Abstract
Corynosoma obtuscens Lincicome, 1943 (Acanthocephala: Polymorphidae) is synonymised with Corynosoma australe Johnston, 1937 based on combined morphological and molecular evidence. Morphological comparison of C. obtuscens (24 males and 27 females) collected from a California sea lion Zalophus californianus (Lesson) in California, USA, with the type-specimens of C. obtuscens and C. australe, and with published data on C. australe collected from different hosts and regions showed no significant differences. The levels of genetic divergence in the cox1 sequences obtained from C. obtuscens from a California sea lion in the present study and C. australe from otariid seals from Argentina and penguins from Brazil ranged between 1.4–1.6% and was considered to represent intraspecific variability. Additionally, cox1 sequences were generated for Andracantha phalacrocoracis (Yamaguti, 1939), Corynosoma semerme (Forssell, 1904), C. strumosum (Rudolphi, 1802), C. validum Van Cleave, 1953 and C. villosum Van Cleave, 1953. Our results revealed inconsistency in the identification of material used as a source of the previously published sequence data for C. obtuscens and C. magdaleni Montreuil, 1958.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morphological similarity among species belonging to the acanthocephalan genus Corynosoma Lühe, 1904 have been documented by several researchers (Johnston & Edmonds, 1953; Zdzitowiecki, 1984; Stryukov, 2004; Sardella et al., 2005). The similarity between Corynosoma australe Johnston, 1937 and Corynosoma obtuscens Lincicome, 1943 was first noted by Johnston & Edmonds (1953) in their survey of acanthocephalans of marine mammals from sub-Antarctic Auckland and Campbell Islands. Later, Zdzitowiecki (1984) also noted the close resemblance between these two species based on specimens of C. australe from the leopard seal Hydrurga leptonyx (Blainville) from the South Shetland Islands, Antarctica. The only character used to distinguish between these two species was the location of spines on the ventral surface of the body in females. The ventral surface of the body in females of C. obtuscens is completely covered with spines from the anterior part to the genital opening, whereas females of C. australe are characterised by the presence of a small spine-free zone between the somatic and genital spines on the ventral surface of the body. According to the original descriptions and subsequent re-descriptions of these two species, no other morphological differences were identified (Johnston, 1937; Lincicome, 1943; Johnston & Edmonds, 1953; Van Cleave, 1953a, b; Smales, 1986; Hernández-Orts et al., 2017b).
Corynosoma obtuscens was described from a California sea lion Zalophus californianus (Lesson) in California (Lincicome, 1943), and C. australe was described from an Australian sea lion Neophoca cinerea (Péron) in South Australia (Johnston, 1937). Both species usually parasitise marine mammals (Johnston, 1937; Lincicome, 1943; Johnston & Edmonds, 1953; Van Cleave, 1953a, b; Smales, 1986; Sardella et al., 2005; Aznar et al., 2012; Hernández-Orts et al., 2017b) and rarely, terrestrial mammals (Cabrera et al., 1990; Castro et al., 2004; Tantaleán et al., 2007) and birds (Hoberg & Ryan, 1989; Hernández-Orts et al., 2017b). They supposedly use amphipod crustaceans as intermediate hosts and various species of marine fishes as paratenic hosts (Brownell, 1975; Tantaleán & Huiza, 1994; Andrade et al., 1997; Aznar et al., 2004, 2006; Tantaleán et al., 2005; Chero et al., 2014). Corynosoma australe is more broadly distributed than C. obtuscens and was reported throughout much of the southern hemisphere from South Australia, Antarctica, South Africa to South America while the latter was found only along the Pacific coasts of North and South America (Van Cleave, 1953b; Zdzitowiecki, 1984; Ionita et al., 2008; Aznar et al., 2012; Fonseca, 2016; Hernandez-Orts et al., 2017b; Lisitsyna et al., 2018).
Difficulties in differentiation between C. australe and C. obtuscens based on morphological characters alone questioned their status as independent species and warranted an in-depth analysis using an additional source of characters. In this study we used previously reported material collected from California sea lions on the California Pacific coast and identified as C. obtuscens (see Lisitsyna et al., 2018) to determine the taxonomic status of this species using DNA sequences. Additionally, we compared our material with the type-specimens of C. obtuscens from the Smithsonian National Museum of Natural History and the original description by Lincicome (1943) as well as with the type-specimens of C. australe from the South Australian Museum and original and recently published detailed descriptions (Johnston, 1937; Zdzitowiecki, 1984; Smales, 1986; Sardella et al., 2005; Hernández-Orts et al., 2017b). We used newly obtained and previously published cox1 sequences of nine species of the genus Corynosoma to reconstruct their phylogenetic relationships.
Materials and methods
Morphological examination
Morphological variability of C. obtuscens was studied using material collected from an approximately two-year-old female of Z. californianus necropsied at the Marine Mammal Center (TMMC), Sausalito, California, USA in 2015 (see Lisitsyna et al., 2018 for details). The sample contained 1,201 specimens of acanthocephalans (542 males and 659 females). Arrangement of the spines on the ventral surface was examined in 659 specimens of females. Morphology of acanthocephalans was studied on temporary total mounts cleared in Berlese’s medium and on specimens stained with iron acetocarmine permanently mounted in Canada balsam. Photomicrographs were made using the Zeiss Axio Imager M1 compound microscope equipped with DIC optics and a digital imaging system.
