Abstract
With only six recognised genera, the family Clinostomidae Lühe, 1901 remains a global research interest of parasitologists and ecologists. Recent efforts have focused on providing molecular data to investigate species diversity, elucidate life-cycles, and make inferences on the group’s evolutionary history. Of the clinostomid genera, the monotypic Ithyoclinostomum Witenberg, 1926 has remained more enigmatic compared to the commonly encountered Clinostomum Leidy, 1856. Recent morphological and molecular evidence from metacercariae suggests a second Ithyoclinostomum species may exist in freshwater cichlids in Central America and Mexico. In a recent survey of great blue herons Ardea herodias L. from commercial catfish production farms in Mississippi, USA, two specimens of an abnormally large (> 20 mm) clinostomid were encountered in the oesophagus of a single bird. These specimens were identified as an Ithyoclinostomum sp. morphologically distinct from the only nominal species Ithyoclinostomum dimorphum (Diesing, 1850). Using morphological and molecular data these adult specimens were confirmed as conspecific with the larval metacercariae previously described from Central America and Mexico and represent the novel species, Ithyoclinostomum yamagutii n. sp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinostomid trematodes are cosmopolitan parasites found as adults in the buccal cavity and oesophagus of birds, reptiles and occasionally mammals, including humans (Kanev et al., 2002). The family Clinostomidae Lühe, 1901 currently contains four subfamilies for its six recognised genera (Kanev et al., 2002; Caffara et al., 2019). The subfamily Clinostominae Lühe, 1901 is comprised of two genera: Clinostomatopsis Dollfus, 1932 and the well-known type-genus Clinostomum Leidy, 1856. The Euclinostominae Yamaguti, 1958 and Ithyoclinostominae Yamaguti, 1958 both contain only one genus, Euclinostomum Travassos, 1928 and Ithyoclinostomum Witenberg, 1926, respectively. Lastly, Nephrocephalinae Travassos, 1928 contains two genera, Nephrocephalus Odhner, 1902 and Odhneriotrema Travassos, 1928, uniquely found in the buccal cavity and oesophagus of reptiles (Kanev et al., 2002). Recent efforts at unraveling the species diversity of the Clinostomidae have focused heavily on Clinostomum spp. and the inclusion of molecular data has revealed an unprecedented global diversity of species (Gustinelli et al., 2010; Caffara et al., 2011; Locke et al., 2015; Rosser et al., 2017, 2018; Sereno-Uribe et al., 2013, 2018). Comparably, limited studies have applied these molecular techniques to answer questions of higher-level taxonomic placement of the genera within their subfamilies (Caffara et al., 2016, 2019; Woodyard et al., 2017; Briosio-Aguilar et al., 2018), but molecular data are still lacking for the genera Clinostomatopsis and Nephrocephalus.
The genus Ithyoclinostomum was originally established for an adult clinostomid from the oesophagus of piscivorous birds from Brazil with a long, narrow body capable of reaching several cm in length (Witenberg, 1926; Kanev et al., 2002). Currently the genus is represented only by the type-species, Ithyoclinostomum dimorphum (Diesing, 1850) from Ardea cocoi L., Ardea alba L., Nycticorax nycticorax (L.), and Tigrisoma lineatum (Boddaert) from South American localities (Diesing, 1850; Lent & Freitas, 1937; Dias et al., 2003; Pinto et al., 2004). Ithyoclinostomum dimorphum is the largest described clinostomid trematode, capable of reaching 100 mm in length. There are limited recorded accounts of adults of this enigmatic trematode (Braun, 1899; Travassos, 1928; Lent & Freitas, 1937; Dias et al., 2003; Pinto et al., 2004) and the current morphological basis for the genus (and its lone species) is based on a small number (< 5) of adult specimens (Braun, 1901; Lent & Freitas, 1937; Dias et al., 2003).
Metacercariae morphologically resembling I. dimorphum have been reported from a variety of freshwater fish in Central and South American localities (see Briosio-Aguilar et al., 2018 and references therein). More recently metacercariae identified as an Ithyoclinostomum sp., but lacking the elongate and attenuated anterior region characteristic of I. dimorphum were encountered in cichlid fish in Mexico and Costa Rica (Briosio-Aguilar et al., 2018), which provided the first molecular data for an Ithyoclinostomum sp. It should be noted, however, that Briosio-Aguilar et al., (2018) were tentative in their generic identification of these specimens owing to the distinct morphological differences from I. dimorphum. However, they did provide sufficient evidence to support their assertion this likely represents a novel species of Ithyoclinostomum, on the occasion the adult stage is encountered and described.
In a recent survey of piscivorous birds in Mississippi, USA adult clinostomids morphologically resembling the metacercariae described in Briosio-Aguilar et al. (2018) were collected. Using morphological and molecular data these two stages are united and herein is proposed a second nominal species, Ithyoclinostomum yamagutii n. sp.
Materials and methods
Trematode collection and morphological characterisation
Thirteen great blue herons Ardea herodias (L.) were collected from four commercial catfish farms in Lowndes and Noxubee County, Mississippi, USA from April to May 2019. Herons were collected pondside using shotguns (IACUC QA 2853) and transported on ice to the Parasitology Laboratory at the College of Veterinary Medicine, Mississippi State University, Mississippi State, Mississippi, USA. Sex was determined at necropsy.
