Abstract
Ingwenascaris n. g. (Nematoda: Heterocheilidae) is established to accommodate Ingwenascaris sprenti n. g., n. sp., described from the stomach of Crocodylus niloticus Laurenti (Reptilia: Crocodylidae) in South Africa, based on light and scanning electron microscopy studies of its morphology. The new genus can be distinguished from other heterocheilid genera through a combination of its characters, including the pronounced asymmetry of each subventral lip due to an alate ventral margin and a non-alate margin facing the dorsal lip, the presence of continuous ridges of triangular denticles along the free labial margins, the lack of interlocking processes or a rostral plate, interlabia being indistinct or represented by small lateral interlabia between the dorsal and ventral lips only, the absence of prominent interlabial longitudinal cuticular ridges, the presence of lateral alae that are fused with the subventral lips, the presence of lateral caudal alae in both sexes, spicules of males that are composed of handle and alate blade, the presence of a gubernaculum, the number and arrangement of male caudal papillae and the position of the vulva near the anterior and middle third of the body in females. Ingwenascaris sprenti n. g., n. sp. represents the sixth heterocheilid genus parasitising African crocodilians. Trispiculascaris assymmetrica (Ortlepp, 1932) (syn. Porrocaecum assymmetricum Ortlepp, 1932) from a Central African crocodile is transferred to the new genus as I. assymmetrica (Ortlepp, 1932) n. comb. The genus Trispiculascaris Skrjabin, 1916 is considered a genus incertae sedis. An identification key to the genera of the family Heterocheilidae is presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Old World crocodilians are represented by three crocodylid genera on the African continent, Crocodylus Laurenti, Osteolaemus Cope and Mecistops Gray (see Ross, 1989; McAliley et al., 2006; Eaton et al., 2009). In turn, these crocodiles are hosts to a diverse endoparasite fauna, including pentastomids, acanthocephalans, digenean trematodes and nematodes (Huchzermeyer, 2003). Of the latter group, ascaridids of the Heterocheilidae Henry & Railliet, 1912, are among the predominant taxa (Huchzermeyer, 2003). Though little is known about the biology of these ascaridids found in crocodilian, chelonian, sirenian, fish and elasmobranch definitive hosts (Anderson, 2000; Mašová et al., 2010), field and experimental studies suggest that larvae of the Heterocheilidae infect intermediate and/or paratenic hosts with a strong aquatic connection, such as, for example, frogs and fishes (Walton, 1937; Moravec & Kaiser, 1994; Vieira et al., 2010). Sprent (1977, 1980a) speculated that crocodiles and sirenians might be infected when ingesting crustacean intermediate hosts, either as part of their diet in the case of young crocodiles or accidentally when feeding on sea grasses in the case of the herbivorous sirenians.
During a recent disease survey of the Nile crocodile, Crocodylus niloticus Laurenti, in the Kruger National Park, South Africa, several nematode taxa were collected from these animals, including a new species of Micropleura von Linstow, 1906 from the peritoneal cavity (Junker & Mutafchiev, 2017). A number of specimens from the stomach of some of the same crocodiles could be allocated to the family Heterocheilidae, based on characters of their lips, the presence of a ventriculus and a caecum extending over half the length of the oesophagus. They did, however, differ from all of the currently recognised genera within the family in a combination of characters and are described below as a new genus and species.
Materials and methods
Nile crocodiles were collected from various perennial rivers in the Kruger National Park, South Africa, during July 2010 and February 2011, as part of a study on their blood chemistry and disease ecology parameters. During post-mortem examination, aliquots were made of the ingesta of the stomach, fixed in hot saline and stored in 10% formalin until worm recovery. Nematodes collected were stored in 70% ethanol. For morphological observation, specimens were cleared in lactophenol and studied as temporary mounts under a compound light microscope. Specimens used for SEM were dehydrated through a graded ethanol series, immersed in hexamethyldisilazane for 20 min, air-dried, coated with gold in a JEOL JFS 1200 coater and examined using a JEOL JSM 5510 microscope at an accelerating voltage of 10 kV. All measurements are in micrometres unless otherwise indicated. Metrical data are given as the range followed by the mean in parentheses. The type- and voucher specimens were deposited in the helminthological collections of the Museum für Naturkunde, Berlin, Germany (Entozoa ZMB) and the National Collection of Animal Helminths, ARC-Onderstepoort Veterinary Institute, South Africa (NCAH).
Superfamily Ascaridoidea Baird, 1853
Family Heterocheilidae Henry & Railliet, 1912
Ingwenascaris n. g.
