Abstract
Most studies on dactylogyrid monogeneans in Argentina have been carried out during 1980s and 1990s. Many of these species have been later synonymised and other remain under a confusing taxonomic status, particularly those parasitising Cyphocharax voga (Hensel) (Teleostei: Curimatidae). In order to clarify the identity of dactylogyrids, new material was collected from fishes in Lake Chascomús, Buenos Aires Province, Argentina. A total of four species was found in the gills of C. voga. Two known species, Curvianchoratus singularis (Suriano, 1980) Suriano, 1986 and Palombitrema triangulum (Suriano, 1981) Suriano, 1997, are redescribed and their generic and specific status discussed, and two new species are described. Urocleidoides surianoae n. sp. can be distinguished from its congeners by having an anterior medial projection in the ventral bar and a laminar ligament connecting the base of the male copulatory organ and accessory piece. Annulotrematoides bonaerensis n. sp. differs from its congeners principally by having a ventral bar with an anterior medial projection. The diversity of dactylogyrids harboured by C. voga indicates the need of further studies in the Pampas region, which will provide interesting and valuable sources of evidence for future zoogeographical and evolutionary research on dactylogyrids in the Neotropics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most studies on monogenean parasites of freshwater fishes in Argentina have been carried out during the 1980s and 1990s, and were focused mainly on dactylogyrid parasites of Siluriformes and Characiformes from the River Paraná basin and shallow lakes in the Buenos Aires Province. During these years, a total of 23 species of monogenean were recorded and described (Suriano, 1980, 1981, 1983, 1985, 1986a, b, 1997a, b; Gutierrez & Suriano, 1992; Suriano & Incorvaia, 1995). Eleven of these species belong to the family Dactylogyridae and were recorded on fishes from a unique shallow lake from the Pampas region of Argentina, the Lake Chascomus (Suriano, 1980, 1981, 1983, 1986a, b, 1997b). Three of these species, Curvianchoratus singularis (Suriano, 1980) Suriano, 1986, Palombitrema triangulum (Suriano, 1981) Suriano, 1997 and Charachitecium chascomusensis (Suriano, 1981) Rossin & Timi, 2014, were described parasitising Cyphocharax voga (Hensel) (Characiformes: Curimatidae) from Lake Chascomús. This host was misidentified as Pseudocurimata gilberti (Quoy & Gaimard) by Suriano (1980, 1981) and then as Curimata gilberti (Quoy & Gaimard) by Suriano (1997b), both junior synonyms of Cyphocharax gilberti (Quoy & Gaimard) (see Vari, 1992). This species does not reach latitudes as southern as Lake Chascomús (Vari, 1992), where only C. voga has been reported (Colautti et al., 2015). All these three parasites of C. voga have had a complicate systematic history with none of them persisting with the original nomenclature. Curvianchoratus singularis was originally described as the type-species of Notodiplocerus Suriano, 1980, and later transferred by Suriano (1986a) to Curvianchoratus Hanek, Molnar & Fernando, 1974. Palombitrema triangulum was described by Suriano (1981) as Androspira triangula, the type-species of Androspira Suriano, 1981, and then transferred to Palombitrema Price & Busing, 1968 by Suriano (1997b). Simultaneously, A. chascomusensis Suriano, 1981 was described by Suriano (1981) from C. voga and Oligosarcus jenynsii (Günter) (Characidae) and later transferred to P. chascomusense by the same author (Suriano, 1997b); but recently, Rossin & Timi (2014) transferred this species to Characithecium Mendoza-Franco, Reina & Torchin, 2009 based on examination of material from O. jenynsii from different localities, including Lake Chascomús.
In order to clarify the identity of dactylogyrid monogeneans that parasitise C. voga, new material from Lake Chascomús was collected. Two species already described by Suriano (1980, 1981) were found in the gills, C. singularis and P. triangulum; both species are redescribed and their generic status is discussed. Additionally, two new species were discovered; these are also described in this paper.
