Abstract
Members of the genus Lueheia Travassos, 1919, are endoparasites of birds, particularly passerines, throughout the Americas. Adults of Lueheia sp., (Plagiorhynchidae Golvan, 1960; Porrorchinae Golvan, 1956) were recovered from the intestine of the American robin (Turdus migratorius phillipsi Bangs) in Mexico City, and two other species of acanthocephalans identified as Porrorchis nickoli, (Plagiorhynchidae: Porrorchinae) Salgado-Maldonado and Cruz-Reyes, 2002 and Centrorhynchus microcephalus (Bravo-Hollis, 1947) Golvan, 1956 (Centrorhynchidae Van Cleave, 1916), were recovered from the Virginia opossum (Didelphis virginiana Allen) and groove-billed ani (Crotophaga sulcirostris Swainson), respectively in southeastern Mexico. Specimens of three species were sequenced at two molecular markers, the small subunit (SSU) and large subunit (LSU) of the nuclear rDNA and compared with other sequences available in GenBank. Maximum likelihood and Bayesian inference analyses of the combined (LSU + SSU) dataset and each individual dataset revealed that the specimens of Lueheia sp. formed an independent lineage, which is recognized herein as a new species, Lueheia aztecae n. sp., representing the fifth species of the genus in the Americas, and the second in the Nearctic region. The new species can be morphologically distinguished from the other five species in the genus by having a cylindrical proboscis, armed with 24–26 longitudinal rows with 9–10 hooks each. Phylogenetic inference performed with the combined dataset consisting of two genes (LSU + SSU) revealed that Lueheia aztecae n. sp. and P. nickoli belonging to subfamily Porrorchinae, formed two independent lineages, indicating that the subfamily is paraphyletic. Porrorchis nickoli and C. microcephalus formed a clade with other species of the genus Centrorhynchus, suggesting that P. nickoli should be transferred to genus Centrorhynchus, to form C. nickoli n. comb. In addition, we briefly discuss the ecological associations between the members of the families Plagiorhynchidae and Centrorhynchidae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of Plagiorhynchidae Golvan, 1960 are acanthocephalans that use birds, mammals, and rarely reptiles as definitive hosts and are distributed worldwide (Smales 2002; Amin 2013). Currently, the family includes three subfamilies: Porrorchinae Golvan, 1956, with six genera; Sphaerechinorhynchinae Golvan, 1956, represented by a single genus; and Plagiorhynchinae Meyer, 1931, with two genera (see Amin 2013). Golvan (1956) reviewed the taxonomy of Plagiorhynchidae and recognized the subfamily Porrorchinae with five genera. Currently, the subfamily includes approximately 37 species, classified into six genera: Porrorchis Fukui, 1929; Oligoterorhynchus Monticelli, 1914; Lueheia Travassos, 1919; Pseudolueheia Schmidt and Kuntz 1967; Owilfordia Schmidt and Kuntz, 1967; and Pseudogordiorhynchus Golvan, 1957 (Amin 2013). The genus Lueheia is morphologically diagnosed by having a large cylindrical body with a small subglobular to semispherical proboscis armed with numerous strong hooks, a short and spineless neck, a double-walled cylindrical proboscis receptacle, a cerebral ganglion located in the middle of proboscis receptacle, and 4 to 10 long and slender lemnisci. The male possesses 2 spherical to oblique testes in tandem, placed in the anterior region of the body and long, tubular cement glands. The genital pore is terminal or subterminal, the eggs are oval or elongated, and the fertilization membrane exhibits polar prolongation (Smales 2013). The taxonomy of the genus Lueheia was evaluated recently by Smales (2013), recognizing five species: one distributed in North America (Lueheia adlueheia, (Werby 1938)), three in South America, (Lueheia lueheia Travassos, 1919; Lueheia cajabambensis Machado Filho and Nicanor Ibañez, 1967; Lueheia inscripta (Westrumb 1821)), and one in Asia (Lueheia karachiensis Khan, Bilqees and Muti-ur-Rahman, 2005).
