Abstract
A new species of Eimeria Schneider, 1875 (Apicomplexa: Eimeriidae) is described from faecal samples of two of three southern short-tailed shrews, Blarina carolinensis (Bachman) (Soricidae) from southeastern Oklahoma, USA. Oöcysts of Eimeria tkachi n. sp. are subspheroidal to ovoidal with a rough-pitted, tan colored, bi-layered wall, measure 16.5 × 15.2 µm, and have a length/width (L/W) ratio of 1.1; both micropyle and oöcyst residuum are absent, but polar granule(s) are present. Sporocysts are ovoidal, 9.5 × 6.5 µm, L/W 1.4; a distinct button-like Stieda body is present, but the sub-Stieda and para-Stieda bodies are absent and the sporocyst residuum is composed of large globules distributed throughout the sporocyst. Sporozoites have a spheroidal anterior refractile body, a subspheroidal posterior refractile body, and one centrally-located nucleus. This is the smallest eimerian described thus far from the Soricidae, the initial description of a coccidian from B. carolinensis, and the first from any shrew from Oklahoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The state of Oklahoma is thought to support at least five species of shrews, including southern short-tailed shrew, Blarina carolinensis (Bachman), Elliot’s short-tailed shrew, B. hylophaga (Elliot), least shrew, Cryptotis parva (Say), desert shrew, Notiosorex crawfordi (Coues), and southeastern shrew, Sorex longirostris Bachman (see Taylor & Wilkinson, 1988; Caire et al., 1989). However, Pfau et al. (2011) suggested that the northern short-tailed shrew, B. brevicauda (Say) could occur in eastern Oklahoma. One of these, B. carolinensis ranges from southern Illinois to the Gulf of Mexico and from eastern Texas to the Atlantic Ocean (Reid, 2006). In Oklahoma, B. carolinensis is known only from Le Flore and McCurtain counties (Caire et al., 1989; Braun et al., 2011). It is listed as vulnerable (S3) in the state by NatureServe (NatureServe Explorer, 2015).
A great deal of information is available on the natural history and ecology of B. carolinensis, including information on its helminth parasites (McCay, 2001). Although there are reports of coccidians from numerous other North American shrew species (Duszynski & Upton, 2000), nothing, to date, has been published on coccidia from B. carolinensis. The present study provides a description of an eimerian infecting B. carolinensis in southeastern Oklahoma, USA.
Materials and methods
During November 2016, three adult B. carolinensis were collected by hand or with Victor® snap-traps from Hochatown, McCurtain County, Oklahoma. Fresh fecal samples were placed in individual vials containing 2.5% (w/v) aqueous potassium dichromate (K2Cr2O7). Samples were initially examined for coccidia after flotation in Sheather’s sugar solution (specific gravity 1.30) and two were found to contain numerous unsporulated oöcysts. Specimens were then placed in individual Petri dishes containing a small layer of K2Cr2O7 and allowed to sporulate for one week at room temperature (c. 23°C). Samples were further examined after flotation using an Olympus BX53 light microscope. Measurements were taken on 42 sporulated oöcysts from a single shrew using Olympus© cellSens 1.14 digital software and reported in micrometers (µm) with the ranges followed by the means in parentheses; photographs were taken on 49 sporulated oöcysts using Nomarski interference-contrast optics. Oöcysts of the new species were c.37 days old when measured and photographed. Host vouchers (alcohol-fixed specimens) were accessioned into the Henderson State University Collection, Arkadelphia, Arkansas, USA. Photosyntypes of sporulated oöcysts were accessioned into the Harold W. Manter Laboratory of Parasitology (HWML), Lincoln, Nebraska, USA.
Results
Two of the three (67%) B. carolinensis were found to be passing coccidian oöcysts with an eimerian that we describe here as new.
Family Eimeriidae Minchin, 1903
Genus Eimeria Schneider, 1875
Eimeria tkachi n. sp.
Type-host: Southern short-tailed shrew, Blarina carolinensis (Bachman, 1837) (Mammalia: Soricomorpha: Soricidae) (adult male, voucher HSU 953, collected 29.ix.2016).
Type-locality: Halibut Bay Road, Hochatown (34°09′55.152″N, 94°45′35.8776″W), McCurtain County, Oklahoma, USA.
Type-material: Photosyntypes of sporulated oöcysts are deposited as HWML 103064.
Prevalence: In 2 of 3 (67%).
Sporulation time: Oöcysts were completely sporulated within 7 days at 23°C.
Site of infection: Unknown; oöcysts recovered from faeces.
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Eimeria tkachi n. sp. is urn:lsid:zoobank.org:act:3D6BDAA5-8334-4EF1-9A52-2E22F5313106.
Etymology: The specific epithet is given in honor of Dr Vasyl V. Tkach (University of North Dakota, Grand Forks, North Dakota, USA) for his work on the parasites of North American shrews.