Materials and methods
Thirteen specimens of C. voga, caught by trawl in Lake Chascomús (35°35′S, 58°01′W) Buenos Aires Province, Argentina, during May 2013 and May 2015, were examined for gill monogeneans. Gills were removed and shaken individually in vials containing hot water (70°C), and then the water was removed and replaced by formalin 4% for fixation. Monogeneans were removed from fixed gills and sediments with the aid of dissecting needles and droppers under stereoscopic microscopy. Some specimens were stained with Gomori’s trichrome and mounted in Canada balsam for study of their soft anatomy; other specimens were cleared and mounted in Hoyer’s mounting media (Thatcher, 2006) or in wet mounts using 10 % sodium dodecyl sulphate (SDS) (Wong et al., 2006) or subjected to proteolytic digestion to obtain tissue-free sclerites, using proteinase K solution (INBIO®) to an equal volume (1:1) of ATL lysis buffer enzyme (Qiagen®) for the study of sclerotised structures (Fannes et al., 2015). Drawings were made with the aid of a drawing tube attached to a light microscope equipped with differential interference contrast. Measurements were made by taking the distances in straight lines between extreme points of the structures measured (body length includes the haptor) following Mizelle & Klucka (1953) and Kritsky & Boeger (2002) and are given in micrometres as the range followed by the mean in parentheses. The distribution and numbering of hook pairs follow Mizelle (1936; see Mizelle & Price, 1963) and the procedure to determining the direction of the coil of the male copulatory organ (MCO) follows the suggestions of Kritsky et al. (1985). The type- and voucher specimens are deposited in the Helminthological Collection of the Museo de La Plata (MLP), La Plata, Argentina.
Family Dactylogyridae Bychowsky, 1933
Genus Annulotrematoides Kritsky & Boeger, 1995
Annulotrematoides bonaerensis n. sp.
Type-host: Cyphocharax voga (Hensel) (Characiformes: Curimatidae).
Type-locality: Lake Chascomús, Buenos Aires Province, Argentina (35°35′S, 58°01′W).
Type-material: Holotype MLP-He 7144 and 5 paratypes MLP-He 7145 were deposited in the Helminthological Collection of the Museo de La Plata, La Plata, Argentina.
Site on host: Gill filaments.
Etymology: The specific name alludes to the region where the type-locality of his species is situated, the Buenos Aires Province.
Description (Figs. 1–7, 32–36)
[Based on 10 specimens.] Small dactylogyrids with annulated body surface, 206–231 (218) long; greatest width 58–67 (62) usually at mid-body. Cephalic lobes 3 pairs, 1 lateral and 2 anterior. Head organs 3 pairs. Eyespots 4, frequently dissociated at level of pharynx; accessory granules often present in cephalic region, anterior to pharynx. Pharynx circular, muscular 11–14 (13) wide; oesophagus short. Haptor globose, 37–53 (44) long, 52–57 (55) wide. Ventral anchor 32–35 (34) long, base 14–16 (15) wide; with elongate superficial root, developed deep root, curved shaft, and curved, short distal point. Dorsal anchor 26–30 (28) long; base 10–14 (12) wide; with well-developed roots, superficial root elongate with transversal wing, curved shaft and straight point. Ventral bar 24–27 (25) long, with bi-lobed anteromedial projection and expanded ends. Dorsal bar widely U-shaped, 20–27 (22) long, with slightly enlarged ends, directed backwards. Hooks similar in shape and size, 12–28 (22) long, with protruding thumb and curved point; shank comprised of 2 subunits; proximal subunit expanded, filamentous hook loop extending anterior to union of shank subunits. MCO J-shaped, with expanded base and straight distal end, 25–28 (27) long. Accessory piece clamp-shaped, comprising 2 subunits; ventral subunit larger and serving as guide for MCO, presenting handle attached to laminar ligament articulating MCO. Total length of accessory piece including the ligament and handle 22–26 (24) long, accessory piece 14–19 (17) long. Gonads in tandem; testis post-ovarian; seminal vesicle a distal dilation of vas deferens; single prostatic reservoir present. Vitellarium moderately dense throughout trunk except in regions of reproductive organs; oviduct, oötype and uterus not observed. Vaginal aperture slightly sclerotised, sinistral; vaginal canal directed posteriorly, connecting with globular seminal receptacle. Seminal receptacle post-equatorial, anterior to germarium. Eggs not observed.