The acanthocephalans in Mexico have received a great attention recently, and much effort has been made to incorporate morphological and molecular characters in order to describe and delineate the biodiversity of this group of parasites (see Monks 2001; García-Varela and Nadler 2005; Guillén-Hernández et al. 2008; García-Varela and Pérez-Ponce de León 2008; López-Caballero et al. 2015; Pinacho-Pinacho et al. 2018; García-Varela et al. 2019). In a checklist of acanthocephalans from Mexico, a total of 77 taxa were recognized (García-Prieto et al. 2010). In the current study, adult acanthocephalans were collected from the intestine of the groove-billed ani (Crotophaga sulcirostris Swainson), and were identified as Centrorhynchus microcephalus (Bravo-Hollis, 1947) Golvan, 1956. The acanthocephalans associated with the Virginia opossum (Didelphis virginiana Allen) were identified as Porrorchis nickoli Salgado-Maldonado and Cruz-Reyes, 2002, and finally, the acanthocephalans found in the American robin (Turdus migratorius phillipsi Bangs) corresponded to an undescribed species of the genus Lueheia.
The objectives of the present research were to (1) provide a morphological description of the new species (2) identify the systematic position of the genera Lueheia and Porrorchis belonging to subfamily Porrorchinae, and (3) reconstruct the phylogenetic relationships among members of the families, Centrorhynchidae and Plagiorhynchidae by using sequences of the near-complete small (SSU) and large (LSU) subunit of the nuclear rDNA. We then used the resulting phylogenetic trees as a framework to discuss host-parasite associations and begin to understand the evolutionary history of this group of acanthocephalans.
Material and methods
Specimens collecting, DNA isolation, and morphological analyses
During a helminthological survey in central and southeastern Mexico, acanthocephalans were collected from intestine of the groove-billed ani (C. sulcirostris), in Tlacatalpan, Veracruz (18° 36′ 0′′ N; 95° 39′ 0′′ W), the Virginia opossum (D. virginiana) in Los Tuxtlas, Veracruz (18° 34′ 21′′ N; 95° 04′ 30′′ W), and an American robin (T. migratorius phillipsi) found dead in Mexico City (see Table 1). The acanthocephalans recovered were immersed in distilled water for 10–12 h at 4 °C. Specimens were subsequently preserved in 100% ethanol and stored at 4 °C. For taxonomic identification, some specimens were stained with Mayer’s paracarmine, dehydrated in graded ethanol series, cleared in methyl salicylate, and mounted as permanent slides using Canada balsam. All the specimens were examined using a bright-field Leica DM 1000 LED microscope (Leica, Wetzlar, Germany). Measurements were taken using the Leica Application Suite microscope software. The measurements are presented in micrometers (μm) unless otherwise stated; with the mean followed by the range in parenthesis. Drawings were made with the aid of a drawing tube. For scanning electron microscope (SEM) observations, some individuals were dehydrated through a graded series of ethyl alcohol, and then critical-point dried with carbon dioxide. These specimens were mounted on metal stubs with silver paste, coated with gold, and examined in a Hitachi Stereoscan model SU1510 (Hitachi High-Technologies, Tokyo, Japan) at 15 kV. The acanthocephalans recovered from groove-billed ani were identified as Centrorhynchus microcephalus, and the parasites from Virginia opossum were identified as Porrorchis nickoli. Finally, the specimens from the American robin were assigned to the genus Lueheia. All the specimens were deposited in the Colección Nacional de Helmintos (CNHE: C.microcephalus No. 7074; P. nickoli Nos. 9512, 9513), Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City. Definitive avian hosts were identified using the field guides of Howell and Webb (1995) and the American Ornithologists’ Union (1998). The mammals were shot by local hunters or caught with tomahawk traps and then injected intraperitoneally with an overdose of sodium pentobarbital. The opossums were dissected within the following 4 h, and all organs were examined under a stereomicroscope (see Acosta-Virgen et al. 2015).