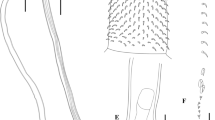
Thirty characters were measured to analyse morphological variability of C. obtuscens. Measurements for each specimen were taken from the digital images using Zeiss Axio Vision4 camera analysis software. Measurements of trunk spines were taken as shown in Fig. 1. All measurements are in micrometres unless otherwise stated. Trunk length does not include proboscis, neck or bursa. Minimum (min), maximum (max) and mean values, standard deviation (SD) and coefficient of variation (CV) were calculated for each character.
Diagram of measurements of trunk spines for specimens of C. obtuscens Lincicome, 1943. A, Male; B, Female. Abbreviations: ads, anterior dorsal spines; mds, middle dorsal spines; pds, posterior dorsal spines; avs, anterior ventral spines; mvs, middle ventral spines; pvs, posterior ventral spines; gs, genital spines
Morphological analysis included comparison of the present specimens with the original description for C. obtuscens by Lincicome (1943) and C. australe by Johnston (1937) as well as with re-descriptions of the latter species supplemented with molecular data (Zdzitowiecki, 1984; Smales, 1986; Sardella et al. 2005; Hernández-Orts et al., 2017a, b).
The type-specimens of C. obtuscens from the US National Parasite Collection of the Smithsonian National Museum of Natural History: holotype USNM1395376, allotype USNM1387619 and paratypes USNM1337543 (2 males and 4 females) were examined. The type-specimens of C. australe held in the Australian Helminthological Collection, South Australian Museum: holotype and allotype AHC 41273 and paratypes AHC 42227, 42228, 42229 (4 males and 2 females) were also examined.
The specimens used for molecular study (Table 1) were cut frontally; the dorsal part of the worm was used for DNA extraction; the ventral part, including the proboscis, was left as a molecular voucher (hologenophore sensu Pleijel et al., 2008) for morphological examination on temporary mounts. Therefore, only twelve morphological characters were measured from each hologenophore. Specimens of Andracantha phalacrocoracis (Yamaguti, 1939) and C. strumosum (Rudolphi, 1802) from Z. californianus and C. semerme (Forssell, 1904), C. validum Van Cleave, 1953 and C. villosum Van Cleave, 1953 from northern fur seals Callorhinus ursinus (L.) used for molecular studies were selected from the Parasitological collection of the Department of Parasitology, I. I. Schmalhausen Institute of Zoology NAS of Ukraine deposited by Kuzmina et al. (2012) and Lisitsyna et al. (2018). Hologenophores (PCIZ-A-1/1–9) were deposited in the Parasitological collection of the Department of Parasitology, I. I. Schmalhausen Institute of Zoology NAS of Ukraine after morphological examination. Forty specimens of C. obtuscens (20 males and 20 females) collected from Z. californianus were deposited in the collection of the Harold W. Manter Laboratory of Parasitology at the University of Nebraska, Lincoln, Nebraska, USA (accession number P-2018-044; HWML 110491).
DNA sequencing and phylogenetic analysis
Total genomic DNA was isolated from the dorsal part of ethanol-fixed specimens using the Kapa Express Extract kit (Kapa Biosystems, Cape Town, South Africa) (Table 1). A 623 nucleotide (nt) long fragment of the mitochondrial cytochrome c oxidase 1 (cox1) gene was amplified using the forward primer #507 (5′-AGT TCT AAT CAT AAR GAT ATY GG-3′) (Nadler et al., 2006) and the reverse primer HC02198 (5′-TAA ACT TCA GGG TGA CCA AAA AAT CA-3′) (Folmer et al., 1994) using annealing temperature of 40°C. In addition, we amplified and sequenced a 1,620 nt long fragment of the 18S rRNA gene for C. obtuscens (GenBank: MK119255). The forward primer (5′-AGA TTA AGC CAT GCA TGC GTA AG-3′) and the reverse primer (5′-TGA TCC TTC TGC AGG TTC ACC TAC-3′) published by Garey et al. (1996) were used for 18S rDNA amplification with annealing temperature of 60°C. The PCR products were sent to Inqaba Biotechnical Industries (Pty) Ltd. (Pretoria, South Africa) for purification and sequencing. The PCR primers were used for sequencing. Contiguous sequences were assembled and edited using Geneious ver. 9.1 (Biomatters, Auckland, New Zealand).
Newly-generated sequences were used to perform a basic local alignment search tool (BLAST) analysis against the sequences deposited in the GenBank database to compare and reveal closely related sequences. The novel cox1 sequences of Corynosoma spp. were aligned with sequences of Corynosoma spp. from GenBank with reference to the amino acid translation using the invertebrate mitochondrial code (translation table 5) (Telford et al., 2000) with MUSCLE implemented in Mega v.6 (Tamura et al., 2013). A newly obtained cox1 sequence of A. phalacrocoracis was included as the outgroup. Phylogenetic relationships were assessed via Bayesian inference (BI) and maximum likelihood (ML) analyses. Prior to BI and ML analyses, the best-fitting model, HKY+I+G was estimated with jModelTest 2.1.2 (Darriba et al., 2012). BI was performed using MrBayes software (ver. 3.2.3) (Ronquist et al., 2012) run on the CIPRES portal (Miller et al., 2010). Markov chain Monte Carlo (MCMC) chains were run for 3,000,000 generations, log-likelihood scores were plotted and only the final 75% of trees were used to produce the consensus trees by setting the ‘burn in’ parameter at 750. The results were visualised in Tracer ver. 1.6 (Rambaut et al., 2014) to assess convergence and proper sampling and to identify the ‘burn-in’ period. ML analysis was performed using PhyML version 3.0 (Guindon et al., 2010) with a non-parametric bootstrap validation based on 100 replicates. Trees were visualised using the FigTree ver. 1.4 software (Rambaut et al., 2012). Genetic distance matrices (uncorrected p-distance) were calculated in MEGA ver. 6.