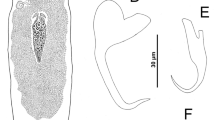
Urocleidoides triangulus (Suriano, 1981) n. comb. 16, Whole mount, ventral view; 17, Male copulatory complex, ventral view; 18, Vaginal sclerite; 19, Vagina, ventral view; 20, Ventral bar; 21, Dorsal bar; 22, Ventral anchor; 23, Dorsal anchor; 24, Hooks (a, hook 1; b, hook 4; c, hook 7; d, hook 5). Scale-bars: 16, 20 μm; 17–19, 5 μm; 20–24, 10 μm
Curvianchoratus singularis (Suriano, 1980). 25, Whole mount, composite drawing, ventral view; 26, Male copulatory complex, ventral view; 27, Ventral bar, ventral view; 28, Ventral anchor; 29, Hooks (a, pair 2; b, pair 6); 30, Dorso-median subunit; 31, Dorsal subunit. Scale-bars: 25, 25 μm; 26, 5 μm; 27, 28, 30, 31, 10 μm; 29, 2 μm
Annulotrematoides bonaerensis n. sp. 32, Ventral bar; 33, Dorsal bar; 34, Ventral anchor; 35, Dorsal anchor; 36, MCO. Figs. 37–43 Urocleidoides surianoae n. sp. 37, Ventral bar; 38, Dorsal bar; 39, Hook; 40, Ventral anchor; 41, Dorsal anchor; 42, Vaginal sclerite; 43, MCO. Scale-bars: 32–36, 38–40, 42–43, 10 μm; 37, 43, 20 μm
Remarks
Annulotrematoides Kritsky & Boeger, 1995 includes so far only four species parasitising characifor fishes in Brazil, i.e. A. amazonicus Kritsky & Boeger, 1995 from Psectrogaster rutiloides (Kner) (Curimatidae); A. bryconi Cuglianna, Cordeiro & Luque, 2003 from Brycon cephalus (Günther) (Characidae) and Salminus brasiliensis (Cuvier) (Characidae); and A. glossophallus Cohen, Kohn & Boeger, 2012 and A. parisellei Cohen, Kohn & Boeger, 2012 from Salminus brasiliensis (see Kritsky & Boeger, 1995; Cuglianna et al., 2003; Cohen et al., 2012). Although the morphology of the MCO (not coiled tube) of A. bonaerensis n. sp. closely resembles that of A. amazonicus, A. glossophallus and A. parisellei, the general morphology of the haptoral sclerites, especially the hooks and anchors, of the new species resembles more closely that of A. amazonicus. However, A. bonaerensis n. sp. can be easily distinguished from A. amazonicus by the morphology of the copulatory complex and ventral and dorsal bars. The MCO of A. amazonicus is an arcuate tube whereas the MCO of A. bonaerensis n. sp. is J-shaped, with expanded base and straight distal end. Finally, both A. bonaerensis n. sp. and A. amazonicus parasitise hosts belonging to the same fish family, the Curimatidae; this fact may explain the morphological similarities between these species.
Genus Urocleidoides Mizelle & Price, 1964
Urocleidoides surianoae n. sp.
Type-host: Cyphocharax voga (Hensel) (Characiformes: Curimatidae).
Type-locality: Lake Chascomús, Buenos Aires Province, Argentina (35°35’S, 58°01’W).
Type-material: Holotype MLP-He 7146 and 5 paratypes MLP-He 7147 were deposited in the Helminthological Collection of the Museo de La Plata, La Plata, Argentina.
Site on host: Gill filaments.
Etymology: The new species is named after Dr. Delia Mabel Suriano in recognition of her contribution to the knowledge of the dactylogyrid fauna of Argentina.