DNA extraction, PCR amplification, sequencing, and phylogenetic analyses
Specimens of each species were placed individually in tubes and digested overnight at 56 °C in a solution containing 10 mM Tris-HCl (pH 7.6), 20 mM NaCl, 100 mM Na2-EDTA (pH 8.0), 1% Sarkosyl, and 0.1 mg/ml proteinase K. Following digestion, DNA was extracted using DNAzol reagent (Molecular Research Center, Cincinnati, Ohio) according to the manufacturer’s instructions. Two regions of nuclear ribosomal DNA (rDNA) were amplified using the polymerase chain reaction (PCR). Near-complete 18S rDNA (~ 1,800 bp) was amplified using 2 overlapping PCR fragments of 1,000 bp. The primers used for SSU amplicon 1 were forward 5′-AGATTAAGCCATGCATGCGT-3′ and reverse 5′-AACTTTTCGTTCTTGATTAATG-3′; for amplicon 2, forward 5′-GCAGCGCGGTAATTCCAGCTC-3′ and reverse 5′-GCAGGTTCACCTACGGAAA-3′. Near-complete 28S rDNA (~ 2,900 bp) was amplified using 3 overlapping PCR fragments of 1200–1300 bp. Primers for LSU amplicon 1 were forward 5′-CAAGTACCGTGAGGGAAAGTTGC-3′ and reverse 5′-CAGCTATCCTGAGGGAAAC-3′; amplicon 2 were forward 5′-ACCCGAAAGATGGTGAACTATG-3′ and reverse 5′- CTTCTCCAACGTCAGTCTTCAA-3′; and for amplicon 3, forward 5′- CTAAGGAGTGTGTAACAACTCACC-3′ and reverse 5′-CTTCGCAATGATAGGAAGAGCC-3′ (García-Varela and Nadler 2005). The PCRs (25-μl final volume) consisted of 10 μM of each primer, 2.5 μl of 10× buffer, 2 mM MgCl2, and 1 U of Taq DNA polymerase (Platinum Taq, Invitrogen Corporation, Carlsbad, California, USA). PCR cycling parameters for rDNA amplifications included denaturation at 94 °C for 3 min, followed by 35 cycles of 94 °C for 1 min, annealing at 50–58 °C (optimized for each rDNA amplification) for 1 min, and extension at 72 °C for 1 min, followed by a post-amplification incubation at 72 °C for 7 min. Sequencing reactions were performed with the primers mentioned above using ABI Big Dye (Applied Biosystems, Boston, Massachusetts) terminator sequencing chemistry, and reaction products were separated and detected using an ABI 3730 capillary DNA sequencer. Contigs were assembled and base-calling differences resolved using Codoncode Aligner version 5.1.5 (Codoncode Corporation, Dedham, Massachusetts). Sequences obtained in the current research for SSU and LSU of C. microcephalus, P. nickoli, and Lueheia sp. were aligned with other sequences downloaded from GenBank dataset (see Table 1). Sequences of each molecular marker were aligned separately using the software Clustal W (Thompson et al. 1997) after a combined alignment (LSU + SSU) was performed. A nucleotide substitution model was selected for each molecular marker and the combined dataset using jModelTest version 2.1.7 (Posada 2008) applying the Akaike criterion. The best nucleotide substitution models for each and the combined dataset were GTR + G + I. Phylogenetic trees were inferred through maximum likelihood (ML) with the program RAxML version 7.0.4 (Stamatakis 2006). A GTRGAMMAI substitution model was used, and 10,000 bootstrap replicates were run to assess nodal support. We also analyzed our data in a Bayesian framework using MrBayes 3.2.2 (Ronquist et al. 2012), with two Markov chain (MCMC) runs for 10 million generations, sampled every 1000 generations, a heating parameter value of 0.2 and burn-in of (25%). Trees were edited using FigTree version 1.4.0 (Rambaut 2012).
Results
Morphological description
Class Palaeacanthocephala Meyer, 1931.
Order Polymorphida Petrochenko, 1956.
Family Plagiorhynchidae Golvan,1960.
Subfamily Porrorchinae Golvan, 1956.
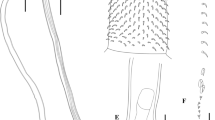
Lueheia aztecae n. sp. García-Varela and Andrade-Gómez; Figs. 1a–f
Description based on 14 specimens (seven males and seven females).
General: Porrorchinae with characters of the genus Lueheia. Living specimens of white color. Sexual dimorphism evident; females larger than males. Proboscis subglobular, armed with 24–26 longitudinal rows, with 8–10 hooks with simple roots per row; largest hooks located at the mid-proboscis (42–61 long). First hooks in row 29–34 long, last hooks in row 42–48 long, other hooks 38–52 long. Neck small and spineless, cone shaped. Proboscis receptacle double-walled, with an oval cephalic ganglion in the middle. Lemnisci 4–6, slender, of different lengths, inserted at the base of the neck. Genital pore subterminal in both sexes.