Diagnosis
Anterior extremity bearing three lips not set-off from body, each with single continuous row of triangular denticles around its free margin; interlabia indistinct or slightly developed between the dorsal and subventral lips only, interlocking processes, postlabial groove and rostral plate absent. Dorsal lip with alate lateral margins. Subventral lips similar in shape, each distinctly asymmetrical due to alate ventral margin and non-alate margin facing dorsal lip. Lateral alae originate at base of subventral lips. Deirids and excretory pore near level of nerve-ring. Oesophagus muscular, terminating in lobed ventriculus. Intestinal caecum extending anteriorly for more than half oesophageal length. Caudal alae, lateral, present in both sexes. In females, vulva situated near anterior and middle third of body, without salient lips. Amphidelphic. Eggs thin-shelled, containing morula. In males, spicules similar in shape and size, with distinct bipartite handle and blade. Gubernaculum present. Parasitic in the stomach of Crocodylidae. Type-species: Ingwenascaris sprenti n. sp. Other species: Ingwenascaris assymmetrica (Ortlepp, 1932) n. comb. [syns. Porrocaecum assymmetricum Ortlepp, 1932 and Trispiculascaris assymmetrica (Ortlepp, 1932) Spent, 1983].
Etymology: The generic name is derived from the isiZulu word “ingwenya” for crocodile (Doke et al., 1977), and refers to the crocodilian host of the type-species.
Ingwenascaris sprenti n. sp.
Type-host: Crocodylus niloticus Laurenti (Reptilia: Crocodylidae).
Type-locality: Type-specimens were collected from two crocodiles in the Kruger National Park, South Africa: one adult female from the Crocodile River (25°27′S, 31°58′E; 12.vii.2010; host no. 1), and one subadult male from Silolveni Dam (24°49′S, 31°50′E; 15.vii.2010; host no. 2).
Site in host: Stomach.
Prevalence: 67% (6 of 9 crocodiles were infected).
Intensity of infection: A mean of 463, with a range of 1–1,827.
Type-specimens: Holotype: ZMB E.7620 (1 male; host no. 1); paratypes: ZMB E.7621 (10 males and 10 females; host no. 1); NCAH.6.1 (6 males and 5 females; host no. 1); NCAH.6.2 (2 males and 2 females; host no. 2).
Voucher-specimens: ZMB E.7622 (SEM stub, fragments of females; host no. 1); ZMB E.7623 (SEM stub, fragments of males; host no. 1); NCAH.6.3 (9 males and 5 females; host no. 1).
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new genus and new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Ingwenascaris n. g. is urn:lsid:zoobank.org:act:F7D900FF-77EF-4F47-B744-973DF6FB92F5 and the LSID for Ingwenascaris sprenti n. sp. is urn:lsid:zoobank.org:act:EA9147A7-BC36-4247-A52D-980D24C1D437.
Etymology: The species is named after the late Professor John F. A. Sprent, University of Queensland, Australia, in recognition of his vast contribution to the taxonomy and systematics of ascaridoid nematodes as well as their host-parasite relationships.
Description (Figs. 1–2)
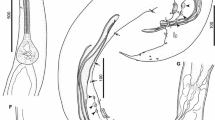
General. Small-sized heterocheilid nematodes. Body slender, tapering towards anterior extremity and with conspicuously elongated, sharply pointed tail in both sexes. Cuticle bearing distinct transverse striations and numerous short interstrial longitudinal ridges along entire length of body (Fig. 2C). Head with 3 large alate lips; interlabia absent, slight interlabial elevation between dorsal and subventral lips observed in some specimens (Fig. 2A1). Anterior half of subventral lips with continuous ridge of 35–40 triangular denticles around their free margin; denticles larger posteriorly (Figs. 1B2, 1B4, 2A). Dorsal lip larger than subventral lips, with rounded anterior outline, widening at level between first and second third of its length and rapidly narrowing posteriorly; continuous row of denticles along free margin anterior to double papillae. Anterior prolongations of labial pulp broad and stubby, originating at same level together with more slender, lateral prolongations (Figs. 1B3, 2A1). One large double papilla situated at the end of each of the lateral prolongations. Subventral lips similar in shape, each distinctly asymmetrical due to alate ventral margin and non-alate margin facing dorsal lip, each bearing 1 subventral double papilla, 1 smaller lateral papilla and 1 amphid (Figs. 1B1, B2, 2A2). Lateral alae originate at base of subventral lips (Fig. 1B1) and terminate near level of midbody. Caudal alae, narrow, lateral, present in both sexes. Somatic papillae present along entire body (Fig. 2C, D), distance between papillae within rows c.163–217 in one female measured. Postdeirids spatula-like, 8–9 high (Fig. 2E). Oesophagus long, slightly widening anterior to terminal ventriculus (Fig. 1A). Ventriculus shorter than wide, with several short lobes (Fig. 1D). Intestinal caecum dorsal to oesophagus, with narrow lumen and extending forward for more than half the length of the oesophagus (Fig. 1A). Nerve-ring surrounding oesophagus at posterior level of its first fourth, excretory pore slightly posterior to it. Deirids slightly posterior to excretory pore; short and stubby (Fig. 2B). Anterior width of intestine similar to combined width of caecum and ventriculus (Fig. 1D).