A complete parasitology necropsy was performed on each bird within 15 hours of collection. The oral cavity was examined for attached clinostomid trematodes and all were removed and placed in physiologic saline (0.9% NaCl). The entire gastrointestinal tract was removed and cinched into sections (oesophagus, proventriculus, small intestine, and cecum with large intestine) using plastic zip ties. Each section was excised and placed into a container with physiologic saline. These sections of intestinal tract were opened longitudinally, and the mucosal lining and organ contents were gently scraped into a 38-µm aperture brass sieve. Helminths were relaxed by pouring boiling saline over the screened contents and organs prior to preservation in 70% molecular biology grade ethanol (modified from Pritchard & Kruse, 1982).
Two abnormally large (> 20 mm) clinostomid trematodes (Fig. 1) were observed in the oesophagus of a single female great blue heron. These were individually relaxed and preserved as previously described. Each worm was individually placed into a sterile Petri dish and submerged in 70% molecular ethanol. With the aid of a dissecting microscope (Olympus SZ60, Olympus Optical Co. Ltd., Tokyo, Japan), sections of the right posterior margin of each worm were excised using sterile scalpels to create hologenophore specimens for further morphological and molecular characterisation. Care was taken to excise only parenchyma tissue and not cut through any morphologically informative organs. Each excised section of tissue was placed individually into separate microcentrifuge tubes for genomic DNA extraction.
Trematode staining and morphological characterisation
Hologenophores (n = 2) were transitioned from 70% ethanol to distilled water through a decreasing series of 1-hour ethanol washes (50% and 30%) before staining with Van Cleave’s hematoxylin. Worms were dehydrated, cleared in methyl salicylate, and mounted in Canada balsam. The line drawing was made using a camera lucida and digitized using Adobe Illustrator CC 2019 (Adobe, San Jose, California, USA). Morphological data were recorded using an Olympus BX53 microscope with differential interference contrast microscopy optics, DP74 digital camera and the accompanying cellSens v. 1.18 software (Olympus Optical Co. Ltd.). Morphological data are reported in micrometres, unless otherwise stated, and are represented as the range followed by the mean in parentheses. The holotype has been deposited in the Smithsonian Institution, National Museum of Natural History, Washington, DC, USA.
DNA extraction and molecular characterisation
Total genomic DNA was extracted from excised tissue sections from each hologenophore using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, California, USA). A c.4,200-bp region of ribosomal DNA spanning the 18S rRNA gene, internal transcribed spacer 1 region (ITS1), 5.8S rRNA gene, ITS2 region, and partial 28S rRNA gene was sequenced from a single specimen using the primers and protocols reported in Rosser et al. (2018). The ITS regions and partial 28S rRNA gene were sequenced from both clinostomid specimens. Additionally, the ITS regions and partial 28S rRNA gene were amplified and sequenced from an archived specimen of Odhneriotrema incommodum (Leidy, 1856) isolate 1603326 of Woodyard et al. (2017). A new set of primers were designed to amplify a c.600-bp region of the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene: Clino_gen_F: 5′-GAT TGA ATC TGT GTG ATC CTT ATT-3′ and Clino_gen_R: 5′-CAC AAA TAT GAC TAA TAA CCC CAA-3′. Briefly, for the new cox1 primers, each 20-µl polymerase chain reaction consisted of 7 µl of nuclease-free water, 10 µl of Phusion Green Hot Start II High-Fidelity PCR Master Mix (Thermo Fisher Scientific, Inc., Waltham, Massachusetts, USA), 10 µM of each primer, and 1 µl of genomic DNA (c.10 ng/µl). Cycling parameters for the cox1 primers were 98°C for 3 min, 40 cycles at 98°C for 10 s, 55°C for 30 s, 72°C for 1 min, and a final extension step at 72°C for 10 min.
Amplicons were passed electrophoretically through 0.8% sodium borate buffered agarose gels in the presence of ethidium bromide (0.5 µg/ml). Each amplicon was compared to a concurrently run molecular weight ladder (HyperLadder™ 50 bp, Bioline, London, UK) to determine the appropriate size band for excision. Target amplicons were excised and purified using the QIAquick Gel Extraction Kit (Qiagen Inc.). Each product was bidirectionally sequenced (Eurofins MWG Operon LLC, Louisville, Kentucky, USA) using both forward and reverse primers for each target. Chromatograms were aligned and annotated using Geneious Prime® 2019.2.1 (Biomatters Ltd., Auckland, New Zealand) and ambiguous bases were resolved manually. Each sequence was compared to publicly available sequences of other clinostomid trematodes through a BLASTN search of the National Center for Biotechnology Information non-redundant nucleotide database (NCBI nr/nt) (Altschul et al., 1990).
Phylogenetic analysis
Published sequences derived from representatives of the Clinostomidae were downloaded from the NCBI nr/nt database. Downloaded sequences were comprised of representatives from all sequenced clinostomid genera wherein ITS1, 5.8S rRNA gene, ITS2, 28S rRNA gene, and cox1 gene sequence data were available. ITSx v. 1.0.11 was used to extract the ITS1, 5.8S rRNA gene, and ITS2 region from each downloaded sequence (Bengtsson-Palme et al., 2013). Sequences were aligned using MAFFT v. 1.3.7 in Geneious Prime® (Katoh et al., 2002; Katoh & Standley, 2013). Alignments were trimmed and any positions containing at least one gap were removed before concatenation for a final alignment containing 2,086 positions. The Bayesian information criterion was used to select the nucleotide substitution model that best fits the data for each aligned region (Nei & Kumar, 2000; Kumar et al., 2016): cox1 codon position 1 (TN93 + I; 144 positions), cox1 codon position 2 (JC; 144 positions), cox1 codon position 3 (HKY + G; 144 positions), ITS1 region (K2 + G; 463 positions), 5.8S rRNA gene (JC; 157 positions), ITS2 region (GTR + G + I; 294 positions), and 28S rRNA gene (HKY + G; 740 positions). Bayesian inference analysis was performed using MrBayes v. 3.2.6 (Ronquist & Huelsenbeck, 2003) with Markov chain Monte Carlo searches of two simultaneous runs of four chains with sampling occurring every 100th tree for 1,000,000 generations. With the value of the standard deviation of split frequencies < 0.01, the first 25% of trees were discarded as “burn-in” and posterior probabilities were calculated from the remaining trees. Maximum likelihood analysis was performed using IQ-Tree on the IQ-Tree web server (Nguyen et al., 2015; Trifinopoulos et al., 2016) with the same concatenated alignment and partitioning scheme as previously described. Branch support was tested by the ultrafast bootstrap method (Minh et al., 2013) with 1,000 pseudoreplicates. In addition to the aforementioned molecular methods of tree construction, a cladogram depicting the currently accepted morphology-based classification of clinostomids (Kanev et al., 2002) was made using a text editor. Phylogenetic trees and the morphology-based cladogram were analysed in FigTree 1.4.2 (Rambaut, 2014) and refined in Adobe Illustrator CC 2019 (Adobe). Pairwise distances were calculated using Geneious Prime®.