Male: (Based on seven mounted adult specimens and one analyzed by SEM.) Trunk 7.1 mm (4–10.5 mm) × 1.5 mm (1.0–2.3 mm); maximum width at hind-trunk level. Proboscis 430 (287–531) × 348 (291–520). Proboscis subglobular, armed with 24–26 longitudinal rows of hooks, with 8–10 hooks each row (Fig. 2a), largest hooks located at the mid-proboscis (42–61 long). First hooks in row 29–34 long, last two hooks in row 42–48 long, other hooks 38–52 long. Neck 228 (178–272) × 344 (244–433). Proboscis receptacle 1.13 mm (0.83–1.35 mm) × 287 (228–357). Lemnisci 1.9 mm (1.5–2.6 mm). Testes ovoid, in tandem, posterior to the proboscis receptacle. Anterior testis 1.18 mm (0.69–1.5 mm) × 501 (215–818). Posterior testis 1.09 mm (0.73–1.31 mm) × 469 (334–625). Cement glands, four tubular, 2.25 mm (1.6–3.1 mm) long. Säfftigen’s pouch 729 (552–983) long. Copulatory bursa 519 (351–680) × 576 (364–700).
Female: (Based on seven mounted gravid specimens and one analyzed by SEM.) Trunk 12.4 mm (10.2–15.3 mm) × 2.0 (1.4–2.3 mm) (Fig. 1b). Proboscis 555 (505–628) × 365 (333–418). Proboscis subglobular, armed with 24–26 longitudinal rows of hooks, with 8–10 hooks each row. Largest hooks located at the mid-proboscis (43–48 long). First hooks in row 28–35 long, last two hooks in row 29–42 long, other hooks 32–53 long. Neck 260 (213–302) × 366 (343–400). Proboscis receptacle 1.3 mm (1.26–1.37 mm). Lemnisci of unequal lengths. Uterine bell with a thick body wall 539 (473–606) long. Uterus long 870 (723–964); vagina complex with two sphincter muscles 255 (220–276) long; gonopore subterminal (Fig. 3c). Mature eggs, containing a fully developed acanthor, fusiform, with polar prolongations in the middle fertilization membrane 67 (60–72) × 24 (22–27) (Fig. 3d).
Phylogenetic trees using maximum likelihood and consensus Bayesian Inference for the combined (LSU + SSU) data set (a), LSU data set (b), and SSU data set (c) Numbers near internal nodes show ML bootstrap percentage (BP) values and Bayesian posterior probabilities (BPP). Scale bars represent the branch length
Taxonomic summary
Host: Turdus migratorius phillipsi (Bangs) (American robin), Passeriformes.
Site of infection: Intestine.
Locality: Border between Tlalpan and Xochimilco municipalities, Mexico City, Mexico (19°16′ 34.63′′ N 99° 08′ 30.46′′ W).
Type-material: Holotype; (CNHE: 11247), allotype (CNHE: 11248); Paratype (CNHE: 11249).
Representative DNA sequences: MT161620 (SSU), MT161665 (LSU).
Etymology: The specific epithet refers to the Azteca, a Mesoamerican civilization who dominated Central Mexico during the early thirteenth century and founders of Tenochtitlán (where Mexico City is currently located). ZooBank registration: The Life Science Identifier (LSID) of the article is urn:lsid: zoobank.org:pub: CE6ABC27-DF7D-4DE6-9815-BCF52538978B. The LSID for the new name Lueheia aztecae n. sp. is urn:lsid:zoobank.org:act:1334EDE1-8E00-40BB-B25E-2CF833C66B03.
Taxonomic remarks
The new species belongs to the genus Lueheia in having a subglobular proboscis, small and spineless neck, double-walled proboscis receptacle with an oval cephalic ganglion in the middle, 4–6 slender lemnisci of different lengths inserted at the base of the neck and eggs with polar prolongations in the middle fertilization membrane. Lueheia aztecae n. sp. can be morphologically distinguished by its cylindrical proboscis armed with 24–26 longitudinal rows with 9–10 hooks each (vs 20–22 longitudinal rows with 8–9 hooks each in L. lueheia (Table 2), 28–30 longitudinal rows with 9–12 hooks each in L. inscripta, 28 longitudinal rows with 9–10 hooks each in L. adlueheia, and 20–40 longitudinal rows with 10–14 hooks each in L. karachiensis; Table 2). The new species also differs from L. adlueheia from North America by having larger eggs (60–72 × 22–27 vs 36–41 × 12–15; see Table 2).