Description (Figs. 8–15, 37–43)
[Based on 10 specimens.] Body short and delicate 170–221 (193) (n = 9) long; 45–63 (55) wide, with annulated surface. Cephalic region with 2 more developed lateral lobes and 2–3 small anterolateral poorly developed lobes. Eyespots 2 pairs, anterior to pharynx; anterior pair smaller than posterior pair. Pharynx muscular, circular 11–18 (14) wide; oesophagus short. Haptor sub-hexagonal 32–49 (38) long, 41–54 (45) wide, with short peduncle. Anchors similar in shape, with developed roots; protruding superficial root with rounded flange on junction with deep root; deep root rectangular, well developed, shaft curved, with small point. Ventral anchor 28–35 (30) long, base 12–16 (14) wide; dorsal anchor 21–29 (25) long, base 10–14 (12) wide. Ventral bar straight, 22–30 (26) long, with expanded ends and elongate anteromedial process, 5–7 (6) long. Dorsal bar straight, 20–27 (24) long, with expanded bi-lobed ends and medial posterior constriction. Hooks dissimilar in size, each with depressed thumb, delicate point and straight shank, comprising 2 subunits (distal subunit expanded); hook pairs 1 and 5 reduced in size, 14–18 long, remaining 17–28 long; filamentous hook loop extending anterior to union of shank subunits. MCO reverse J-shaped, with expanded proximal end. Accessory piece complex, clamp-shaped, comprising 2 subunits; anterior subunit serving as guide for de MCO, posterior subunit spoon-shaped. Laminar ligament joins base of MCO and accessory piece. Total length of copulatory complex, 20–25 (23), MCO 22–29 (27) long, accessory piece 9–10 (9) long (n = 4). Testis post-ovarian, seminal vesicle a distal dilation of vas deferens; single prostatic reservoir present. Vitellarium moderately dense throughout trunk, except in regions of reproductive organs; oviduct, oötype and uterus not observed. Vaginal aperture slightly sclerotised, ventrolateral, sinistral; vaginal canal slightly sclerotised, connecting with globular seminal receptacle; vaginal sclerite present, robust, sinistral, long rod distally hooked, 22–25 (24) long. Eggs not observed.
Remarks
The new species can be readily differentiated from all other congeners by having a ventral bar with an anterior medial projection. Furthermore, most of the 20 species of Urocleidoides (sensu stricto) have a MCO not articulated to an accessory piece (Kritsky et al., 1986), although for U. reticulatus Mizelle & Price, 1964 and U. paradoxus Kritsky, Thatcher & Boeger, 1986, it has been proposed that the base of MCO “may articulate” with an accessory piece (Kritsky et al., 1986; Ergens & Moravec, 1989). Therefore the laminar ligament joining the base of the MCO and the accessory piece seems to be unique among congeners. On the basis on these differences, a new species, Urocleidoides surianoae n. sp. is proposed.
Urocleidoides triangulus (Suriano, 1981) n. comb.
Syns Androspira triangula Suriano, 1981; Palombitrema triangulum Suriano, 1997
Host: Cyphocharax voga (Hensel) (Characiformes: Curimatidae).
Locality: Lake Chascomús, Buenos Aires Province, Argentina (35°35’S, 58°01’W).
Voucher material: Five voucher specimens (MLP-He 7148) were deposited in the Helminthological Collection of the Museo de La Plata, La Plata, Argentina.
Site on host: Gill filaments.