Family Clinostomidae Lühe, 1901
Genus Ithyoclinostomum Witenberg, 1926
Ithyoclinostomum yamagutii n. sp.
Type-host: Ardea herodias (L.) (Pelicaniformes: Ardeidae), great blue heron, ♀.
Type-locality: Lowndes County, Mississippi, USA.
Type-material: The holotype (USNM 1548282) is deposited in the Smithsonian Institution, National Museum of Natural History, Washington, D.C., USA.
Infection parameters: Prevalence: 7.7% (1 out of 13 birds); abundance: range 0–2; mean intensity 2.0 worms per infected bird.
Site in host: Oesophagus.
Representative DNA sequences: GenBank MN696159-MN696162 (ribosomal regions) and MN696163-MN696164 (cox1 gene).
Etymology: The specific epithet is in reference to the renowned Satyu Yamaguti for establishing the subfamily Ithyoclinostominae and his eminence in the field of trematode systematics.
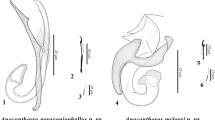
Description (Figs. 1–4)
[Based on the holotype and 1 paratype. All measurements taken from Van Cleave’s hematoxylin stained and Canada balsam mounted gravid worms, see Table 1.] Body large, linguiform, dorsally convex, ventrally concave, widest at midpoint, 24,218–25,238 × 6,088–6,505 (24,728 × 6,297). Tegument lacking surface spines. Anterior extremity contains incomplete oral collar-like fold surrounding oral sucker. Oral sucker subterminal, small, 429–641 × 807–839 (535 × 823). Pharynx present, 600 × 467 (n = 1). Oesophagus absent. Intestinal caeca simple, bifurcate just posterior to pharynx and laterally extend almost to posterior extremity of body. Ventral sucker, large, located near anterior extremity of the body, close to oral sucker, 2,219–2,436 × 2,108–2,462 (2,327 × 2,285). Small, tegumental papillae visible on the ventral surface just anterior to ventral sucker (Fig. 4A). Distance between oral and ventral sucker 1,089–1,889 (1,489).
Photomicrographs of morphological details of Van Cleave’s hematoxylin-stained Ithyoclinostomum yamagutii n. sp. ex Ardea herodias from commercial catfish operation in Lowndes County, Mississippi, USA. A and inset figure, Tegumental papillae (arrows) anterior to the ventral sucker of holotype. B, Dorsal opening (arrowhead) of Laurer’s canal on the tegumental surface of the paratype. Scale-bars: A, B, 1 mm; A inset, 200 µm
Testes 2, tandem, median, intercaecal, deeply lobed, located in upper region of posterior fourth of body. Anterior testis with 4 lobes, H-shaped, 1,995–2,024 × 2,661–2,708 (2,010 × 2,685), anterior lobes longer than posterior lobes. Posterior testis with 4 lobes, X-shaped, 1,502–2,000 × 1,944–2,084 (1,751 × 2,014), lobes roughly the same size. Distance between testes 672–914 (793). Cirrus-sac ovoid, small, median to slightly dextral, 639–649 × 742–776 (644 × 759), just anterior to anterior testis. Cirrus not observed. Distance from posterior margin of posterior testis to body terminus, 2,838–2,874 (2,856).
Ovary small, intertesticular, dextral, ovoid, smooth, 630–698 × 493–591 (664 × 542). Mehlis’ gland large. Seminal receptacle present, with Laurer’s canal opening on dorsal surface at level of anterodextral margin of posterior testis (Fig. 4B). Uterine duct intracaecal, emerging from oötype region and extending anteriorly along sinistral margin of anterior testis before opening into uterine sac, details difficult to discern due to the presence of eggs. Uterine sac, well developed, 11,166–11,560 (11,363) long, filled with thousands of eggs, occupying almost entire median field between ventral sucker and anterior testis. Distance from posterior margin of ventral sucker to anterior margin of uterine sac 1,445–1,771 (1,608). Metraterm muscular, leads from posterior margin of uterine sac into genital atrium. Genital pore just pretesticular, median vitelline fields restricted mostly to lateral margins of body, begin at level of ventral sucker and extend to posterior extremity of body. Vitelline follicles small, in clusters, median and lateral relative to caeca except for gonadal region where they are primarily laterally positioned. Vitelline follicles confluent only posterior to gonads. Eggs (n = 30) operculate, 135–145 × 83–90 (139 ± 2.0 × 86 ± 1.5), often located within uterine sac, uterine duct, and oötype region.