Phylogenetic analyses
The combined dataset including two genes (LSU + SSU) consisted of 30 terminals with 4888 sites (including gaps), with GTR + G + I as the best model. The phylogenetic tree inferred with ML and Bayesian inference (BI) recovered Polymorphida as a monophyletic group with strong bootstrap support (100%) and Bayesian posterior probability (1.0). The phylogenetics trees showed three main clades (Fig. 3a). The first clade contained 10 genera of Polymorphidae, (Andracantha Schmidt, 1975; Corynosoma Lühe, 1904; Bolbosoma Porta, 1908; Southwellina Witenberg, 1932; Hexaglandula Petrochenko, 1950; Ibirhynchus García-Varela, Pérez-Ponce de León, Aznar and Nadler, 2011; Arhythmorhynchus Lühe, 1911; Profilicollis Meyer, 1931; Pseudocorynosoma Aznar, Pérez-Ponce de León and Raga 2006; and Polymorphus Lühe, 191, with strong bootstrap support (100%) and Bayesian posterior probability (1.0). The second clade had strong bootstrap support (100%) and a high Bayesian posterior probability (1.0) and included Plagiorhynchus Luhë 1911 with two subgenera (Plagiorhynchus Luhë, 1911 and Prosthorhynchus Kostylew, 1915), which are members of Plagiorhynchidae. The third clade included six species of Centrorhynchidae (Centrorhynchus globocaudatus (Zeder, 1800); Centrorhynchus aluconis (Müller, 1780); Centrorhynchus conspectus Van Cleave and Pratt, 1940; Centrorhynchus microcephalus; Centrorhynchus globirostris Amin, Hechmann Wilson Keele and Khan, 2015; Centrorhynchus nahuelhuapensis Steinauer, Flores and Rauque 2020; and Centrorhynchus sp.) plus Porrorchis nickoli (Plagiorhynchidae), with a high bootstrap support (100%) and a high Bayesian posterior probability (1.0). The phylogenetic position of Lueheia aztecae n. sp., as sister to Centrorhynchus and Porrorchis was supported, with only 58% bootstrap in maximum likelihood analysis and an 0.88 posterior probability in the Bayesian analysis. The LSU dataset consisted of 29 terminals and 3093 sites (including gaps), with GTR + G + I as the best model. The tree topologies inferred with LSU dataset from rDNA (Fig. 3b) had the same branching order among the families from Polymorphida as the ML and Bayesians trees inferred with the combined (LSU + SSU) dataset, with minor differences regarding the position of C. aluconis. The SSU dataset consisted of 28 terminals with 1795 sites (including gaps), with GTR + G + I as the best model. The tree topologies inferred with SSU dataset were not the same because their taxon sampling differed. Nevertheless, they were similar to the topologies inferred with the combined (LSU + SSU) dataset, i.e., the SSU tree also recognized the three families from Polymorphida (Fig. 3c). One of the major differences between the trees inferred with SSU and combined (LSU + SSU) datasets was the systematic position of Lueheia aztecae n. sp. which was placed sister taxa to Polymorphidae with a weak bootstrap support (55%) and Bayesian posterior probability (0.52). The other taxa sampled in this study, namely, C. microcephalus and P. nickoli were consistently placed within the genus Centrorhynchus Lühe 1911, in all phylogenetic analyses (see Figs. 3a–c).
Discussion
The taxonomic history and species composition of Lueheia are complex and problematic, due in part to the incomplete morphological descriptions of the adult worms. Smales (2013) reviewed the taxonomy and recognized five species, L. lueheia, L. adlueheia, L. cajabambensis, L. inscripta, and L. karachiensis. However, the same author considered L. karachiensis to potentially belong to genus Centrorhynchus due to the morphological and ecological similarities between the two genera. Additionally, L. karachiensis was described from Asia, whereas the other congeneric species are distributed in the Americas. Given the current evidences, we agree with Smales (2013), that L. karachiensis may not belong to genus Lueheia; however, this hypothesis should be tested in a formal phylogenetic analysis. Therefore, the species described herein, Lueheia aztecae n. sp. represents the fifth species of the genus distributed in the Americas, and the second species of this genus recorded in the Nearctic region. Morphologically, Lueheia aztecae n. sp. is distinguished from L. adlueheia, which is also distributed in the Nearctic region by having a cylindrical proboscis, armed with 24–26 longitudinal rows with 9–10 hooks (vs 28 longitudinal rows with 9–10 hooks in L. adlueheia). The species L. adlueheia was described from the western robin (Turdus migratorius propinquus Ridgway), in Seattle, Washington, USA (Werby 1938). This bird occurs from British Columbia to southwestern Mexico (Kemper and Taylor 1981; American Ornithologists’ Union 1998). Lueheia aztecae n. sp. was found in a subspecies of the American robin (Turdus migratorius phillipsi). This passerine bird has a distribution from central to southern Mexico (see American Ornithologists’ Union 1998).