Description (Figs. 16–24; 44–47)
[Based on 12 specimens.] Body small, delicate, 262–421 (320) (n =11) long; greatest width 50–73 (62) usually at mid-body. Cephalic lobes 1 pair. Cephalic glands not observed. Eyespots absent. Pharynx ovate, muscular 17–28 × 15–22 (20 × 17); oesophagus short. Haptor rhomboidal, 54–66 (60) long, 63–79 (70) wide. Ventral anchor with developed roots; superficial root more developed than deep root, shaft triangular, wider at base, narrowing abruptly in curved point. Dorsal anchor with well-developed superficial root and poorly developed deep root; shaft triangular ending in right point. Ventral anchor 33–42 (37) long, base 22–28 (25) wide; dorsal anchor 31–37 (34) long; base 22–26 (24) wide. Ventral bar straight with single pair of bi-lobed processes on each extremity, 20–24 (22) long. Dorsal bar robust, V-shaped, with prominent postero-median process; small suture present at distal end of process, 30–33 (31) long. Hooks dissimilar in shape, each with protruding thumb, delicate point and robust shank, pairs 1, 5 and 7 reduced in size, pair 7 barrel-shaped, with small distal bulbous base and curved point; pair 1, 14-17 (15) long; pair 5, 11–14 (13) long, pair 7, 11–13 (12) long, remaining hooks 24–32 (30) long. Filamentous hooklet loop extending anterior to union of shank subunits. MCO not articulated with accessory piece, a coiled tube with 2.5–3 counter-clockwise rings and bulbous base, 5–6 long, with prominent circular sclerotised brim, 2–3 (2) (n = 5) long. Accessory piece complex, comprising 2 subunits; internal piece fork-shaped; external piece curved, serving as guide for MCO, total length 18–23 (21). Gonads in tandem; testis posterior to ovary; seminal vesicle a distal dilation of vas deferens, looping before entering base of MCO; single prostatic reservoir present. Vitellarium moderately dense throughout trunk, except in regions of reproductive organs; oviduct, oötype and uterus not observed. Vaginal aperture slightly sclerotised, lateral, sinistral; vagina a long sclerotised tube, directed posteriorly and coiled before connecting with seminal receptacle. Seminal receptacle median, anterior to germarium. Vaginal sclerite present, sinistral, delicate and difficult to observe, flexible, curved rod with dilated base, 3–4 long, ending in a distal hook 12–17 (15) in total length. Eggs not observed.
Urocleidoides triangulus (Suriano, 1981) n. comb. 44, Haptoral complex; 45, Ventral anchor and dorsal bar; 46, Dorsal anchor and ventral bar; 47, Vaginal sclerite (black arrow), vagina (white arrow), 47a, vaginal sclerite. Figs. 48–50 Curvianchoratus singularis (Suriano, 1980). 48, Haptoral complex; 49, Dorsomedial anchor; 50, Dorsal anchor. Scale-bars: 44, 48, 20 μm; 45–47, 49, 50, 10 μm
Remarks
The detailed morphological examination of the newly-collected specimens on C. voga from the type-locality and their comparison with the descriptions of the species provided by Suriano (1981, 1997b) as Androspira triangula and later as Palombitrema triangulum, showed that these are conspecific by having identical morphology of the copulatory complex, hooks and internal organs. It is noteworthy that no type-material was deposited by the author for this species. The morphology of the copulatory complex and haptoral structures does not agree with the generic diagnosis of Palombitrema Price & Bussing, 1968. This genus is characterised by having the MCO directly articulated to the accessory piece, hook pairs 6 and 7 different in shape compared with hook pairs 1–5, pair 7 being also considerably larger, and dorsal anchors larger than ventral anchors and with poorly developed roots (Price & Bussing, 1968; Kritsky & Leiby, 1972; Kritsky & Thatcher, 1974; Mendoza-Franco et al., 1999, 2003, 2009). The present material, on the other hand, has the MCO not articulated to accessory piece and possesses a vaginal sclerite that was not observed in previous descriptions of the species. The presence of a vaginal sclerite, according to Kritsky et al. (1986), is a generic diagnostic character of Urocleidoides Mizelle & Price 1964 and recently Rosim et al. (2011) postulated that the presence of a ventral bar with enlarged ends is also a character that could be considered as diagnostic for this genus. Consequently, P. triangulum is transferred to Urocleidoides as U. triangulus (Suriano, 1981) n. comb. Currently, 20 valid species of Urocleidoides are recognised, all described on Neotropical freshwater fishes of nine families belonging to the orders Characiformes, Cyprinodontiformes and Gymnotiformes (see Moreira et al., 