Excretory pore ventrally subterminal with an irregularly Y-shaped vesicle.
Remarks
Adult specimens of I. yamagutii n. sp. in this study are morphologically similar to metacercariae described in Briosio-Aguilar et al. (2018) and both fit the generic description of Ithyoclinostomum according to Baer (1933), Lent & Freitas (1937), Skrjabin (1947), Travassos et al. (1969) and Kanev et al. (2002). This generic identity is reached by I. yamagutii n. sp. having a pre-testicular cirrus-sac, deeply lobed testes, a greater body length relative to species of the other genera in the family Clinostomidae, and the portion of the body between the ventral sucker and anterior testis being void of internal organs (Lent & Freitas, 1937; Travassos et al., 1969; Kanev et al., 2002). However, I. yamagutii n. sp. differs from the sole species within the genus, I. dimorphum, in several qualitative and quantitative features. The overall body of I. dimorphum is elongated and attenuated anteriorly and may reach up to 100 mm in length. Ithyoclinostomum yamagutii n. sp. is approximately 1/5 the length of I. dimorphum and is not elongated and attenuated anteriorly to the extent of I. dimorphum (Table 1). The ventral sucker dimensions of I. yamagutii n. sp. are consistently larger (> 2 mm in length and width) than those of I. dimorphum (< 1.8 mm in diameter). Similarly, the dimensions of both testes and ovary of I. dimorphum are larger than those of I. yamagutii n. sp. and the placement of the cirrus-sac in I. dimorphum is consistently antero-dextral relative to the anterior testis compared to the median to slightly dextral positioning in I. yamagutii n. sp. The ovary of I. dimorphum is described as being lobed in all accounts of adults and metacercariae, compared to the smooth ovary in accounts of I. yamagutii n. sp. adults and metacercariae (Briosio-Aguilar et al., 2018). The distance between the posterior margin of the ventral sucker and anterior margin of the uterine sac is considerably longer in I. dimorphum (63,3265 vs 1,608 µm). While body morphometric data may exhibit plasticity as trematodes develop, both specimens in this study were gravid and more rigid characters such as egg dimensions can also be used to contrast I. yamagutii n. sp. with I. dimorphum: eggs of I. yamagutii n. sp. are distinctly larger than those of I. dimorphum (135–145 × 83–90 vs 100–107 × 57–64 µm).
As noted by Briosio-Aguilar et al. (2018) although the metacercaria and adult stages of I. yamagutii n. sp. are superficially similar to those of Clinostomatopsis, they differ in a number of taxonomically informative characters. Although similar in body plan and distribution of the gonads toward the posterior extremity of the body, Clinostomatopsis spp. differ in having an intertesticular cirrus-sac and overall body size comparable to the other genera within the family (i.e. < 20 mm) and do not approach the size of either I. dimorphum or I. yamagutii n. sp. (Kanev et al., 2002; Lunaschi & Drago, 2009).
Lent & Freitas (1937) provided measurements from a single adult of I. dimorphum collected from the oesophagus of A. cocoi from Brazil which have been used to newly calculate commonly used ratios in describing clinostomids and other dimensions previously not measured (see Table 1). In their description of metacercariae of an Ithyoclinostomum sp., Briosio-Aguilar et al. (2018) appear to have misinterpreted the length and width metrics of the specimen of I. dimorphum described in Lent & Freitas (1937) by swapping the measurements for length with those of width (see Table 2 of Briosio-Aguilar et al., 2018). Additionally, the ratios calculated by Briosio-Aguilar et al. (2018) are also affected by this error and have been clarified in Table 1 of this study. Regardless of these mishaps, we agree with Briosio-Aguilar et al. (2018) in their assertion that their metacercariae were of a previously undescribed Ithyoclinostomum sp. Metrical data between specimens in this study and those previously reported for I. dimorphum support the erection of a second species, Ithyoclinostomum yamagutii n. sp.
Molecular data
Ithyoclinostomum yamagutii n. sp. shared highest similarity for all sequenced regions with data obtained from the metacercariae of Ithyoclinostomum sp. of Briosio-Aguilar et al. (2018) collected from Cichlasoma urophthalma (Günther), Herichthys deppii (Heckel), and Vieja melanura (Günther) from Mexico and Amphilophus alfari (Meek), Amphilophus longimanus (Günther) and Parachromis managuensis (Günther) from Costa Rica (Table 2). Sequence similarities > 99 % at these regions suggest the Ithyoclinostomum sp. of Briosio-Aguilar et al. (2018) and I. yamagutii n. sp. are conspecific and are well within the limits of other molecular studies on clinostomid trematodes (Sereno-Uribe et al., 2013, 2018; Locke et al., 2015; Pérez-Ponce de León et al., 2016; Rosser et al., 2017, 2018)
Phylogenetic analyses assessing the position of Ithyoclinostomum have been previously performed using both individual single-locus and concatenated alignments (Briosio-Aguilar et al., 2018). Herein, phylogenetic analyses using a concatenated alignment of cox1, ITS regions, and 28S rDNA sequence data, I. yamagutii n. sp. and the metacercariae of Briosio-Aguilar et al. (2018) were within a monophyletic clade basal to Clinostomum and Euclinostomum and with Odhneriotrema as the most basal genus in the family Clinostomidae (Fig. 5). The phylogenetic positioning of the genera with available sequence data is in agreement with the morphological based taxonomy of genera within their subfamilies according to the most recent clinostomid classification scheme (Kanev et al., 2002).