Ingwenascaris sprenti n. sp. ex Crocodylus niloticus. A, Anterior extremity, female, ventral view, note excretory pore (arrowhead) and deirids (arrows); B, Head region, female, apical view (B1), dextral view (B2), dorsal view (B3) and ventral view (B4), note lateral alae (arrowheads); C, Transverse section at level of posterior half of oesophagus, note oesophagus and intestinal caecum, female; D, Region of oesophago-intestinal junction, lateral view, note slightly lobed ventriculus; E, Posterior extremity male, dextral view; F, Tail, male, dextral view (F1) and ventral view (F2), note phasmids (arrowheads) and caudal ala (arrow); G, Right spicule, dextral view, note end of handle (arrowhead); H, Gubernaculum, dextral view (H1) and dorsal view (H2); I, Terminal part of vagina, note folds (at level of arrowhead) delimiting border between vagina vera and vagina uterina; J, Vagina and uteri, dissected; note vulva (arrow); K, Distal part of uterus with seminal receptacle and proximal part of oviduct with spermatheca (arrow); L, Tail, female, ventral view (L1) and dextral view (L2), note caudal alae (arrows) and phasmids (arrowheads); M, Egg
Ingwenascaris sprenti n. sp. ex Crocodylus niloticus. A, Cephalic region, female, dorsal view (A1) and lateral view (A2), note cuticular elevation between subventral and dorsal lip (asterisk); B, Deirid; C, Somatic papilla; D, Region of vulva, note vulva (arrow), position of somatic papillae (arrowheads) and lateral alae; E, Right postdeirid; F, Tail with protruding spicule, dextral view, note papillae (arrowheads) and phasmid (arrow); G, Cloacal region, subventral view, note complex single ventral precloacal papilla (arrowhead) and three pairs of adcloacal papillae
Male [Based on ten males.] Body 10.3–12.9 (11.3) mm long. Maximum width of body 354–499 (403) near midbody; body width at cloaca 107–133 (123). Cuticle c.6–8 thick in dorsal and ventral region, distance between transverse striations c.12–14 at level of midbody. Lateral alae with maximum width up to 30 at level of oesophagus. Left and right postdeirid situated dorsally to lateral midline at 6.3 mm and 3.9 mm from anterior extremity, respectively, in specimen 11.0 mm long. Total length of oesophagus, including ventriculus, 1,795–2,283 (2,005), i.e. 17.1–18.9 (18.0)% of body length; ventriculus 86–114 (96) long, 122–170 (140) wide. Caecum 1,148–1,522 (1,278) long, i.e. 58.9–70.2 (63.7)% of total oesophagus length; width at base 121–214 (147). Nerve-ring, excretory pore and deirids at 540–628 (570), 590–838 (667) and 648–885 (767), respectively, from anterior extremity. Reflexion of testis 1–520 (207) posterior to oesophago-intestinal junction. Ventral precloacal cuticle starting at 1,558–2,581 (2,113) from tail tip, extending posteriorly to approximately level of cloaca, comprising bands of thickened cuticle c.15–31 wide; distance between bands 16–31. Narrow lateral caudal alae extending from approximately level of cloacal aperture posteriorly to approximately level of last pair of caudal papillae (Fig. 1E, F). Caudal papillae comprising a complex median structure anterior to rim of cloaca and 10 pairs of papillae. Median semi-circular protuberance anterior to cloaca bears four papilla-like structures arranged in a transverse line on a rectangular field (Fig. 2G). Ten pairs of papillae arranged as follows (Figs. 1E, F, 2F): 3 pairs of subventral precloacal papillae, spaced widely apart; 3 pairs of subventral paracloacal papillae in straight line adjacent to cloaca, 1 just anterior to cloacal opening, 1 at cloacal opening and 1 just posterior to it; 3 pairs of subventral postcloacal papillae on posterior half of tail, and 1 lateral postcloacal pair situated at level of first subventral pair. Phasmids lateral, on level of second subventral pair of postcloacal papillae. Spicules equal, 816–984 (928) long, including bipartite handle 109–143 (123) long, with narrow most proximal part followed by wider part; handle immediately followed by 49–108 (80) long expansion of blade; blade alate, alae terminating in distal sixth of its length (Fig. 1G). Gubernaculum well-sclerotised, 197–227 (207) long, slender, with rounded distal tip in lateral view and pointed tip in dorsoventral view (Fig. 1H). Tail curved ventrally, with elongated, pointed tip; 298–374 (339) long or 2.5–3.5 (3.0)% of body length.