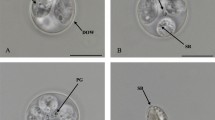
Sporulated oöcyst
Oöcyst (n = 42) tan, rough-pitted wall, subspheroidal to ovoidal; 14–19 × 13–18 (16.5 × 15.2), length/width (L/W) ratio 1.0–1.2 (1.1). Wall bi-layered, c.1.2 thick, inner layer c.0.4, outer layer c.0.8. Micropyle and oöcyst residuum absent, polar granule(s) present.
Sporocyst
Sporocyst (n = 42) four, colourless, smooth, ovoidal 8–11 × 6–7 (9.5 × 6.5); L/W ratio 1.3–1.6 (1.4); wall single-layered, c.0.4 thick. Distinct button-like Stieda body present, sub-Stieda and para-Stieda bodies absent; sporocyst residuum formed by large globules distributed throughout the sporocyst.
Sporozoites
Sporozoites (not measured) two, sausage-shaped; with a spheroidal anterior refractile body and subspheroidal posterior refractile body and one centrally located nucleus.
Remarks
We limit the comparison of our new form only to those eimerian species from North American shrews of the family Soricidae (see Duszynski & Upton, 2000) (Table 1). When compared to the oöcysts and sporocysts of both Eimeria blarinae Todd, French & Levine, 1986 from B. brevicauda from Illinois, and Eimeria brevicauda Hertel & Duszynski, 1987 from B. brevicauda from Ohio (Todd et al., 1986; Hertel & Duszynski, 1987), oöcysts and sporocysts of the new species are significantly smaller. In addition to size differences, E. brevicauda possesses a sub-Stieda body (unlike the new species), and E. blarinae supposedly has an oöcyst residuum (unlike the new species) but no polar granules (as originally described). However, Duszynski & Upton (2000) mentioned that there was some confusion about the internal structure of oöcysts of E. blarinae described by Todd et al. (1986). These authors did not distinguish between polar granules and an oöcyst residuum in E. blarinae, the latter internal structure of which was thought to be present as “composed of clear homogenous globules of two sizes.” So we are left wondering if E. blarinae indeed does possess an oöcyst residuum or actually what would be referred to as polar granules. In addition, the line drawing of E. blarinae does not distinguish between these structures, there are no photomicrographs of the species, and it hasn’t been reported in another shrew since its original description over 30 years ago.
The new species is most similar in size to Eimeria cryptotis McAllister & Upton, 1989 and Eimeria whitakeri Upton & McAllister, 1991 from C. parva in Texas (Table 1) but both have prominent sub-Stieda bodies (McAllister & Upton, 1989; Upton & McAllister, 1991). In addition, E. beringiacea Lynch & Duszynski, 2008 from Sorex tundrensis Merriam from Alaska, USA, and Siberia, Russia, is also similar in size to the new species but it possesses a sub-Stieda body and does not have a polar granule (Lynch & Duszynski, 2008). Compared to all the eimerians known from shrews (Duszynski & Upton, 2000; Lynch & Duszynski, 2008), the new species represents the smallest species in the genus.
Based on the differences in morphological and morphometric characteristics noted in the present study, E. tkachi is considered a species new to science. Moreover, it is the first eimerian reported from the southern short-tailed shrew as well as the only species reported from Oklahoma shrews.
Discussion
It has been more than a decade and a half since Duszynski & Upton (2000) summarized the coccidians of Soricimorphs (formerly Insectivora) of the world. Since then, Milek & Seville (2003) reported coccidians from shrews from Wyoming, and Lynch & Duszynski (2008) described two species and added others from shrews in Alaska and Siberia, Russia. Here, we provide an update with what we believe to be 12 species known from North American soricid host species (Table 1).
Duszynski & Upton (2000) listed 23 species of shrews within six genera that have served as hosts for various coccidians (both isosporans and eimerians). However, with over 300 species within 23 genera, that number represents only 8% of all soricid shrews reported to have coccidians (see Bray & Stockley, 2005). In addition, previous examination of the faeces of 14 additional B. carolinensis from two counties (Conway and Union) in Arkansas between July 2012 and April 2013 failed to find any to be passing coccidian oöcysts, so the prevalence of infection with coccidia may be quite low among some shrew populations. Obviously, many species are yet to be examined and future surveys will surely increase our knowledge of shrew coccidians by reporting new host and geographic distributional records, as well as including the possibility of discovering additional species new to science.
References
Braun, J. K., Vitt, L. J., Caldwell, J. P., Mares, M. A., & Revelez, M. A. (2011). Mammals from Le Flore County, Oklahoma. Southwestern Naturalist, 56, 410–417.
Bray, D., & Stockley, P. (2005). Coccidian parasite faunas and shrew life history traits: a comparative analysis. In: Merritt, J. F., Churchfield, S., Hutterer, R. & Sheftel, B. I. (Eds) Advances in the Biology of Shrews II, Special Publication 01. Pennsylvania, USA: International Society of Shrew Biologists (ISSB), pp. 349–356.