2015). Urocleidoides triangulus n. comb. can be easily differentiated from most congeners based on the general morphology of the anchors and by the characteristic shape of the dorsal bar (postero-median process). Only tree species of Urocleidoides possess a medial projection on the posterior margin of the dorsal bar, U. curimatae Molnar, Henek & Fernado, 1974, U. neotropicalis Mendoza-Franco & Reina, 2008 and U. piriatiu Mendoza-Franco & Reina, 2008 (see Mendoza-Franco & Reina, 2008). However, in all three species the medial projection is notably slender than bar arms, whereas the opposite relationship is observed in U. triangulus n. comb. Furthermore, U. triangulus n. comb. differs from both U. neotropicalis and U. piriatiu in having a triangular shaft of the ventral anchor. Urocleidoides curimatae shares this character with U. triangulus n. comb., but can be differentiated by the number of coils and the length of the MCO (3 coils and 125 µm in U. triangulus vs 1.5 coils and 16–19 µm in U. curimatae) as well as by the morphology of the accessory piece (Molnar et al., 1974). Kritsky et al. (1986) studied two paratypes of U. curimatae and confirmed that Molnar et al. (1974) confounded the vaginal sclerite as the vagina in these specimens; similarly, the presence of a vaginal sclerite was not observed by Suriano (1981, 1997b). The internal anatomy of U. curimatae was not confirmed by Kritsky et al. (1986); however, the gonads are described as ovate and the testis as post-ovarian in the original description (see Molnar et al., 1974). These features are in agreement with the internal morphology of U. triangulus n. comb. (see Kritsky et al., 1986).
Genus Curvianchoratus Hanek, Molnar & Fernando, 1974
Curvianchoratus singularis (Suriano, 1980) Suriano, 1986
Syn. Notodiplocerus singularis Suriano, 1980
Host: Cyphocharax voga (Hensel) (Characiformes: Curimatidae).
Locality: Chascomús Lake, Buenos Aires Province, Argentina (35°35′S, 58°01′W).
Voucher material: Five voucher specimens (MLP-He 7149) were deposited in the Helminthological Collection of the Museo de La Plata, La Plata, Argentina.
Site on host: Gill filaments.
Description (Figs. 25–31; 48–50)
[Based on 10 specimens.] Body short, robust, dorso-ventrally flattened, 272–393 (318) long; greatest width 84–177 (130) usually at mid-body. Tegument smooth. Cephalic lobes 3 pairs, one terminal and 2 lateral. Head organs (5 pairs) and cephalic glands present. Eyespots 4, anterior pair smaller than posterior pair. Pharynx oval, muscular 19–36 × 16–33 (27 × 25); oesophagus short. Haptor peduncle short, haptor sub-quadrate, 37–64 (51) long, 64–102 (79) wide. Haptoral architecture complex, with M-shaped ventral bar, 33–39 (36) in total length. Ventral anchor of dactylogyrid type, 20–26 (24) long, base 13–17 (14) wide; with moderately developed deep root and well-developed superficial root; shaft slightly curved, with straight distal point. Dorsal anchor greatly modified and composed of 2 subunits; dorso-median subunit total length 20–26 (24), greatest width 13–17 (14); dorsal subunit 28–46 (33) long, 5–8 (6) wide. Dorso-median subunit semicircular, with well-developed superficial and deep roots, both covered by small tubercles; dorsal protuberance present at base of subunit articulating with dorsal subunit; distal end sickle-shaped. A spoon-shaped piece articulates with distal part of dorso-median subunit, anterior to its sickle-shaped end, its handle is inserted in cavity of shaft, concave distal end of this piece harbours point of anchor. Dorsal subunit elongate, with well-developed deep root, covered by small tubercles; superficial root absent, distal point clamp-shaped. Hooks similar in size and shape, 10–15 (14) long, with small distal bulbous base, elongate slender shank, protruding thumb and curved shaft, filamentous hooklet loop about half shank length. MCO a coiled tube with 1.5 counter-clockwise coils, 61–100 (79) long; base of MCO moderately expanded, connected to accessory piece by ligament. Accessory piece 12–14 (13) long, pincer-shaped, composed of 2 subunits, one with expanded end and bifurcate point, the other serving as guide to MCO. Gonads overlapping, testis dorsal to germarium; seminal vesicle large, as distal dilation of vas deferens; single prostatic reservoir present. Vitellarium dense throughout trunk, except in regions of reproductive organs; oviduct, oötype and uterus not observed. Vaginal aperture strongly sclerotised, sinistral; vagina a long sigmoid sclerotised tube; seminal receptacle large, median, ventral to anterior part of germarium. Eggs not observed.