Results
Morphological variability of C. obtuscenscollected fromZ. californianus
Morphological variability of 30 characters of C. obtuscens was studied for males (n = 24) and females (n = 27) (Table 2). The following characters were most variable (CV > 10%) in both sexes: length of dorsal spines zone, length of somatic spines, number of spiniform hooks on the proboscis, length of the neck and lemnisci. The width of the testes and Sáfftigen’s pouch in males demonstrated a high level of variability due to the physiological condition of the worms. The following characters were least variable (CV < 6%): the number of the longitudinal rows of hooks on the proboscis and the number of hooks in a row, the length of the trunk in males, and the number of the rooted hooks on the proboscis and egg length in females (Table 2).
Morphological and morphometric comparison of C. obtuscensandC. australe
Morphological and morphometric data for C. obtuscens in our material was compared (Tables 3, 4) to the original descriptions of C. obtuscens and C. australe (see Johnston, 1937; Lincicome, 1943) and subsequent morphological descriptions of C. australe (see Zdzitowiecki, 1984; Smales, 1986; Sardella et al. 2005). Our specimens had only insignificant differences compared to the descriptions of both species mentioned above. These minor differences were observed in the width of the proboscis in both sexes [males: 182–265 µm in the present specimens vs 144–180 µm in C. obtuscens according to Lincicome (1943), 100–200 µm in C. australe according to Smales (1986), 160–203 µm in C. australe according to Zdzitowiecki (1984); females: 202–297 vs 180–204 vs 170–230 vs 187–242 µm, respectively], the number of hooks per row [11–14 in the present specimens vs 12–13 in C. obtuscens according to Lincicome (1943), 13–14 in C. australe according to Johnston (1937), 12–15 in C. australe according to Smales (1986), 11–15 in C. australe according to Zdzitowiecki (1984), 12–14 in C. australe according to Sardella et al. (2005)] and the number of spiniform hooks [2–4 in the present specimens vs 3–4 in C. australe according to Johnston (1937)]. The greatest differences were found in the size of the eggs. In our material, eggs of C. obtuscens were substantially larger (85–108 × 30–39 µm) than indicated in the original description of this species (68–90 × 20–28 µm) by Lincicome (1943) and larger than the eggs of C. australe [75–85 × 23–29 µm according to Johnston (1937); 84–96 × 28–36 µm in Smales (1986) and 66–82 × 23–32 µm in Zdzitowiecki (1984)], but smaller than the eggs of C. australe [92–115 × 27–42 µm in Sardella et al. (2005)].
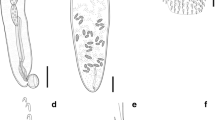
Arrangement of the spines on the ventral surface of the body in females, the character considered as the most reliable for the differentiation between these two species, was found to be unstable. A small spine-free zone on the ventral surface of the body of female between somatic and genital spines was observed in the allotype of C. obtuscens (USNM1387619) (Fig. 2A). However, this was not observed in 5 female paratypes (Fig. 2B–F). This spine-free zone between the somatic and genital spines was observed only in 3 out of 659 females in our material. A short (540 µm) spine free zone was observed in the allotype female of C. australe as well as in the 2 paratypes (300 and 330 µm). A small spine free zone was figured but not commented on by Smales (1986) (see figure 11 in Smales, 1986) for C. australe.
Photomicrographs of the type-specimens of females of C. obtuscens Lincicome, 1943 ex Zalophus californianus (Lesson), posterior end of body, ventral view. A, Allotype, USNM 1387619; B, Paratype, USNM 1337543 #4; C, Paratype, USNM 1337543 #7; D, Paratype, USNM 1337543 #8; E, Paratype, USNM 1337543 #12; F, Paratype, USNM 1337543 #13. Spine-free zone indicated with an arrow. Scale-bars: 100 μm
Molecular analyses
DNA was successfully amplified from specimens representing six acanthocephalan species (Table 1). Morphometric data for the hologenophores of Corynosoma spp. and A. phalacrocoracis are provided in the Supplementary Table S1. Illustrations for the voucher specimens of Corynosoma spp. are presented in the Supplementary Figure S1 and A. phalacrocoracis in figures 1D, 2D, 2K in Lisitsyna et al. (2018). Newly generated partial cox1 sequences for species of the genus Corynosoma were aligned together with 16 sequences of Corynosoma spp. from GenBank for phylogenetic analyses (Table 1). The final alignment was 573 nt long.
