + H2O.
Literature data indicate that magnesium oxide prevents the caking of particles of the active phase of CuO-ZnO catalysts [11]. We should note that the surface of the ZnO-CuO/Al2O3/cordierite sample after maintenance in a methanol–argon stream at ~500 °C is a continuous conglomerate of particles without visible boundaries due to caking [7].
In addition to stabilization of the highly dispersed state of the active phase, MgO decreases the reducibility of cupric oxide, possibly, due to formation of a mixed oxide system MgCu2O3 [12]. This hypothesis is supported by the thermal-programmed reduction results: the temperature range and temperature for the maximum of hydrogen absorption by the surface of the ZnO-CuO-MgO/Al2O3/cordierite sample (350–550 °C, T m = 425 °C) significantly exceed the corresponding values for ZnO-CuO/Al2O3/cordierite (220–365 °C, T m = 305 °C) (Fig. 1b). These differences in reducibility are apparently manifest in the less sharp decrease in the activity of the catalysts modified with magnesium oxide in the steam conversion of methanol for reagent ratio CH3OH/H2O = 1/2 (2.5-1.6 moles H2/mole CH3OH/270-415 °C) in comparison with the decrease found for the binary ZnO-CuO sample (2.2-0.7 moles H2/mole CH3OH/270-320 °C). Higher temperatures for the decrease in the activity of ZnO-CuO-MgO/Al2O3/cordierite are observed both in steam and combined reforming than found for ZnO-CuO/Al2O3/cordierite. In contrast to the case of the ZnO-CuO-MgO/Al2O3/cordierite catalyst, the temperature dependence of the hydrogen yield in the presence of ZnO-CuO/Al2O3/cordierite shows a minimum in the range 350–400 °C also when there is a ten-fold excess of water in the reaction mixture. Furthermore, the values of \( {Y}_{{\mathrm{H}}_2} \) in the presence of ZnO-CuO/Al2O3/cordierite are somewhat lower than for the MgO-modified catalyst with a sharp increase in the selectivity relative to CO formation (S CO→50%), which may be attributed to predominance of methanol decomposition. The presence of methane in the products of steam and combined reforming of methanol (\( {S}_{{\mathrm{CH}}_4}\approx 1\%-4\%,350-550{}^{\circ}\mathrm{C} \)) is apparently due to a side-reaction methanation: CO + 3H2 → CH4 + H2O.
Thus, the ZnO-CuO/Al2O3/cordierite catalyst modified with magnesium oxide gives a higher hydrogen yield in the steam and steam-oxygen conversion of methanol along with decreased selectivity relative to carbon monoxide formation as a side-product in the broad temperature range from 230 to 550 °C. Extrema are noted on the temperature dependence curve for \( {Y}_{{\mathrm{H}}_2} \) (a minimum at 320–400 °C). Promotion by magnesium oxide and an excess of water in the reaction mixture (CH3OH/H2O = from 1/5 to 1/10) level out the sharp decrease in the hydrogen yield. This effect is attributed to a decrease in the reducibility of cupric oxide, which is the active component of the catalyst.
References
S. Krummrich and J. Llabrés, Int. J. Hydrogen Energy, 40, 5482–5486 (2015).
N. Laosiripojana and S. Assabumrungrat, Chem. Eng. Sci., 61, 2540–2549 (2006).
K. Geissler, E. Newson, F. Vogel, et al., Phys. Chem. Chem. Phys., 3, 289–293 (2001).
S. T. Yong, C. W. Ooi, S. P. Chai, and X. S. Wu, Int. J. Hydrogen Energy, 38, 9541–9552 (2013).
S. Schuyten and E. E. Wolf, Catal. Lett., 106, 7–14 (2006).
S. Sá, H. Silva, L. Brandão, et al., Appl. Catal. B, 99, 43–57 (2010).
A. Yu. Kapran, S. O. Soloviev, and S. N. Orlyk, React. Kinet. Mech. Catal., 101, 343–353 (2010).
A. Yu. Kapran, S. N. Orlyk, and S. O. Soloviev, React. Kinet. Mech. Catal., 114, 135–145 (2015).
S. A. Solov’ev, Ya. P. Kurilets, and S. N. Orlik, Teor. Éksp. Khim., 39, No. 1, 50–54 (2003). [Theor. Exp. Chem., 39, No. 1, 58–63 (2003) (English translation).]
T. Valdés-Solís, G. Marbán, and A. B. Fuertes, Catal. Today, 116, 354–360 (2006).
A. Basile, A. Parmaliana, S. Tosti, et al., Catal. Today, 137, 17–22 (2008).
H. Von Drenkhahn and H. Müller-Buschbaum, Z. Anorg. Allg. Chem., 418, 116–120 (1975).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Éksperimental’naya i Teoreticheskaya Khimiya, Vol. 51, No. 4, pp. 206–210, July-August, 2015.
Rights and permissions
About this article
Cite this article
Kapran, A.Y., Orlyk, S.N. Effect of Magnesium Oxide on the Catalytic Properties of ZnO-CuO-MgO/Al2O3/Cordierite in Steam and Steam-Oxygen Reforming of Methanol. Theor Exp Chem 51, 210–215 (2015). https://doi.org/10.1007/s11237-015-9418-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-015-9418-6