The phylogenetic analyses obtained with the combined dataset of two genes (LSU + SSU) revealed that Lueheia aztecae n. sp. and P. nickoli, both members of the subfamily Porrorchinae Golvan, 1956 were not grouped together. For instance, Lueheia aztecae n. sp. was placed in an independent lineage (Fig. 3a), whereas P. nickoli was nested within the genus Centrorhynchus (Fig. 3a). Porrorchis nickoli was originally described from the gray four-eyed opossum, (Philander opossum Linnaeus) in southeastern Mexico (Salgado-Maldonado and Cruz-Reyes 2002). This is the only recorded species of the genus in the Americas, whereas the other 21 species of Porrorchis Fukui, 1929 are distributed in Eurasia, Madagascar, and Australia, mostly in association with birds and rarely, with terrestrial mammals (Lisitsyna et al. 2012). Morphologically, our specimens of P. nickoli possessed a cylindrical trunk, a subglobular proboscis armed with 22–24 rows of 7–8 hooks each (Figs. 4a–c), a cylindrical and double-walled proboscis receptacle, a cerebral ganglion located at the mid-receptacle, lemnisci of equal size, oval testes arranged in tandem, four elongate cement glands, and elliptical eggs without polar prolongation. These characteristics of our newly collected material clearly correspond with those reported in the original description by Salgado-Maldonado and Cruz-Reyes (2002). In addition, Amin et al. (2015) reviewed and emended the genus Centrorhynchus to include species with a globular proboscis, a character also present in P. nickoli. Based on the current morphological evidence plus the phylogenetic position of P. nickoli in our analyses, P. nickoli should most likely be transferred to Centrorhynchus to form Centrorhynchus nickoli n. comb. To date, seven species of Centrorhynchus have been described from North America, primarily in association with birds of prey: Centrorhynchus spinosus (Kaiser, 1893) Van Cleave, 1924; Centrorhynchus californicus Millzner, 1924; Centrorhynchus conspectus, C. microcephalus; Centrorhynchus wardae Holloway, 1958 (junior synonym of C. conspectus); Centrorhynchus kuntzi Schmidt and Neiland, 1966, and Centrorhynchus robustus Richardson and Nickol, 1995 (Richardson and Nickol 1995; Amin 2013). In the current study, specimens of C. microcephalus were collected from groove-billed ani (C. sulcirostris). Morphologically, C. microcephalus is characterized by having a constricted, cylindrical proboscis armed with 30–33 longitudinal rows of 16–17 hooks each (Figs. 5a–c). The proboscis receptacle is double walled, the lemnisci are equal in size, and the cerebral ganglion is located at the mid-receptacle, two elliptoid testes, and four elongate cement glands. The eggs display polar prolongations (Bravo-Hollis, 1947; Richardson and Nickol 1995; Richardson et al. 2010). The phylogenetic analyses inferred with each molecular marker and the combined (LSU + SSU) dataset, consistently placed C. microcephalus within a clade with other species of Centrorhynchus, such as C. nickoli n. comb, Centrorhynchus aluconis (type species), Centrorhynchus globocaudatus, Centrorhynchus conspectus, Centrorhynchus globirostris, and Centrorhynchus nahuelhuapensis.
The phylogenetic relationships inferred with the combined dataset of two molecular markers, (LSU + SSU), represents the most compressive analysis to date and provides the first insight into the ecological associations between the parasites and their definitive hosts. We inferred that birds were the ancestral definitive hosts for Centrorhynchidae and Plagiorhynchidae with a secondary and independently event of colonization of mammals.