Female [Based on ten gravid females; the number of measurements, where different from ten, is included in parentheses.] Body 11.8–14.6 (13.1) mm long. Maximum body width 440–550 (507); width near vulva 362–530 (430). Cuticle c.7–9 thick in dorsal and ventral region, distance between transverse striations c.12–15 at level of vulva. Lateral alae with maximum width up to 35 at level of oesophagus. Caudal alae originating at short distance anterior to anus and terminating shortly before tail tip (Fig. 1L). Left and right postdeirid situated dorsally to lateral midline at 7.5 mm and 4.6 mm from anterior extremity, respectively, in specimen 14.0 mm long with vulva situated at 4.3 mm from anterior extremity. Nerve-ring, excretory pore and deirids at 565–688 (613), 649–730 (686) and 754–940 (864), respectively, from anterior extremity. Total length of oesophagus, including ventriculus, 1,993–2,471 (2,228), or 15.9–18.2 (17.0)% of body length; ventriculus 80–113 (95) long, 131–185 (145) wide. Caecum 1,172–1,561 (1,379) long, i.e. 52.5–66.6 (61.9)% of total oesophagus length; 140–219 (170) wide at base. Rectum short, conical, with thickened walls (Fig. 1L). Tail slender, elongate, straight or slightly curved ventrally, 652–830 (744) long or 5.2–6.1 (5.7)% of body length, with short terminal spike (Fig. 1L). Phasmids at 175–241 (208) from tail tip. Vulva pre-equatorial, at 3,452–4,039 (3,724; n = 9) from anterior extremity or 26.7–29.6 (28.2; n = 9)% of body length. Vagina directed posteriorly, composed of muscular vagina vera, 300–355 long (n = 2) and vagina uterina 2,160–2,240 (n = 2) long, gradually expanding in distal direction (Fig. 1I). Distal quarter of vagina uterina lined with dense layer of delicate microvilli obscuring eggs (Fig. 1J). Uteri two, amphidelphic, slightly shorter than vagina uterina; their distal part modified into seminal receptacle covered by microvilli; microvilli less dense than those observed in distal part of vagina uterina (Fig. 1J). Oviducts long, tubular with high columnar epithelium; proximal enlargement, probably serving as additional spermatheca, present (Fig. 1K). Eggs ovoid to round in uterus (round when dissected free), thin shelled, 60–77 × 51–66 (67 × 59), at morula-stage (Fig. 1M).
Discussion
The present material conforms to the characters of the family Heterocheilidae, as defined by Sprent (1980a) as subfamily and restored to family rank by Fagerholm (1991) in the following characters: morphology of the cephalic end, deirids and excretory pore situated near nerve-ring, presence of lateral alae, oesophagus with posterior lobed glandular ventriculus, well-developed intestinal caecum, pre-equatorial vulva, uterus didelphic-amphidelphic, spicules complex with distinct handle and blade, blade with alate midsection but bare tip, and well-sclerotised gubernaculum.
Currently the following ten genera are included within the family Heterocheilidae (the genus name is followed by the number of species in parentheses): Heterocheilus Diesing, 1839 (n = 2; Sprent, 1980a, 1983a), Typhlophoros von Linstow, 1906 (n = 2; Sprent, 1999), Trispiculascaris Skrjabin, 1916 (n = 2; Skrjabin, 1916; Sprent, 1983b), Multicaecum Baylis, 1923 (n = 2; Sprent, 1979; Mašová et al., 2010), Paradujardinia Travassos, 1933 (n = 1; Sprent, 1980a), Dujardinascaris Baylis, 1947 (n = 21; Sprent, 1977, 1990; Machida et al., 1992; Sprent et al., 1998; Moravec & Jirků, 2014), Brevimulticaecum Mozgovoy in Skrjabin, Shikobalova & Mozgovoy, 1951 (n = 9; Sprent 1979, 1990; Mašová et al., 2010), Hartwichia Chabaud & Bain, 1966 (n = 1; Chabaud & Bain, 1966), Ortleppascaris Sprent, 1978 (n = 4; Sprent, 1978; Zhao et al., 2016) and Krefftascaris Sprent, 1980 (n = 2; Sprent, 1980b; Tkach et al., 2010).
The genus Trispiculascaris was erected to accommodate Ascaris helicina Molin, 1860 sensu Skrjabin, 1916, collected from an unspecified crocodilian in East Africa (Skrjabin, 1916). Later on, the material described by Skrjabin was named Trispiculascaris trispiculascaris Travassos, 1920, and placed within the family Ascaridae (see Travassos, 1920). A number of authors (Hartwich, 1957, 1974; Chabaud, 1965), considered the genus a possible junior synonym of Dujardinascaris, speculating that Skrjabin (1916) had failed to observe the intestinal caecum and ventriculus. Sprent (1983b) recognised Trispiculascaris as a valid genus within the Heterocheilinae and assigned another crocodilian species to it, T. assymmetrica. The latter had been described, as P. assymmetricum, based on two females obtained from the stomach of a not further identified crocodile from Central Africa (Ortlepp, 1932). In order to study the genital tract as well as the caecum and ventriculus, Ortlepp (1932) had dissected one of the two females, but Sprent (1983b) re-examined the remaining intact specimen and used this to supplement Ortlepp’s (1932) description.