BI and ML phylogenetic analyses produced a tree topology (Fig. 3) consistent with that in Hernández-Orts et al. (2017a, b). The five newly-generated sequences of C. obtuscens were identical. They formed a strongly supported clade with the previously published sequences of C. australe. The genetic divergence (uncorrected p-distance) between the sequences of C. obtuscens and sequences of C. australe ranged between 1.1–1.6% (6–9 nt difference). The 18S rDNA sequence divergence between C. obtuscens in the present study and C. obtuscens from GenBank (JX442169) was 0.4% (7 nt).
Phylogenetic relationships inferred from Bayesian inference (BI) and maximum likelihood (ML) analyses for species of Corynosoma based on cox1 sequence data. Nodal support from the three analyses is indicated as BI/ML; values < 0.95 (BI) and < 70 (ML) are not shown. The newly generated sequences are indicated by bold typeface. The scale-bar indicates the number of substitutions per site
Our sequence of C. strumosum, appeared in a strongly supported clade with sequences of C. strumosum from Phoca vitulina L. and Pusa hispida spp. saimensis (Gmelin), sequences of Corynosoma magdaleni Montreuil, 1958 from Pusa hispida saimensis (Nordquist) published by García-Varela & Pérez-Ponce de León (2008) and from Phoca vitulina L. published by Waindok et al. (2018) and sequence of “Candidatus Corynosoma nortmeri sp. nov.” sensu Waindok et al. (2018). The genetic divergence between our sequence of C. strumosum and sequences of C. strumosum retrieved from GenBank (EF467870, EF467871) ranged between 3.2–3.4% (18–19 nt). Our sequence differed from the sequence of C. magdaleni (EF467872) by 2.6% (15 nt) and sequence of C. magdaleni (MF078642) by 5.8% (33 nt). Difference between sequences of C. magdaleni and “Candidatus Corynosoma nortmeri sp. nov.” published Waindok et al. (2018) was 0.2% (1 nt).
Novel cox1 sequence of C. semerme clustered with the sequences of C. semerme (GenBank: MF00127) published by Waindok et al. (2018) and C. obtuscens (GenBank: JX442192) published by García-Varela et al. (2013) (Fig. 3). Both sequences of C. semerme were identical. The genetic divergence between these isolates and isolate of C. obtuscens was 1.1% (6 nt). Sequence of C. validum obtained in the present study and a previously published sequence of this species clustered with C. villosum in a strongly supported clade. The intraspecific divergence among sequences of C. validum was 0.9% (5 nt).
Interspecific divergence observed within cox1 dataset for the genus Corynosoma ranged between 5.3–17.1% (33–97 nt) with C. validum and C. villosum exhibiting the lowest interspecific divergence, whereas C. validum and Corynosoma enhydri Morozov, 1940 showed the highest sequence divergence.
Discussion
Difficulties in species delineation within the genus Corynosoma have been a lingering problem for a long time (Zdzitowiecki, 1984; Stryukov, 2004; Sardella et al., 2005; Hernández-Orts et al., 2017a, b). It was noted, for instance, that the morphologically similar species have heteropolar geographical distribution (Stryukov, 2004). For example, Corynosoma pseudohamanni Zdzitowiecki, 1984 widely distributed in the Antarctic differs from morphologically similar species Corynosoma erignathi Stryukov, 2000 distributed in the Arctic, in body size, the arrangement of the somatic spines and the number of large hooks.
Our study showed that the characters commonly used to distinguish among species of Corynosoma such as the diameter of the foretrunk disk, the length of the dorsal spined zone, the length of the neck and lemnisci, the width of the testes and Sáfftigen’s pouch, are variable (Table 2) and cannot be used for species differentiation. Our attempt to find additional characters, e. g. the length of anterior, median and posterior somatic spines ventrally and dorsally, did not provide satisfactory results because these characters also proved to be variable.
One of the relatively stable characters in our study was the size of eggs with the coefficient of variation being 4.5–6.6%. The eggs of C. obtuscens in our study were larger than the eggs of C. obtuscens reported by Lincicome (1943) and C. australe reported by Johnston (1937), Smales (1986) and Zdzitowiecki (1984), but smaller than the eggs of C. australe reported by Sardella et al. (2005). Similar results were shown for other species of Corynosoma (see Popov & Fortunato, 1987; Amin et al., 2011). Popov & Fortunato (1987) showed that the egg size of C. strumosum from a ringed seal Pusa hispida (Schreber) from the Barents, Bering and East Siberian Seas, and from the same host from the Sea of Okhotsk was one of the most variable population level characters. Amin et al. (2011) also reported substantial variation of the egg size in specimens of C. strumosum from different population of the Caspian seal Pusa caspica (Gmelin).
The variation in the arrangement of the somatic spines in different species of Corynosoma was documented previously (Stryukov, 2004; Aznar et al., 2016). It was found that one of the main diagnostic characters of C. erignathi, namely the ventral surface entirely covered with spines in males, was present only in 75% of males; the rest of males possessed a spine-free zone between the somatic and genital spines (Stryukov, 2004). Interestingly, in Corynosoma cetaceum Johnston & Best, 1942 females showed a higher variability in the spination of the ventral surface than males (Aznar et al., 2016). These authors did not observe similar variation in other species of Corynosoma, including C. australe. Zdzitowiecki (1984) reported the ventral surface of the body completely covered with spines in 25% individuals of C. australe in his material collected from the leopard seal Hydrurga leptonyx (Blainville) in South Shetlands. Later, Sardella et al. (2005) described the presence of ventral somatic armature that covered 89.2 (84.6–100)% of trunk length in adults and 87.4 (85–89)% in cystacanths of C. australe from the South American fur seal Arctocephalus australis (Zimmerman) and the stripped weakfish Cynoscion guatucupa (Cuvier), respectively. Thus, these authors clearly pointed out the variability of this character in C. australe.