References
Acosta-Virgen K, López-Caballero J, García-Prieto L, Mata-López R (2015) Helminths of three species of opossums (Mammalia, Didelphidae) from Mexico. Zookeys 511:131–152. https://doi.org/10.3897/zookeys.511.9571
American Ornithologists’ Union (AOU). (1998) Check-list of North American birds. 7th ed. 829 pp. Washington, DC, AOU
Amin OM (2013) Classification of the Acanthocephala. Folia Parasitol 60:273–305
Amin OM, Heckmann RA, Wilson E, Keele B, Khan A (2015) The description of Centrorhynchus globirostris n. sp. (Acanthocephala: Centrorhynchidae) from the pheasant crow, Centropus sinensis (Stephens) in Pakistan, with gene sequence analysis and emendation of the family diagnosis. Parasitol Res 114:2291–2299
Bravo-Hollis M (1947) Gordiorhynchus microcephalus n. sp., acantocephalo parasito de un pajaro (Cassidix mexicanus mexicanus Gmelin). Anales Inst Biol Univ Nac Auto Mex 18:499–506
García-Prieto L, García-Varela M, Mendoza-Garfias B, Pérez-Ponce de León G (2010) Checklist of the Acanthocephala in wildlife vertebrates of Mexico. Zootaxa 2419:1–50
García-Varela M, Nadler SA (2005) Phylogenetic telationships of Palaeacanthocephala (Acanthocephala) inferred from SSU and LSU rRNA gene gequences. J Parasitol 91:1401–1409
García-Varela M, Pérez-Ponce de León G (2008) Validating the systematic position of Profilicollis Meyer, 1931 and Hexaglandula Petrochenko, 1950 (Acanthocephala: Polymorphidae) using cytochrome c oxydase (Cox 1). J Parasitol 94:212–217
García-Varela M, Pérez-Ponce de León G, Aznar FJ, Nadler SA (2009) Systematic position of Pseudocorynosoma and Andracantha (Acanthocephala, Polymorphidae) based on nuclear and mitochondrial gene sequences. J Parasitol 95:178–118. https://doi.org/10.1645/GE-1538.1
García-Varela M, Pérez-Ponce de León G, Aznar FJ, Nadler SA (2011) Erection of Ibirhynchus gen. nov. (Acanthocephala: Polymorphidae), based on molecular and morphological data. J. Parasitol 97: 97–105. https://doi.org/10.1645/GE-2350.1
García-Varela M, Pérez-Ponce de León G, Aznar FJ, Nadler SA (2013) Phylogenetic relationships among genera of Polymorphidae (Acanthocephala), inferred from nuclear and mitochondrial gene sequences. Mol Phylogenet Evol 68:176–184. https://doi.org/10.1016/j.ympev.2013.03.029
García-Varela M, Park JK, Hernández-Orts JS, Pinacho-Pinacho CD (2019) Morphological and molecular data on a new species of Plagiorhynchus Lühe, 1911 (Acanthocephala: Plagiorhynchidae) from the long-billed curlew (Numenius americanus) from northern Mexico. J Helminthol 22. https://doi.org/10.1017/S0022149X19000543
Golvan YJ (1956) Acanthocéphales d’oiseaux Troisième note. Revision des especes européenes de la sous-familledes Plagiorhynchinae A. Meyer, 1931 (Polymorphidae). Ann Parasitol Hum Comp 3:350–384
Guillén-Hernández S, García-Varela M, Pérez-Ponce de León G (2008) First record of Hexaglandula corynosoma (Travassos, 1915) Petrochenko, 1958 (Acanthocephala: Polymorphidae) in intermediate and definitive host in Mexico. Zootaxa 1873:61–68
Howell SNG, Webb S (1995) A guide to the birds of Mexico and Northern Central America. 851pp. New York, Oxford University Press
Kemper LD, Taylor MJ (1981) Seasonal reproductive changes in the American robin (Turdus migratorius L.) of the Pacific Northwest. Can J Zool 59:212–217. https://doi.org/10.1139/z81-035
Lagrue C, Heaphy K, Presswell B, Poulin R (2016) Strong association between parasitism and phenotypic variation in a supralittoral amphipod. Mar Ecol Prog Ser 553:111–123
Lisitsyna O, Tkach VI, Bush S (2012) New records of Acanthocephalans from birds in the Philippines with a description of a new Porrorchis species identification keys for the genus. J Parasitol 98:1176–1184. https://doi.org/10.1645/GE-3116.1
López-Caballero J, Mata-López R, García-Varela M, Pérez Ponce de Léon G (2015) Genetic divergence of Oligacanthorhynchus microcephalus (Acanthocephala:Archiacanthocephala: Oligacanthorhynchidae), parasite of three species of opossum (Mammalia:Didelphidae) across Central and Southeastern Mexico. Com Parasitol 82:175–186. https://doi.org/10.1654/4742.1