Phylogenetic tree and cladogram of the Clinostomidae. A, Phylogenetic tree constructed from concatenated alignment of cox1 gene, ITS1, 5.8S rRNA gene, ITS2, and 28S rRNA gene sequence data. Numbers above the branches represent Bayesian posterior probabilities and maximum likelihood ultrabootstrap support values based on 1,000 pseudoreplicates (< 0.7/70 are indicated by –). Taxa sequenced in this study are in bold. B, Cladogram constructed based on the current morphological placement of the genera within the subfamilies of the Clinostomidae. Scale-bar: number of nucleotide substitutions per site
Discussion
Ithyoclinostomum is now comprised of two species, the type-species I. dimorphum and the newly described I. yamagutii n. sp. While these two species differ markedly in terms of overall body length, with adult and metacercariae of I. dimorphum capable of reaching > 100 mm in length and those of I. yamagutii n. sp. being approximately 1/5th the size of I. dimorphum, the distribution and shape of their remaining morphological characters suggest them to be congeneric. In both species, there is great distance between the ventral sucker and remaining internal organs, the gonads are located in the posterior fourth of the body, the cirrus-sac and genital pore are pretesticular, and the testes are deeply lobed compared to the other genera in the family. In addition to the qualitative features, dimensions of reproductive organs and eggs can also be used to distinguish the two species. Testes size and shape of I. yamagutii n. sp. metacercariae (Briosio-Aguilar et al., 2018) and adults (this study) are notably conserved in overall morphological shape and their distinctly lobed nature. This is in accordance with metacercariae and adults of other clinostomids (Caffara et al., 2019).
Briosio-Aguilar et al. (2018) speculated that host specificity and geographical restriction also separate these two species. Ithyoclinostomum dimorphum has thus far been reported from only South American localities and as metacercariae parasitising characiform fish (Braun, 1901; Lent & Freitas, 1937; Szidat, 1969; Weiblen & Brandão, 1992; Machado et al., 1996; Gallio et al., 2007; Paraguassú & Luque, 2007; Belei et al., 2013; Benigno et al., 2014; Reis, 2014; Costa et al., 2015; Delgado et al., 2017; Chagas de Souza et al., 2018). In contrast, I. yamagutii n. sp. has been reported in Central and North American localities and in cichlid fish hosts (Briosio-Agular et al., 2018). We tentatively support Briosio-Aguilar et al. (2018) in their identification of the single clinostomid metacercariae from Parachromis managuensis (syn: Cichlasoma managuense) (Günther) in Nicaragua of Aguirre-Macedo et al. (2001) as consistent with their isolates and therefore likely conspecific with I. yamagutii n. sp. However, no molecular data exist to fully confirm this speculation. Similarly, Dias et al. (2003) describe the qualitative ultrastructural morphology of adult clinostomid trematodes collected from A. cocoi from Brazil and reported as I. dimorphum. Morphometric data are not provided but based on the published figures (namely figure 1 and its accompanying scale-bar) these specimens are much shorter than the minimum metric of described I. dimorphum adults (~8.3 mm compared to 60 mm) (Dias et al., 2003). These adults do share similarity to those of I. yamagutii n. sp. in that the worms are stouter and not as elongate as I. dimorphum and also have tegumental papillae present along the body surface just anterior to the ventral sucker (Dias et al., 2003). In the absence of morphological data from stained and mounted specimens or molecular data, the identity of the adult clinostomids of Dias et al. (2003) may represent that of another clinostomid, possibly another genus.
Witenberg (1926) established the genus Ithyoclinostomum for specimens of an adult clinostomid with long, narrow bodies capable of reaching several centimeters in length (up to 100 mm) from the oesophagus of piscivorous birds collected in Brazil. Originally named Distoma dimorphum by Diesing (1850) and later refined into several species by Braun (1899), Baer (1933) and later Skrjabin (1947) considered Ithyoclinostomum to be within the subfamily Clinostominae alongside Clinostomum and Euclinostomum. However, Yamaguti (1958) later erected the subfamily Ithyoclinostominae for the genus. Kanev et al. (2002) agreed that Ithyoclinostominae and Ithyoclinostomum are valid and their key represents the most recent and comprehensive assessment of the Clinostomidae, but without the more recent synonymy of Clinostomoides as a junior synonym of Clinostomum (see Caffara et al., 2019). In all keys to the subfamily and genus, body length is listed as the principle distinguishing feature separating Ithyoclinostomum from other clinostomid taxa (Witenberg, 1926; Lent & Freitas, 1937; Kanev et al., 2002) with only some making mention of the distinct deeply lobate nature of the testes (Lent & Freitas, 1937; Yamaguti, 1958, 1971; Travassos et al., 1969). These features are observed in I. dimorphum and now in I. yamagutii n. sp. and could suggest body length may need to be reserved as a species-level distinguishing feature within the genus rather than at the level of subfamily. Conversely, the distinct deeply lobed testes and pretesticular cirrus-sac, previously considered species-level descriptors of I. dimorphum, should now be elevated to diagnostic features of the genus as a whole. This is consistent with the subfamily diagnosis of Yamaguti (1958, 1971). The present authors reassert Yamaguti’s (1958) original diagnosis of the subfamily Ithyoclinostominae and genus Ithyoclinostomum as valid, but amend these diagnoses to account for the inclusion of I. yamagutii n. sp. as follows:
Subfamily Ithyoclinostominae Yamaguti, 1958
Genus Ithyoclinostomum Witenberg, 1926
Diagnosis
Body slender or stout, attenuated anteriorly, > 20 mm and up to 100 mm long. Oral sucker small, surrounded by anterior collar-like thickening of body. Pharynx present. Oesophagus indistinct or absent. Caeca simple, without lateral branches or diverticula, terminating near posterior extremity of body. Ventral sucker near anterior extremity of body, close to oral sucker. Testes tandem, deeply lobed, near posterior extremity of body. Cirrus-sac small, ovoid, pretesticular or anterodextral relative to anterior testis. Genital pore immediately pretesticular, median or submedian. Ovary ovoid, lobate or smooth, submedian, intertesticular. Uterus ascending anteriorly, but not beyond anterior limit of vitelline follicles. Space between ventral sucker and anterior limit of vitelline field free of internal organs. Parasitic as metacercaria in freshwater fish and as adult in buccal cavity and oesophagus of piscivorous birds. South, Central and North America.