Our results confirm the variability in the arrangement of the somatic spines on the ventral surface of the body in females of C. obtuscens. The spine-free zone between the somatic and genital spines was rarely seen in females in our material. This zone was observed in less than 1% of examined specimens. Thus, due to the variability of this character, it cannot be used for differentiation between C. obtuscens and C. australe. Therefore, these two nominal species lack reliable morphological differences.
Comparison of cox1 sequence data for C. obtuscens obtained in the present study with the previously published sequences of C. australe demonstrated a low genetic divergence (1.1–1.6%) that corresponds to intraspecific level. Therefore, based on the combined molecular and morphological evidence, we consider C. obtuscens a junior synonym of C. australe.
Since our molecular and morphological data confirmed the identity of C. australe and C. obtuscens, the information on hosts and distribution previously reported for the species under these two names needs to be combined. Corynosoma australe appears to be the only species of the genus Corynosoma parasitising pinnipeds in both hemispheres. It was originally described by Johnston (1937) from Neophoca cinerea in Australia and subsequently reported under this name from New Zealand, South Shetland Islands in Antarctica, South and South-West Africa, southern coast of Australia, Argentinian Patagonia and across the Atlantic coast of South America (Johnston, 1937; Johnston & Edmonds, 1953; Morini & Boero, 1960; Delyamure & Parukhin, 1968; Obendorf & Presidente, 1978; Shaughnessy & Ross, 1980; Smales, 1986; Zdzitowiecki, 1986; George-Nascimento & Marín 1992; Stewardson & Fourie, 1998; Aznar et al., 2004; Sardella et al., 2005; Morgades et al., 2006; Aznar et al., 2012; Hernández-Orts et al., 2017b).
The same species was reported under the name C. obtuscens from California, USA and along the both Atlantic and Pacific coasts of South America (Lincicome, 1943; Van Cleve, 1953a, b; Cabrera et al., 1999; Tantaleán et al., 2007; Lisitsyna et al., 2018). Mature ovigerous adult stages of this acanthocephalan species were found in nine species of pinnipeds, penguins, the Andean fox Lycalopex culpaeus Molina and domestic dogs (both natural and experimental infections) thus demonstrating a broad host specificity, although pinnipeds should be considered as the main definitive hosts. Corynosoma australe was also reported from four species of cetaceans and four species of pinnipeds in Argentinean waters (including the Patagonian coast) and southern Brazil (Andrade et al., 1997; Dans et al., 1999; Berón-Vera et al., 2008; Hernández-Orts et al., 2015) as well as from a great shearwater Ardenna gravis (O’Reilly) (Procellariiformes) in Gough Island, Southern Atlantic (Hoberg & Ryan, 1989); however, sexually mature individuals have not been found in these hosts.
Aznar et al. (2012) examined fecal samples from the South American sea lion, Otaria flavescens (Shaw) (Pinnipedia) and franciscanas, Pontoporia blainvillei (Cetacea) from Buenos Aires Province in Argentina and found mature eggs of C. australe only in pinnipeds. It remains unknown why this acanthocephalan species can mature in so unrelated hosts as carnivores and penguins, but not other mammals (cetaceans) or other fish-eating birds.
The relationships among the species of the genus Corynosoma have been studied extensively using DNA sequences, particularly the cox1 gene (García-Varela & Nadler, 2006; García-Varela & Pérez-Ponce de León, 2008; García-Varela et al., 2013; Hernández-Orts et al., 2017a, b). The comparative analysis of sequence data for Corynosoma spp. obtained in this study and data available on GenBank raised a number of taxonomic questions and provided answers to some of these questions.
As evidenced by the results of phylogenetic analyses and distance-based identification, specimen identified as C. obtuscens in the study of Garcia-Varela et al. (2013) clustered with C. semerme obtained in our study in a strongly supported clade (Fig. 3). Morphological comparison of the isolates was not possible due to the lack of morphological characterisation of the specimens in the publication of Garcia-Varela et al. (2013). It should be noted that these species are morphologically similar and can easily be misidentified without a detailed morphological examination. Corynosoma semerme differs from C. obtuscens by the larger number of the longitudinal rows of hooks on the proboscis [22–24 according to Van Cleave (1953b) vs 17–19], the somewhat lower number of the largest hooks in each row [7–9 according to Van Cleave (1953b) vs 8–11], and the length of the blade in the largest hook [up to 67 µm according to Van Cleave (1953b) vs 38–57 µm]. It is clear that the cox1 sequence (JX442192) as well as 18S rDNA sequence (JX442169) of C. obtuscens published by García-Varela et al. (2013) represent in fact C. semerme. A similar error was noted by Hernández-Orts et al. (2017a, b) for the sequence of Corynosoma hannae Zdzitowiecki, 1984 misidentified as C. australe (JX442191; García-Varela et al., 2013).