Monks S (2001) Phylogeny of the Acanthocephala based on morphological characters. Syst Parasitol 48:81–116
Near TJ, Garey JR, Nadler SA (1998) Phylogenetic relationships of the Acanthocephala inferred from 18S ribosomal DNA sequences. Mol Phylogenet Evol 10:287–298
Pinacho-Pinacho CD, García-Varela M, Sereno-Uribe AL, Pérez-Ponce de León G (2018) A hyper-diverse genus of acanthocephalans revealed by tree-base and non-tree based species delimitation methods: ten cryptic species of Neoechinorhynchus in Middle America freshwater fishes. Mol Phylogenet Evol 127:30–45
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256
Rambaut A (2012) FigTree v1.4.0. Institute of Evolutionary Biology. Edinburgh, University of Edinburgh
Richardson JD, Nickol BB (1995) The genus Centrorhynchus (Acanthocephala) in North America with description of Centrorhynchus robustus n. sp., redescription of Centrorhynchus conspectus, and a key to species. J Parasitol 81:767–772
Richardson JD, Monks S, García-Varela M, Pulido-Flores G (2010) Redescription of Centrorhynchus microcephalus (Bravo–Hollis, 1947) Golvan, 1956 (Acanthocephala: Centrorhynchidae) from the Groove-Billed Ani (Crotophaga sulcirostris) in Veracruz, Mexico. Comp Parasitol 77:164–171. https://doi.org/10.1654/4412.1
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61: 539–542.
Salgado-Maldonado G, Cruz-Reyes A (2002) Porrorchis nickoli n. sp. (Acanthocephala: Plagiorhynchidae) from mammals in Southeastern Mexico, first known occurrence of Porrorchis in the Western Hemisphere. J. Parasitol 88:146–152
Smales LR (2002) Plagiorhynchidae Meyer, 1931 (Acanthocephala) from Australasian birds and mammals, with descriptions of Plagiorhynchus (plagiorhynchus) menurae (Johnston, 1912) and P. (P). allisonae n. sp. Syst Parasitol 51:2017–2216
Smales LR (2013) Acanthocephala including the descriptions of new species of Centrorhynchus (Centrorhynchidae) and the redescription of Lueheia scripta (Westrumb, 1821) (Plagiorhynchidae) from birds from Paraguay, South America. Rev. Suisse Zool 120:175–202
Stamatakis A (2006) RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Steinauer M, Flores V, Rauque C (2020) Centrorhynchus nahuelhuapensis n. sp. (Acanthocephala: Centrorhynchidae) from rufous-legged owl (Strix rufipes King) in Patagonia. J Helminthol 94:1–7. https://doi.org/10.1017/S0022149X18001220
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F (1997) The Clustal windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Werby HI (1938) A new genus of Acanthocephala with forked lemnisci. Trans Am Microsc Soc 57:204–212
Acknowledgments
We thank to Luis García-Prieto for providing specimens deposited at the CNHE. Laura Márquez and Nelly López Ortiz for their help with the automatic sequencer. The American robin specimen was collected by Martin Figueroa B. and Paola Chavez; other specimens were collected under the Cartilla Nacional de Colector Científico (FAUT 0202) issued by the Secretaría del Medio Ambiente y Recursos Naturales (SEMARNAT) to MGV. This research was partially supported by grants from the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT-UNAM) IN207219 and IN220113 to MGV and RML respectively. LAG thanks the support of the Programa de Posgrado en Ciencias Biológicas, UNAM, and CONACYT for granting a scholarship to complete his PhD program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Simonetta Mattiucci
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
García-Varela, M., Andrade-Gómez, L., López-Caballero, J. et al. Morphological and molecular data reveal a new species of Lueheia (Acanthocephala: Plagiorhynchidae) from Turdus migratorius (Turdidae) in central Mexico and its phylogenetic implications within the family. Parasitol Res 119, 3221–3231 (2020). https://doi.org/10.1007/s00436-020-06748-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06748-7