Type-species: I. dimorphum (Diesing, 1850).
Other species: I. yamagutii n. sp.
During the review of the literature for this study, the taxonomic authority of the genus Ithyoclinostomum is attributed variably to the years 1925 or 1926 and is correctly represented herein as Ithyoclinostomum Witenberg, 1926. To clarify the placement of the genus Ithyoclinostomum in relation to the other clinostomid genera, sequence data from the type-species are needed, but remain unavailable. Similarly, molecular data are needed from the genera Clinostomatopsis and Nephrocephalus to support their inclusion in the subfamilies Clinostominae and Nephrocephalinae, respectively. Regardless, based on the current phylogenetic placement it appears that the most basal subfamily in the Clinostomidae is Nephrocephalinae as previously observed (Woodyard et al., 2017; Briosio-Aguilar et al., 2018). This study represents a novel locality as this is first record of a species of Ithyoclinostomum in the USA and the great blue heron represents a novel host record for the genus.
The complete life-cycle of either species of Ithyoclinostomum remains unresolved, but likely follows that of other clinostomids. Clinostomids with known life-cycles utilise primarily freshwater planorbid snails as first intermediate hosts, freshwater fish or amphibians as second intermediate hosts, and predatory birds as definitive hosts (Hunter & Hunter, 1934; Edney, 1950; Jhansilakshmibai & Madhavi, 1997; Rosser et al., 2018). Future efforts will focus on determining if the life-cycle of I. yamagutii n. sp. is capable of being completed in the ictalurid catfish aquaculture ponds in Mississippi, USA, where this species was encountered. These aquatic systems are known to sustain the life-cycles for Clinostomum album Rosser, Alberson, Woodyard, Cunningham, Pote & Griffin, 2017 and Clinostomum marginatum (Rudolphi, 1819), and likely Clinostomum poteae Rosser, Baumgartner, Alberson, Noto, Woodyard, King, Wise & Griffin, 2018 where the marsh rams horn snail Planorbella trivolvis (Say) is a host for C. album and C. marginatum (see Overstreet & Curran, 2004; Rosser et al., 2017, 2018).
Herein we report a fourth clinostomid species and a second clinostomid genus from piscivorous birds collected from commercial catfish operations in Mississippi, USA. The description of I. yamagutii n. sp. documents the second named species within the genus, expands the geographical range of the genus to include the USA, and documents another ardeid bird as a host. The diversity and ecology of the clinostomid trematodes warrant further investigation.
References
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology,215, 403–410.
Aguirre-Macedo, M. L., Scholz, T., González-Solís, D., Vidal-Martínez, V. M., Posel, P., Arjona-Torres, G., et al. (2001). Larval helminths parasitizing freshwater fishes from the Atlantic coast of Nicaragua. Comparative Parasitology,68, 42–51.
Baer, J. G. (1933). Note sur un nouveau trématode, Clinostomum lophophallum sp. nov., avec quelques considerations générales sur la famille des Clinostmoidae. Revue Suisse de Zoologie,40, 317–342.
Belei, F., Ferreira, S. R., Perin, L. M., Braga, F. R., Sampaio, W. M. S., de Araújo, J. V., et al. (2013). First report of Austrodiplostomum compactum and Ithyoclinostomum dimorphum in trahira (Hoplias malabaricus) from the middle course of the Rio Doce, Minas Gerais, Brazil. Arquivos do Instituto de Biologia,80, 249–252.
Bengtsson-Palme, J., Ryberg, M., Hartmann, M., Branco, S., Wang, Z., Godhe, A., et al. (2013). Improved software detection and extraction of ITS1 and ITS2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods in Ecology and Evolution,4, 914–919.
Benigno, R. N. M., Knoff, M., Matos, E. R., Gomes, D. C., Pinto, R. M., & Clemente, S. C. S. (2014). Morphological aspects of Clinostomidae metacercariae (Trematoda: Digenea) in Hoplerytrinus unitaeniatus and Hoplias malabaricus (Pisces: Erythrinidae) of the Neotropical region, Brazil. Anais da Academia Brasileira de Ciências,86, 733–744.
Braun, M. (1899). Üeber Clinostomum Leidy. Zoologischer Anzeiger,22, 484–493.
Braun, M. (1901). Die Arten der Gattung Clinostomum Leidy. Zoologische Jahrbücher. Abteilung für Systematik Ökologie und Geographie der Tiere,14, 1–48.
Briosio-Aguilar, R., García-Varela, M., Hernández-Mena, D. I., Rubio-Godoy, M., & Pérez-Ponce de León, G. (2018). Morphological and molecular characterization of an enigmatic clinostomid trematode (Digenea: Clinostomidae) parasitic as metacercariae in the body cavity of freshwater fishes (Cichlidae) across Middle America. Journal of Helminthology,93, 461–474.
Caffara, M., Locke, S. A., Cristanini, C., Davidovich, N., Markovich, M. P., & Fioravanti, M. L. (2016). A combined morphometric and molecular approach to identifying metacercariae of Euclinostomum heterostomum (Digenea: Clinostomidae). Journal of Parasitology,102, 239–248.