The present specimens correspond to females of T. assymmetrica as described by Ortlepp (1932) and Sprent (1983b) in the following characters: pronounced asymmetry of subventral lips; dorsal lip distinctly larger than subventral ones; each lip bearing numerous conspicuous triangular denticles along free margins; pulp of dorsal lip divided into two prominent lateral extensions as well as two anterior extensions; presence of lateral alae, originating as extension of posterior part of subventral lips; caecum extending for more than half the oesophageal length; ventriculus being shorter than wide, with short rounded lobes; excretory pore situated just posterior to nerve-ring; vulva pre-equatorial, situated within first third of body; expansion of vagina into saccular egg chamber, followed by short duct leading into two uteri; egg size; shape and length of tail, presence of caudal alae on the tail; relative position of conspicuous phasmids on tail (see figure 13 in Sprent, 1983b).
The main distinguishing character between the present specimens and T. assymmetrica, however, is the absence of distinct interlabia in the present material, as confirmed by our SEM studies, even though a slight cuticular thickening could be observed between the dorsal and subventral lips in some of the specimens. Contrary to this, both Ortlepp (1932) and Sprent (1983b) mention and/or illustrate the presence of small but distinct interlabia between the dorsal and subventral lips, 40 µm long after Sprent (1983b), while the ventral interlabium is absent. In addition, the females described here differ from those of T. assymmetrica in their smaller body dimensions (11.8–14.6 vs 16.0 mm long and 440–550 vs 700 µm wide), shorter tail (652–830 vs 850 µm) and longer lateral alae extending past the vulva to midbody vs one quarter of the body length (Ortlepp, 1932; this study).
Unfortunately, it was not possible to re-examine the head structures of T. assymmetrica in the present study; the dissected specimen remaining in the NCAH (T2052) does not include the cephalic extremity, and while the specimen catalogue of Professor John F. A. Sprent shows that the intact female was returned to South Africa via airmail on 19 December 1976 (personal communication Dr Mal Bryant, Queensland Museum, Australia, 2017), the specimen could not be located in the NCAH. In the absence of suitable type-specimens for comparison, and given the importance of the absence or presence of interlabia as a taxonomic character, as well as the above mentioned differences, the present specimens are considered a species related to, but distinct from T. assymmetrica. Based on the newly acquired morphological data, we propose to erect a new genus, Ingwenascaris, with Ingwenascaris sprenti n. sp. described from the Nile crocodile as type-species and further assign to it Ingwenascaris assymmetrica (Ortlepp, 1932) n. comb.
The original and only known description of Trispiculascaris, provided by Skrjabin (1916), clearly states that the oesophagus and intestine are “simple, without blind diverticula” and males have wide caudal alae. Contrary to this, Ingwenascaris n. g. possesses a caecum as well as a ventriculus and its caudal alae are narrow. Furthermore, Skrjabin (1916) did not describe the subventral lips as asymmetrical and did not mention the presence of lateral alae.
The systematic position of Trispiculascaris within the Heterocheilidae was adopted based on the assumption that the presence of an intestinal caecum and ventriculus were neglected by Skrjabin (1916). It is thus noteworthy that, in later works, Skrjabin et al. (1951) confirmed the morphology of Trispiculascaris by placing it within the subfamily Ascaridinae Railliet & Henry, 1912, which encompasses genera without ventriculus and intestinal caecum. Based on our current knowledge of the morphology of Trispiculascaris, its position within the Heterocheilidae is not well-grounded and we consider it a genus incertae sedis.
From the remaining heterocheilid genera, Ingwenascaris n. g. can be distinguished through a combination of various characters. The new genus resembles Krefftascaris, known from freshwater turtles, in the absence of distinct interlabia, the presence of lateral alae that originate at the base of the subventral lips and a lobed ventriculus, as well as the vulva being situated in the anterior third of the body of females (Sprent, 1980b; Tkach et al., 2010). However, Ingwenascaris n. g. is characterised by dorsal lips with alate margins and pronounced asymmetrical subventral lips with alate ventral margins but non-alate margins facing the dorsal lips, while in Krefftascaris the lips are non-alate and the subventral lips are symmetrical. Furthermore, the pulp of the dorsal lip of the latter is without prominent anterior or lateral extensions (Sprent, 1980b; Tkach et al., 2010).
Multicaecum is similar to the new genus in having lips with dentigerous ridges, but differs from Ingwenascaris n. g. in having lips that are non-alate, distinct postlabial grooves, the presence of three interlabia, cervical alae that anteriorly do not reach the subventral lips, and the lack of caudal alae in both sexes (Sprent, 1979; Mašová et al., 2010).
Ingwenascaris n. g. is readily distinguished from Hartwichia, Heterocheilus and Typhlophoros by the absence of longitudinal cuticular ridges or plate-like prominences in the interlabial region, which is a prominent character shared by the species of the latter three genera (Chabaud & Bain, 1966; Sprent, 1980a, 1983a, 1999).