The sequence of C. strumosum in the present analyses appeared in a strongly supported clade together with the sequences of this species retrieved from the GenBank database (Fig. 3). Besides, the sequence of C. magdaleni (EF467872) was nested among three sequences of C. strumosum (Fig. 3). Genetic divergence between sequences of C. strumosum and C. magdaleni (EF467872) was only 2.6–3.4% (15–18 nt) which corresponds to the intraspecific divergence (see Hernández-Orts et al., 2017a) and suggested that the sequence published under the name C. magdaleni by García-Varela & Pérez-Ponce de León (2008) represents C. strumosum. The two sequences of C. magdaleni and “Candidatus Corynosoma nortmeri sp. nov.” published by Waindok et al. (2018) differ by 0.2% (1 nt), thus confirming their conspecificity.
In this study, morphological methods combined with DNA sequencing have once again proved to be an extremely precise and efficient approach to the taxonomy of acanthocephalans.
References
Amin, O. M., Heckmann, R. A., Halajian, A., & El-Naggar, A. M. (2011). The morphology of an unique population of Corynosoma strumosum (Acanthocephala, Polymorphidae) from the Caspian seal, Pusa caspica, in the land-locked Caspian Sea using SEM, with special notes on histopathology. Acta Parasitologica, 56, 438–445.
Andrade, A., Pinedo, M. C., & Pereira, J., Jr. (1997). Gastrointestinal helminths of the franciscana, Pontoporia blainvillei, in southern Brazil. Reports of the International Whaling Commission, 47, 669–673.
Aznar, F. J., Cappozzo, H. L., Taddeo, D., Montero, F. E., & Raga, J. A. (2004). Recruitment, population structure, and habitat selection of Corynosoma australe (Acanthocephala) in South American fur seals, Artocephalus australis, from Uruguay. Canadian Journal of Zoology, 82, 726–733.
Aznar, F. J., Crespo, E. A., Raga, J. A., & Hernández-Orts, J. S. (2016). Trunk spines in cystacanths and adults of Corynosoma spp. (Acanthocephala): Corynosoma cetaceum as an exceptional case of phenotypic variability. Zoomorphology, 135, 19–31.
Aznar, F. J., Pérez-Ponce de León, G., & Raga, J. A. (2006). Status of Corynosoma (Acanthocephala: Polymorphidae) based on anatomical, ecological, and phylogenetic evidence, with the erection of Pseudocorynosoma n. gen. Journal of Parasitology, 92, 548–664.
Aznar, F. J., Hernández-Orts, J., Suárez, A. A., García-Varela, M., Raga, J. A., & Cappozzo, H. L. (2012). Assessing host-parasite specificity through coprological analysis: a case study with species of Corynosoma (Acanthocephala: Polymorphidae) from marine mammals. Journal of Helminthology, 86, 156–164.
Berón-Vera, B., Crespo, E. A., Raga, J. A., & Fernández, M. (2007). Parasites of common dolphins (Delphinus delphis) from northern Patagonia, Argentina: the relation with host distribution and diet and comparison with sympatric hosts. Journal of Parasitology, 93, 1155–1159.
Brownell, R. L., Jr. (1975). Progress report on the biology of the franciscana dolphin, Pontoporia blainvillei, in Uruguayan waters. Journal of the Fisheries Research Board of Canada, 32, 1073–1078.
Cabrera, R., Rojas, R., & Davalos, M. (1999). Corynosoma obtuscens Lincicome, 1943 (Acanthocephala: Polymorphidae) in Canis familiaris de la Ciudad de Chincha, Peru. Parasitología al Día, 23, 59–62.
Castro, M., & Martínez, R. (2004). Proceso del desarrollo de Corynosoma obtuscens (Acanthocephala: Polymorphidae) en Canis familiaris y su posible implicancia en salud publica. Parasitología Latinoamericana, 59, 26–30.
Chero, J., Iannacone, J., Cruces, C., Sáez, G., & Alvariño, L. (2014). Community of metazoan parasites of corvine drum Cilus gilberti (Abbott, 1899) (Perciformes: Sciaenidae) in the coastal zone of Chorrillos, Lima, Perú. Neotropical Helminthology, 8, 163–182.
Dans, S. L., Reyes, L. M., Pedraza, S. N., Raga, J. A., & Crespo, E. A. (1999). Gastrointestinal helminths of the dusky dolphin, Lagenorhynchus obscurus, off Patagonian coasts, in the Southwestern Atlantic Ocean. Marine Mammal Science, 15, 649–660.
Darriba, D., Taboada, G. L., Doallo, R., & Posada, D. (2012). jModelTest 2: More models, new heuristics and parallel computing. Nature Methods, 9, 772.
Delyamure, S. L., & Parukhin, A. M. (1968). A new parasite of the South African fur seal. Biologiya Morya, 14, 25–34 (in Russian).
Folmer, O., Black, M., Hoeh, W., Lutz, R., & Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3, 294–299.
Fonseca, M. C. G. (2016). [Taxonomia integrativa e aspecto higiêbico-sanitário de helminthos parasitos de peixes teleósteos marinhos do Estado do Rio de Janeiro, Brasil.] PhD Thesis, Niterói: Universidade Federal Fluminense, 177 pp (In Portuguese).
García-Varela, M., & Nadler, S. A. (2006). Phylogenetic relationships among Syndermata inferred from nuclear and mitochondrial gene sequences. Molecular Phylogenetics and Evolution, 40, 61–72.