Caffara, M., Locke, S. A., Gustinelli, A., Marcogliese, D. J., & Fioravanti, M. L. (2011). Morphological and molecular differentiation of Clinostomum complanatum and Clinostomum marginatum (Digenea: Clinostomidae) metacercariae and adults. Journal of Parasitology,97, 884–891.
Caffara, M., Locke, S. A., Halajian, A., Luus-Powell, W. J., Benini, D., Tedesco, P., et al. (2019). Molecular data show Clinostomoides Dollfus, 1950 is a junior synonym of Clinostomum Leidy, 1856, with redescription of metacercariae of Clinostomum brieni n. comb. Parasitology,146, 805–813.
Chagas de Souza, D. C., Correa, L. L., & Tavares-Dias, M. (2018). Ithyoclinostomum dimorphum Diesing, 1850 (Digenea, Clinostomidae) in Hoplias malabaricus (Erythrinidae) with the first report of infection of the eyes. Helminthologia,55, 343–349.
Costa, D. P. C., Monteiro, C. M., & Brasil-Sato, M. C. (2015). Digenea of Hoplias intermedius and Hoplias malabaricus (Actinopterygii, Erythrinidae) from upper São Francisco River, Brazil. Revista Brasileira de Parasitología Veterinária,24, 129–135.
Delgado, A. E., Tantalean, M. V., Martínez, R. R., & Mondragón, A. M. (2017). Trematodos en Hoplerythrinus unitaeniatus (Erythrinidae) <<Shuyo>> and Pterodoras granulosus (Doradidae) <<Cahuara>> in Yurimaguas, Loreto, Peru. Revista de Investigación Veterinaria Perú,28, 461–467.
Dias, M. L. G. G., Santos, M. J., Souza, G. T. R., Machado, M. H., & Pavanelli, G. C. (2003). Scanning electron microscopy of Ithyoclinostomum dimorphum (Trematoda: Clinostomidae), a parasite of Ardea cocoi (Aves: Ardeidae). Parasitology Research,90, 355–358.
Diesing, K. M. (1850). Systema helminthum, Volume 1. Vindobonae: Braumüller, 679 pp.
Edney, J. M. (1950). Productivity in Clinostomum marginatum (Trematoda: Clinostomatidae). Transactions of the American Microscopical Society,69, 186–188.
Gallio, M., da Silva, A. S., Soares, J. F., da Silva, M. K., Salomão, E. L., & Monteiro, S. G. (2007). Ocorrência de metacercárias de Ithyoclinostomum dimorphum em traíras no Rio Grande do Sul, Brasil: relato de caso. Estudos de Biología,29, 337–339.
Gustinelli, A., Caffara, M., Florio, D., Otachi, E. O., Wathuta, E. M., & Fioravanti, M. L. (2010). First description of the adult stage of Clinostomum cutaneum Paperna, 1964 (Digenea: Clinostomidae) from grey herons Ardea cinerea L. and a redescription of the metacercaria from the Nile tilapia Oreochromis niloticus niloticus (L.) in Kenya. Systematic Parasitology,76, 39–51.
Hunter, G. W., & Hunter, W. S. (1934). The life cycle of the yellow grub of fish. Journal of Parasitology,20, 325.
Jhansilakshmibai, K., & Madhavi, R. (1997). Euclinostomum heterostomum (Rudolphi, 1809) (Trematoda): Life-cycle, growth and development of the metacercaria and adult. Systematic Parasitology,38, 51–64.
Kanev, I., Radev, V., & Fried, B. (2002). Family Clinostomidae Lühe, 1901. In: Gibson, D. I., Jones, A. & Bray, R. A. (Eds), Keys to the Trematoda, Vol. 1 (pp. 113–120). Wallingford, UK: CAB International.
Katoh, K., Misawa, K., Kuma, K., & Miyata, T. (2002). MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research,30, 3059–3066.
Katoh, K., & Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution,30, 772–780.
Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: Molecular Evolutionary Genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution,33, 1870–1874.
Lent, H., & Freitas, J. F. T. (1937). Pesquisas helminológicas realizadas no Estado do Pará. I. Trematoda. Memórias do Instituto Oswaldo Cruz,32, 449–460.
Locke, S. A., Caffara, M., Marcogliese, D. J., & Fioravanti, M. L. (2015). A large-scale molecular survey of Clinostomum (Digenea, Clinostomidae). Zoologica Scripta,44, 203–217.
Lunaschi, L. I., & Drago, F. B. (2009). Digenean parasites of six species of birds from Formosa Province, Argentina. Revista Mexicana de Biodiversidad,80, 39–46.
Machado, M. H., Pavanelli, G. C., & Takemoto, R. M. (1996). Structure and diversity of endoparasitic infracommunities and the trophic level of Pseudoplatystoma corruscans and Schizodon borelli (Osteichthyes) of the High Paraná River. Memórias do Instituto Oswaldo Cruz,91, 441–448.
Minh, B. Q., Nguyen, M. A. T., & von Haeseler, A. (2013). Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution,30, 1188–1195.
Nei, M., & Kumar, S. (2000). Molecular evolution and phylogenetics. New York: Oxford University Press.
Nguyen, L., Schmidt, H. A., von Haesler, A., & Minh, B. Q. (2015). IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Molecular Biology and Evolution,32, 268–274.
Overstreet, R. M., & Curran, S. S. (2004). Defeating diplostomoid dangers in USA catfish aquaculture. Folia Parasitologica,51, 153–165.