Both Dujardinascaris and Paradujardinia, possess lips with interlocking processes (Sprent, 1977, 1980a, 1990; Machida et al., 1992; Sprent et al., 1998; Li et al., 2014; Moravec & Jirků, 2014), structures that are absent in Ingwenascaris n. g. In addition, members of Dujardinascaris and Paradujardinia have a rounded ventriculus without lobes or appendages vs a lobed ventriculus in Ingwenascaris n. g. Cervical alae are absent in Paradujardinia halicoris (Owen, 1833) (see Sprent, 1980a), and usually absent in Dujardinascaris spp. (Sprent et al., 1998), whereas lateral alae originate at the posterior border of the subventral lips in Ingwenascaris n. g. and extend beyond the level of the vulva towards the posterior border of the anterior half of the body. Furthermore, the present material differs from the two genera in the arrangement of the caudal papillae; three pairs of precloacal, paracloacal and postcloacal papillae each in Dujardinascaris (see Sprent et al., 1998), and four precloacal pairs and two para- and postcloacal pairs each in Paradujardinia (see Sprent, 1980a). Lastly, Paradujardinia is the only heterocheilid genus in which a gubernaculum is absent (see Sprent, 1980a).
In having lips with dentigerous ridges, Ingwenascaris n. g. differs from Ortleppascaris and Brevimulticaecum (Sprent, 1978, 1979, 1990; Khalil, 1984; Zhao et al., 2016). Furthermore, in Ortleppascaris the excretory pore is situated closer to the lips than to the nerve-ring (Sprent, 1978; Zhao et al., 2016), whereas in Ingwenascaris n. g. the excretory pore is situated posterior to the nerve-ring. Similarly, the excretory pore is situated in front of or at the level of the nerve-ring in representatives of Brevimulticaecum, and, other than in the new genus, a postlabial groove is present and interlabia are conspicuous (Sprent, 1978, 1979, 1990).
Ingwenascaris n. g. is one of seven genera of the Heterocheilidae parasitic in crocodilians, together with Brevimulticaecum, Dujardinascaris, Hartwichia, Multicaecum, Ortleppascaris and Typhlophoros (see Chabaud & Bain, 1966; Sprent, 1978, 1979, 1980a, 1990, 1999; Sprent et al., 1998). Crocodilians, including Crocodylidae, Alligatoridae and Gavialidae, constitute the largest group of hosts, but the two species of Krefftascaris have been reported from chelonians (Sprent, 1980a; Tkach et al., 2010), and two genera, Heterocheilus and Paradujardinia, are represented by two and one species, respectively, in sirenians (Sprent, 1980a, 1983a). In addition to reptilian and mammalian hosts, a single species of Multicaecum has been found in fish, one species each of Brevimulticaecum has been recorded from fishes and elasmobranchs (Mašová et al., 2010), and two species of Dujardinascaris are known to mature in fish (Moravec & Jirků, 2014). Nevertheless, of the 46 species presently included in the Heterocheilidae, 34 have been described in crocodilian hosts, suggesting that diversification of this family was especially successful in these ancient reptiles.
Key to the genera of Heterocheilidae
1a Lips with well-developed posterior prolongation; prominent interlabial longitudinal cuticular ridges or interlabial cuticular plate-like prominences present ……… 2
1b Lips without posterior prolongation; prominent interlabial longitudinal cuticular ridges or interlabial cuticular plate-like prominences absent; interlabia may be present or absent ……… 4
2a Vulva situated near anterior and middle third of body; gubernaculum weakly developed; parasites of sirenians ……… Heterocheilus
2b Vulva near mid-body; gubernaculum well-sclerotised; parasites of Old World crocodilians ……… 3
3a Males with six pairs of precloacal papillae and thin spicules with weakly developed alae ……… Hartwichia
3b Males with four pairs of precloacal papillae and spicules with well distinct handle and broad alate blade ………. Typhlophoros
4a Lips with lateral interlocking tooth-like prominences or processes ……… 5
4b Lips without lateral interlocking tooth-like prominences or processes ……… 6
5a Lips with tooth-like prominences interlocking with grooves on adjacent lips; males without gubernaculum; parasites of sirenians ……… Paradujardinia
5b Dorsal lip with two convex processes fitting to concave processes of subventral lips; right subventral lip with convex process articulating with concave process of left subventral lip; males with gubernaculum; parasites of crocodilians, known also from African bony fish (Mormyridae and Malapteruridae) ……… Dujardinascaris
6a Excretory pore closer to lips than to nerve-ring; parasites of New and Old World crocodilians ……… Ortleppascaris
6b Excretory pore near level of nerve-ring ……… 7
7a Lips without dentigerous ridges; parasites mainly of crocodilians, also in Neotropical freshwater stingray (Potamotrygonidae) and Australian bony fish (Osteoglossidae) ……… Brevimulticaecum
7b Lips with dentigerous ridges ……… 8
8a Subventral lips pronouncedly asymmetrical due to alate ventral margins and non-alate margins facing dorsal lip; lateral alae starting from base of subventral lips; known from African crocodilians ……… Ingwenascaris
8b Subventral lips symmetrical, similar in size and shape to dorsal lip ……… 9
9a Interlabia and distinct postlabial grooves absent; lateral alae originate at base of subventral lips; parasites of Australian freshwater chelonians (Chelidae) ……… Krefftascaris
9b Interlabia and deep postlabial groves present; lateral alae do not reach subventral lips; known from crocodilians in Africa, Asia and Australia, and African fishes (Arapaimidae) ……… Multicaecum
References
Anderson, R. C. (2000). Nematode parasites of vertebrates. Their development and transmission. 2nd edition. Wallingford: CAB International, 650 pp.