García-Varela, M., & Pérez-Ponce de León, G. (2008). Validating the systematic position of Profilicollis Meyer, 1931 and Hexaglandula Petrochenko, 1950 (Acanthocephala: Polymorphidae) using cytochrome c oxidase (cox 1). Journal of Parasitology, 94, 212–217.
García-Varela, M., Pérez-Ponce de León, G., Aznar, F. J., & Nadler, S. A. (2013). Phylogenetic relationship among genera of Polymorphidae (Acanthocephala), inferred from nuclear and mitochondrial gene sequences. Molecular Phylogenetics and Evolution, 68, 176–184.
Garey, J. R., Near, T. J., Nonnemacher, M. R., & Nadler, S. A. (1996). Molecular evidence for Acanthocephala as a subtaxon of Rotifera. Journal of Molecular Evolution, 43, 287–292.
George-Nascimento, M., & Marín, S. (1992). Efecto de dos especies hospedadoras, el lobo fino austral Arctocephalus australis (Zimmerman) y el lobo marino común Otaria byronia (Blainville) (Carnivora; Otariidae), sobre la morfología y la fecundidad de Corynosoma sp. (Acanthocephala; Polymorphidae). Revista Chilena de Historia Natural, 65, 183–193.
Guindon, S., Dufayard, J. F., Lefort, V., Anisimova, M., Hordijk, W., & Gascuel, O. (2010). New algorithms and methods to estimate Maximum-Likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology, 59, 307–321.
Hernández-Orts, J. S., Smales, L. R., Pinacho-Pinacho, C. D., García-Varela, M., & Presswell, B. (2017a). Novel morphological and molecular data for Corynosoma hannae Zdzitowiecki, 1984 (Acanthocephala: Polymorphidae) from teleosts, fish-eating birds and pinnipeds from New Zealand. Parasitology International, 66, 905–916.
Hernández-Orts, J. S., Brandão, M., Georgieva, S., Raga, J. A., Crespo, E. A., Luque, J. L., & Aznar, F. J. (2017b). From mammals back to birds: Host-switch of the acanthocephalan Corynosoma australe from pinnipeds to the Magellanic penguin Spheniscus magellanicus. PLoS One, 12, e0183809.
Hernández-Orts, J. S., Viola, M. N., García, N. A., Crespo, E. A., González, R., García-Varela, M., & Kuchta, R. (2015). A checklist of the helminth parasites of marine mammals from Argentina. Zootaxa, 3936, 301–334.
Hoberg, E. P., & Ryan, P. G. (1989). Ecology of helminth parasitism in Puffinus gravis (Procellariiformes) on the breeding grounds at Gough Island. Canadian Journal of Zoology, 67, 220–225.
Ionita, M., Varela, M. G., Lyons, E. T., Spraker, T. R., & Tolliver, S. C. (2008). Hookworms (Uncinaria lucasi) and acanthocephalans (Corynosoma spp. and Bolbosoma spp.) found in dead northern fur seals (Callorhinus ursinus) on St. Paul Island, Alaska in 2007. Parasitology Research, 103, 1025–1029.
Johnston, T. H. (1937). Entozoa from the Australian hair seal. Proceedings of the Linnean Society of New South Wales, 62, 9–16.
Johnston, T. H., & Edmonds, S. J. (1953). Acanthocephala from Auckland and Campbell Islands. Records of the Dominion Museum, 2, 55–61.
Kuzmina, T. A., Lisitsyna, O. I., Lyons, E. T., Spraker, T. R., & Tolliver, S. C. (2012). Acanthocephalas in northern fur seals (Callorhinus ursinus) and a harbor seal (Phoca vitulina) on St. Paul Island, Alaska: species, prevalence and biodiversity in four fur seal subpopulations. Parasitology Research, 111, 1049–1058.
Lincicome, D. R. (1943). Acanthocephala of the genus Corynosoma from the California sea lion. Journal of Parasitology, 29, 102–106.
Lisitsyna, O. I., Kudlai, O., Spraker, T. R., & Kuzmina, T. A. (2018). New records on acanthocephalans from California sea lions Zalophus californianus (Pinnipedia: Otariidae) from California, USA. Vestnik Zoologii, 52, 181–192.
Miller, M. A., Pfeiffer, W., & Schwartz, T. (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, LA, 1–8.
Morgades, D., Katz, H. M., Castro, O., Capellino, D., Casas, L., Benitez, G., Venzal, J. M., Moraña, A. (2006). Fauna parasitaria del lobo fino Arctocephalus australis y del león marino Otaria flavescens (Mammalia, Otariidae) en la costa uruguaya. In: Rodríguez-Gallego, M. R., Scarabino, L. & Conde, D. (Eds), Bases para la conservación y el manejo de la costa uruguaya. Uruguay: Fundación Vida Silvestre, pp. 89–96.
Morini, E. G., & Boero, J. J. (1960). Corynosoma otariae n. sp. (Acanthocephala; Polymorphidae) parásito de un lobo marino (Otaria flavescens). Actas y Trabajos del Primer Congreso Sudamericano de Zoología, 2, 229–234.
Nadler, S. A., Bolotin, E., & Stock, S. P. (2006). Phylogenetic relationships of Steinernema Travassos, 1927 (Nematoda: Cephalobina: Steinernematidae) based on nuclear, mitochondrial and morphological data. Systematic Parasitology, 63, 161–181.