Pérez-Ponce de León, G., García-Varela, M., Pinacho-Pinacho, C. D., Sereno-Uribe, A., & Poulin, R. (2016). Species delimitation in trematodes using DNA sequences: Middle-American Clinostomum as a case study. Parasitology,143, 1773–1789.
Pinto, R. M., Barros, L. A., Tortelly, L., Teixeira, R. F., & Gomes, D. C. (2004). Prevalence and pathology of helminths of ciconiiform birds from the Brazilian swamplands. Journal of Helminthology, 78, 259–264.
Pritchard, M. H., & Kruse, G. O. W. (1982). The collection and preservation of animal parasites. Lincoln: University of Nebraska Press, 141 pp.
Rambaut, A. (2014). FigTree: Tree figure drawing tool v. 1.4.2. Institute of Evolutionary Biology, University of Edinburgh. Retrieved from http://tree.bio.ed.ac.uk/.
Reis, T. S. (2014). Caracterização morfológica e molecular de endoparasitoss de Hoplias affinis malabaricus Bloch, 1794 (Characiformes: Erythrinidae) provenientes do Rio Araguaia, Tocantins, Brasil. PhD dissertation, Universidade Feral do Tocantins, Brazil.
Ronquist, F., & Huelsenbeck, J. P. (2003). MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics,19, 1571–1574.
Rosser, T. G., Alberson, N. R., Woodyard, E. T., Cunningham, F. L., Pote, L. M., & Griffin, M. J. (2017). Clinostomum album n. sp. and Clinostomum marginatum (Rudolphi, 1819), parasites of the great egret Ardea alba L. from Mississippi, USA. Systematic Parasitology,94, 35–49.
Rosser, T. G., Baumgartner, W. A., Alberson, N. R., Noto, T. W., Woodyard, E. T., King, D. T., et al. (2018). Clinostomum poteae n. sp. (Digenea: Clinostomidae), in the trachea of a double-crested cormorant Phalacrocorax auritus Lesson, 1831 and molecular data linking the life-cycle stages of Clinostomum album Rosser, Alberson, Woodyard, Cunningham, Pote & Griffin, 2017 in Mississippi, USA. Systematic Parasitology,95, 543–566.
Sereno-Uribe, A. L., García-Varela, M., Pinacho-Pinacho, C. D., & Pérez-Ponce de León, G. (2018). Three new species of Clinostomum Leidy, 1856 (Trematoda) from Middle American fish-eating birds. Parasitology Research,117, 2171–2185.
Sereno-Uribe, A. L., Pinacho-Pinacho, C. D., García-Varela, M., & Pérez-Ponce de León, G. (2013). Using mitochondrial and ribosomal DNA sequences to test the taxonomic validity of Clinostomum complanatum Rudolphi, 1814 in fish-eating birds and freshwater fishes in Mexico, with the description of a new species. Parasitology Research,112, 2855–2870.
Skrjabin, K. I. (1947) Family Clinostomatidae Lühe, 1901. In Trematodes of animals and man: Basic trematodology, Volume 1, 1st edn. Moscow: Academy of Sciences of the USSR, pp. 64–97.
Szidat, L. (1969). Structure, development, and behavior of new strigeatoid metacercariae from subtropical fishes of South America. Journal of the Fisheries Research Board of Canada,26, 753–786.
Travassos, L., Freitas, J. T., & Kohn, A. (1969). Trematódeos do Brasil. Memórias do Instituto Oswaldo Cruz,67, 1–886.
Trifinopoulos, J., Nguyen, L. T., von Haesler, A., & Minh, B. Q. (2016). W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research,44(W1), W232–W235.
Weiblen, A. M., & Brandão, D. A. (1992). Levantamento parasitológico em Hoplias malabaricus Bloch (1794) (traíra) de águas da região de Santa Maria-RS. Ciência Rural,22, 203–208.
Witenberg, G. G. (1926). Versuch einer Monographie der Trematodenunterfamilie Harmostominae Braun. Zoologische Jahrbücher. Abteilung für Systematic, Ökologie und Geographie der Tiere,51, 167–254.
Woodyard, E. T., Rosser, T. G., & Rush, S. A. (2017). Alligator wrestling: Morphological, molecular, and phylogenetic data on Odhneriotrema incommodum (Leidy, 1856) (Digenea: Clinostomidae) from Alligator mississippiensis Daudin, 1801 in Mississippi, USA. Parasitology Research,116, 2981–2993.
Yamaguti, S. (1958). Systema helminthum. Volume I. The digenetic trematodes of vertebrates. New York: Interscience, 1575 pp.
Yamaguti, S. (1971). Synopsis of digenetic trematodes of vertebrates. Volume 1. Tokyo, Japan: Keigaku Publishing Co, 1074 pp.
Acknowledgements
We would like to thank Stephen Clements, Katie Hanson-Dorr, Lanna Durst, and Raleigh Middleton for their assistance in collecting herons for this study.
Funding
This work was supported by the Mississippi State University College of Veterinary Medicine, Office of Research and Graduate Studies Internal Grants Programme and United States Department of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed (IACUC QA 2853).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as CBE79808-8564-42D6-97D1-272A37ACD63D. This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number. This is the version of record.
This article is part of the Topical Collection Digenea.
Rights and permissions
About this article
Cite this article
Rosser, T.G., Woodyard, E.T., Mychajlonka, M.N. et al. Ithyoclinostomum yamagutii n. sp. (Digenea: Clinostomidae) in the great blue heron Ardea herodias L. (Aves: Ardeidae) from Mississippi, USA. Syst Parasitol 97, 69–82 (2020). https://doi.org/10.1007/s11230-019-09892-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-019-09892-6