Chabaud, A. G. (1965). Ordre des Ascaridida. In: Grassé, P. P. (Ed.), Traité de Zoologie: Anatomie, Systématique, Biologie. Némathelminthes (Nématodes, Gordiacés), Rotifères, Gastrotriches, Kinorhynques. Tome IV, Fasc. III. Paris: Masson, pp. 932–1025.
Chabaud, A. G., & Bain, O. (1966). Description de Hartwichia rousseloti n. gen., n. sp., ascaride parasite de crocodile et remarques sur la famille des Heterocheilidae Railliet et Henry, 1912. Bulletin du Muséum National d’Historie Naturelle, 2e Série, 37, 848–853.
Doke, C. M., Malcolm, D. M., & Sikakana, J. M. A. (1977). English and Zulu Dictionary. Johannesburg: Witwatersrand University Press, 342 pp.
Eaton, M. J., Martin, A., Thorbjarnarson, J., & Amato, G. (2009). Species-level diversification of African dwarf crocodiles (genus Osteolaemus): a geographic and phylogenetic perspective. Molecular Phylogenetics and Evolution, 50, 496–506.
Fagerholm, H.-P. (1991). Systematic implications of male caudal morphology in ascaridoid nematode parasites. Systematic Parasitology, 19, 215–228.
Hartwich, G. (1957). Zur Systematik der Nematoden-Superfamilie Ascaridoidea. Zoologische Jahrbücher. Abteilung für Systematik, Ökologie und Geographie der Tiere, 85, 211–252.
Hartwich, G. (1974). Keys to genera of the Ascaridoidea. No. 2. In: Anderson, R. C., Chabaud, A. G. & Willmott, S. (Eds), CIH Keys to the Nematode Parasites of Vertebrates. Farnham Royal: CAB International, 15 pp.
Huchzermeyer, F. W. (2003). Crocodiles: biology, husbandry and diseases. Wallingford: CAB International, 352 pp.
ICZN (2012). International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bulletin of Zoological Nomenclature, 69, 161–169.
Junker, K, & Mutafchiev, Y. (2017). A new camallanid nematode, Micropleura huchzermeyeri n. sp. (Dracunculoidea: Micropleuridae), from the Nile crocodile, Crocodylus niloticus Laurenti (Reptilia: Crocodylidae), in South Africa. Systematic Parasitology, 94, 785–795.
Khalil, L. F. (1984). Brevimulticaecum scleropagi sp. nov. (Ascarididae: Nematoda) from the fish Scleropages jardini in Papua New Guinea. Journal of Natural History, 18, 797–802.
Li, L., Guo, Y.-N., & Zhang, L. P. (2014). Dujardinascaris gigantea sp. n. (Nematoda: Ascaridida) from the critically endangered crocodile Alligator sinensis Fauvel (Reptilia: Crocodylia). Parasitology Research, 114, 801–808.
Machida, M., Araki, J., Regoniel, P. A., & Pontillas, F. A. (1992). Three species of ascaridoid nematodes from crocodile in the Philippines. Bulletin of the National Science Museum. Tokyo Series A, Zoology, 18, 95–102.
Mašová, Š., Moravec, F., Baruš, V., & Seifertová, M. (2010). Redescription, systematic status and molecular characterisation of Multicaecum heterotis Petter, Vassiliadès et Marchand, 1979 (Nematoda: Heterocheilidae), an intestinal parasite of Heterotis niloticus (Osteichthyes: Arapaimidae) in Africa. Folia Parasitologica, 57, 280–288.
McAliley, L. R., Willis, R. E., Ray, D. A., White, P. S., Brochu, C. A., & Densmore, L. D. (2006). Are crocodiles really monophyletic? Evidence for subdivisions from sequence and morphological data. Molecular Phylogenetics and Evolution, 39, 16–32.
Moravec, F., & Jirků, M. (2014). A new ascaridoid nematode, Dujardinascaris mormyropsis sp. n. (Nematoda: Anisakidae), from the osteoglossiform fish Mormyrops anguilloides in Central Africa. Systematic Parasitology, 88, 55–62.
Moravec, F., & Kaiser, H. (1994). Brevimulticaecum sp. larvae (Nematoda: Anisakidae) from the frog Hyla minuta Peters in Trinidad. Journal of Parasitology, 80, 154–156.
Ortlepp, R. J. (1932). Two new ascarids from crocodiles. Journal of the South African Veterinary Medical Association, 111, 70–75.
Ross, C. A. (1989). Crocodiles and alligators. London: Merehurst Press, 240 pp.
Skrjabin, K. I. (1916). [Parasitic trematodes and nematodes collected by the expedition of Prof. V. Dogiel and I. Sokolov in British East Africa.] [Scientific results of the Zoological Expedition to British East Africa and Uganda made by Prof. V. Dogiel and I. Sokolov in the year 1914.] Petrograd, 1, 157 pp (In Russian).