Caire, W., Tyler, J. D., Glass, B. P., & Mares, M. A. (1989). Mammals of Oklahoma (p. 567). Norman, Oklahoma: University of Oklahoma Press.
Duszynski, D. W., & Upton, S. J. (2000). Coccidia (Apicomplexa: Eimeriidae) of the mammalian order Insectivora. Special Publication of the Museum of Southwestern Biology, 4, 1–67.
Henry, D. P. (1932). Observations on the coccidia of small mammals in California, with descriptions of seven new species. University of California Publications in Zoology, 37, 279–290.
Hertel, L. A., & Duszynski, D. W. (1987a). Coccidian parasites (Apicomplexa: Eimeriidae) from insectivores. III. Seven new species from shrews (Soricidae: Soricinae) from Canada, Japan, and the United States. Journal of Parasitology, 73, 172–183.
Hertel, L. A., & Duszynski, D. W. (1987b). Revision of the name Eimeria palustris Hertel & Duszynski, 1987, from shrews (Sorex spp.). Journal of Parasitology, 73, 1288.
ICZN. (2012). International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Zootaxa, 3450, 1–7.
Lynch, A. J., & Duszynski, D. W. (2008). Species of coccidia (Apicomplexa: Eimeriidae) in shrews from Alaska, USA, and northeastern Siberia, Russia, with descriptions of two new species. Journal of Parasitology, 94, 883–888.
McAllister, C. T., & Upton, S. J. (1989). Eimeria cryptotis n. sp. (Apicomplexa: Eimeriidae) from the least shrew, Cryptotis parva (Insectivora: Soricidae), in north-central Texas. Journal of Parasitology, 75, 212–214.
McCay, T. S. (2001). Blarina carolinensis. Mammalian Species, 673, 1–7.
Milek, J. A., & Seville, R. S. (2003). Species of Eimeria and Isospora (Apicomplexa: Eimeriidae) from shrews (Insectivora: Soricidae) in northwestern Wyoming, USA. Comparative Parasitology, 70, 72–77.
NatureServe. (2015). NatureServe Explorer: An online encyclopedia of life [web application]. Version 7.1. NatureServe, Arlington, Virginia. Available http://explorer.natureserve.org. (Accessed: December 13, 2016).
Pfau, R. S., Sasse, D. B., Connior, M. B., & Guenter, I. F. (2011). Occurrence of Blarina brevicauda in Arkansas and notes on the distribution of Blarina carolinensis and Cryptotis parva. Journal of the Arkansas Academy of Science, 65, 61–66.
Reid, F. A. (2006). A field guide to mammals of North America (p. 579). New York: Houghton Mifflin.
Taylor, C. L., & Wilkinson, R. F., Jr. (1988). First record of Sorex longirostris (Soricidae) in Oklahoma. Southwestern Naturalist, 33, 248.
Todd, K. S., Jr., French, R. A., & Levine, N. D. (1986). Eimeria blarinae n. sp. (Protozoa: Apicomplexa) from the least shrew, Blarina brevicauda. Transactions of the American Microscopical Society, 105, 182–184.
Upton, S. J., & McAllister, C. T. (1991). Description of the oöcysts of Eimeria whitakeri n. sp. (Apicomplexa: Eimeriidae) from least shrews, Cryptotis parva (Insectivora: Soricidae). Systematic Parasitology, 20, 59–61.
Wilber, P. G., Duszynski, D. W., Upton, S. J., Seville, R. S., & Corliss, J. O. (1998). A revision of the taxonomy and nomenclature of the Eimeria spp. (Apicomplexa: Eimeriidae) from rodents in the Tribe Marmotini (Sciuridae). Systematic Parasitology, 39, 113–135.
Acknowledgements
We thank Drs. Renn Tumlison (HSU) and Scott L. Gardner and Gabor R. Racz (HWML) for expert curatorial assistance. We also thank Matthew B. Connior (NW Arkansas Community College, Bentonville, Arkansas) for providing faecal samples from B. carolinensis from Arkansas. A scientific collecting permit (number 6477) was provided to C.T.M. by the Oklahoma Department of Wildlife Conservation.
Funding
This study was supported, in part, by a grant from the National Institute of General Medical Sciences (2P20GM103432), National Institutes of Health (NIH) to R.S. Seville. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Additional information
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as urn:lsid:zoobank.org:pub:8198DE76-F4FB-4408-B299-3640114F72B2. This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number. This is the Version of Record.
Rights and permissions
About this article
Cite this article
McAllister, C.T., Seville, R.S. A new eimerian (Apicomplexa: Eimeriidae) from southern short-tailed shrews, Blarina carolinensis (Bachman) (Soricimorpha: Soricidae: Soricinae) from southeastern Oklahoma, USA. Syst Parasitol 94, 711–716 (2017). https://doi.org/10.1007/s11230-017-9730-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-017-9730-8