Obendorf, D. L., & Presidente, P. J. A. (1978). Foreign body perforation of the esophagus initiating traumatic pericarditis in an Australian fur seal. Journal of Wildlife Diseases, 14, 451–454.
Pleijel, F., Jondelius, U., Norlinder, E., Nygren, A., Oxelman, B., Schander, C., Sundberg, P., & Thollesson, M. (2008). Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Molecular Phylogenetics and Evolution, 48, 369–371.
Popov, V. N., & Fortunato, M. E. (1987). Geographical variability of Corynosoma strumosum (Acanthocephala, Polymorphidae), a parasite of sea mammals. Zoologicheskii Zhurnal, 86, 12–18 (In Russian).
Rambaut, A. (2012). FigTree v. 1.4. Molecular evolution, phylogenetics and epidemiology. Edinburgh, UK: University of Edinburgh, Institute of Evolutionary Biology. http://tree.bio.ed.ac.uk/software/figtree/.
Rambaut, A., Suchard, M. A., Xie, D., & Drummond, A. J. (2014). Tracer v1.6. http://beast.bio.ed.ac.uk/Tracer. Accessed 15 August 2014.
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542.
Sardella, N. H., Mattiucci, S., Timi, J. T., Bastida, R. O., Rodrıguez, D. H., & Nascetti, G. (2005). Corynosoma australe Johnston, 1937 and C. cetaceum Johnston & Best, 1942 (Acanthocephala: Polymorphidae) from marine mammals and fishes in Argentinian waters: allozyme markers and taxonomic status. Systematic Parasitology, 61, 143–156.
Shaughnessy, P. D., & Ross, G. J. B. (1980). Records of the Subantarctic fur seal (Arctocephalus tropicalis) from South Africa with notes on its biology and some observations of captive animals. Annals of the South African Museum, 82, 71–89.
Smales, L. R. (1986). Polymorphidae (Acanthocephala) from Australian mammals with description of two new species. Systematic Parasitology, 8, 91–100.
Stewardson, C. L., & Fourie, H. J. (1998). Endoparasites of the cape fur seal Arctocephalus pusillus pusillus from the eastern Cape Coast of South Africa. Transactions of the Royal Society of South Africa, 53, 33–51.
Stryukov, A. A. (2004). [Acanthocephalans of phocid seals from Pacific sector of Antarctic.] PhD Thesis, Simferopol, 302 pp (In Russian).
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729.
Tantaleán, M., & Huiza, A. (1994). Sinopsis de los parásitos de peces marinos de la costa peruana. Biotempo, 1, 53–101.
Tantaleán, M., Mendoza, L., & Riofrio, F. (2007). El zorro Andino, Pseudalopes culpaeus, un nuevo huésped para Corynosoma obtuscens (Acanthocephala) en el Perú. Revista Peruana de Biologia, 14, 51–52.
Tantaleán, M., Sánchez, L., Gómez, L., & Huiza, A. (2005). Acantocéfalos del Perú. Revista Peruana de Biología, 12, 83–92.
Telford, M. J., Herniou, E. A., Russell, R. B., & Littlewood, D. T. J. (2000). Changes in mitochondrial genetic codes as phylogenetic characters: two examples from the flatworms. Proceedings of the National Academy of Sciences of the United States of America, 97, 11359–11364.
Van Cleave, H. J. (1953a). A preliminary analysis of the acanthocephalan genus Corynosoma in mammals of North America. Journal of Parasitology, 39, 1–13.
Van Cleave, H. J. (1953b). Acanthocephala of North American mammals. Illinois Biological Monographs, 23, 179 pp.
Waindok, P., Lehnert, K., Siebert, U., Pawliczka, I., & Strube, C. (2018). Prevalence and molecular characterisation of Acanthocephala in pinnipedia of the North and Baltic Seas. International Journal for Parasitology: Parasites and Wildlife, 7, 34–43.
Zdzitowiecki, K. (1984). Some antarctic acanthocephalans of the genus Corynosoma parasitizing Pinnipedia, with descriptions of three new species. Acta Parasitologica Polonica, 29, 359–377.
Zdzitowiecki, K. (1986). Acanthocephala of the Antarctic. Polish Polar Research, 7, 79–117.
Acknowledgements
The authors thank Dr Frances Gulland, Christine Fontaine, Barbie Halaska, and other colleagues from the Marine Mammal Center (TMMC), Sausalito, California, for their help in parasitological examination of dead California sea lions. The authors thank Dr Yuriy Kuzmin from the Institute of Zoology NAS of Ukraine for his useful comments and assistance with preparation of the manuscript. The images of helminths were made at the Center of collective use of scientific equipment “Animalia” (I. I. Schmalhausen Institute of Zoology NAS of Ukraine).
Funding
This study was partially supported by the North-West University, South Africa to OK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Additional information
This article is part of the Topical Collection Acanthocephala.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lisitsyna, O.I., Kudlai, O., Spraker, T.R. et al. Morphological and molecular evidence for synonymy of Corynosoma obtuscens Lincicome, 1943 with Corynosoma australe Johnston, 1937 (Acanthocephala: Polymorphidae). Syst Parasitol 96, 95–110 (2019). https://doi.org/10.1007/s11230-018-9830-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-018-9830-0