Skrjabin, K. I., Shikhobalova, A. A., & Mozgovoi, A. A. (1951). [Oxyurata and Ascaridata.] In: Skrjabin, K. I. (Ed.) Opredelitel’ Paraziticheskikh Nematod. Vol. 2. Moscow: Izdatel’stvo Akademii Nauk SSSR, 631 pp (In Russian).
Sprent, J. F. A. (1977). Ascaridoid nematodes of amphibians and reptiles: Dujardinascaris. Journal of Helminthology, 51, 253–287.
Sprent, J. F. A. (1978). Ascaridoid nematodes of amphibians and reptiles: Gedoelstascaris n. g. and Ortleppascaris n. g. Journal of Helminthology, 52, 261–282.
Sprent, J. F. A. (1979). Ascaridoid nematodes of amphibians and reptiles: Multicaecum and Brevimulticaecum. Journal of Helminthology, 53, 91–116.
Sprent, J. F. A. (1980a). Ascaridoid nematodes of Sirenians - the Heterocheilinae redefined. Journal of Helminthology, 54, 309–328.
Sprent, J. F. A. (1980b). Ascaridoid nematodes of amphibians and reptiles: Angusticaecum and Krefftascaris n. g. Journal of Helminthology, 54, 55–73.
Sprent, J. F. A. (1983a). Ascaridoid nematodes of sirenians - a new species in the Senegal manatee. Journal of Helminthology, 57, 69–76.
Sprent, J. F. A. (1983b). Ascaridoid nematodes of amphibians and reptiles: Typhlophorus, Hartwichia and Trispiculascaris. Journal of Helminthology, 57, 179–189.
Sprent, J. F. A. (1990). Some ascaridoid nematodes of fishes: Heterocheilinae. Systematic Parasitology, 16, 149–161.
Sprent, J. F. A. (1999). Species of Typhlophoros von Linstow, 1906 (Nematoda: Ascaridoidea) in Old World crocodilians. Systematic Parasitology, 43, 229–236.
Sprent, J. F. A., McKeown, E. A., & Cremin, M. (1998). Dujardinascaris spp. (Nematoda: Ascaridoidea) in Old World crocodilians. Systematic Parasitology, 39, 209–222.
Tkach, V. V., Kuzmin, Y. I., & Snyder, S. D. (2010). Krefftascaris (Nematoda, Ascaridoidea) from Australian side-necked turtles with description of Krefftascaris sharpiloi sp. n. from Chelodina rugosa. Vestnik Zoologii, 44, 3–13.
Travassos, L. (1920). Contribuição para a sistematica dos Ascaroidea. Archivos da Escola Superior de Agricultura e Medicina Veterinaria, 4, 15.
Vieira, K. R. I., Vicentin, W., Paiva, F., Pozo, C. F., Borges, F. A., Adriano, E. A., et al. (2010). Brevimulticaecum sp. (Nematoda: Heterocheilidae) larvae parasitic in freshwater fish in the Pantanal wetland, Brazil. Veterinary Parasitology, 172, 350–354.
Walton, A. C. (1937). The Nematoda as parasites of amphibians. III. Studies on life histories. Journal of Parasitology, 23, 299–300.
Zhao, J. H., Wang, S. S., Tu, G. J., Zhou, Y. K., & Wu, X. B. (2016). Morphological and molecular characterization of Ortleppascaris sinensis sp. nov. (Nematoda: Ascaridoidea) from the Chinese alligator Alligator sinensis. Journal of Helminthology, 90, 303–311.
Acknowledgements
We are grateful to Dr. Danny Govender, Kruger National Park, South Africa, for facilitating and co-ordinating the crocodile study, and to Professor Joop Boomker, University of Pretoria, South Africa, for his invaluable help with the dissection of and preparation of aliquots from the gastrointestinal tracts. Thanks are also due to Mr. Daniel Chipana and Mr. Frans Masubelle, ARC-Onderstepoort Veterinary Institute, South Africa, for their help with parasite recovery from the ingesta. Dr. Mal Bryant, Queensland Museum, Australia, kindly assisted in trying to locate type material of I. assymmetrica n. comb. and has made the late Professor John F. A. Sprent’s collection records available to us.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Additional information
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as BD2D5457-E761-47D6-A405-226AF32C17BE. This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number. This is the Version of Record.
This article is part of the Topical Collection Nematoda.
Rights and permissions
About this article
Cite this article
Junker, K., Mutafchiev, Y. Ingwenascaris n. g. (Nematoda: Ascaridida: Heterocheilidae) established for I. sprenti n. sp. and I. assymmetrica (Ortlepp, 1932) n. comb., parasites of African crocodiles, and an identification key to the genera of the Heterocheilidae. Syst Parasitol 94, 849–859 (2017). https://doi.org/10.1007/s11230-017-9748-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-017-9748-y