Remarks
A detailed morphological examination of the newly-collected specimens on C. voga at the type-locality and their comparison with the original description by Suriano (1980) showed that all are members of the same taxon by having identical morphology of the MCO, accessory piece, hooks and internal organs. It is noteworthy that no type-material of this species was deposited by Suriano (1980). Here, we provide a detailed description of the unique anchors that characterise this genus, unique into dactilogyrids, and used by Hanek et al. (1974) as diagnostic features to erect Curvianchoratus and describe the type-species, C. hexacleidus, a parasite of Steindachnerina argentea (Gill) (syn. Curimata argentea) from Trinidad.
The drawings provided in the original description of C. singularis by Suriano (1980), are oversimplified and lack many details of anchor morphology, omitting roots, the sickle-shaped point of the dorsal anchor, and the accessory piece articulating on its shaft. In the same way, the morphology of the copulatory complex is here re-described and details of the accessory structure and MCO are provided. Additionally, in the original description the vagina is described as dextral, when in fact it was observed in a sinistral position in all specimens studied. Curvianchoratus singularis, originally described as Notodiplocerus singularis by Suriano (1980), was later transferred to Curvianchoratus by Suriano (1986a); however no differential diagnosis regarding the only known congener, C. hexacleidus, was provided.
Using the detailed morphological data for the anchors and copulatory complex provided here, C. singularis can be distinguished from C. hexacleidus mainly by the morphology and morphometry of the MCO (2.5 coils and 80 µm in length vs 17–27 µm in length and curved), ventral bar (33–39 vs 16–21 µm), ventral anchor (20–26 vs 17–18 µm), dorsomedial subunit (45–50 vs 30–28 µm) and dorsal subunit (27–46 vs 26–27 µm). Anchor morphology cannot be compared between these species because no details are provided in the original description of C. hexacleidus.
Discussion
Despite the diversity of monogeneans in the Neotropics is still largely unknown, the Dactylogyridae is the most abundant taxon among reported monogeneans in the continental waters of South America (Thatcher, 2006; Cohen et al., 2013). In Argentina, approximately 35 species of dactylogyrid have been described in several host species from a reduced number of environments, namely rivers La Plata and Paraná, lakes Chascomús and Nahuel Rucá, and several Patagonian lakes (Cohen et al., 2013; Rossin & Timi, 2014), which represent only about 3% of the fish species that inhabit in the regions studied.
Besides the small number of species reported in Argentina, the knowledge of their morphology and taxonomy is still confusing in many aspects. Indeed, some species have complicated taxonomic histories, with many synonymies, incomplete descriptions and even misidentifications of host species (Rossin & Timi, 2014). Some other species need further study, for example, Urocleidoides travassosi (Price, 1938), reported from the gills of Pimelodella laticeps Eigenmann was considered a species incertae sedis by Kristky et al. (1986). The records of some species described for distant localities are also doubtful, e.g. Palombitrema heteroancistrium, a parasite of Astyanax fasciatus (Cuvier) from Costa Rica, Mexico and Panama (Price & Busing, 1968; Mendoza-Franco et al., 2009), which has been reported parasitising Astyanax sp. from Lake Chascomús (Suriano, 1997b). Therefore, clarification of the taxonomic status of many dactylogyrid species, previously described in the Pampas region, is necessary since in many studies carried out in other latitudes these species are compared morphologically in the descriptions of new taxa, included as valid species in checklists, and used as evidence for zoogeographical, phylogenetic or co-evolutionary analyses (Mendoza-Franco & Reina, 2008; Mendoza-Franco et al., 2003, 2007, 2009; Mendoza-Palmero et al., 2012; Cohen et al., 2013; Braga et al., 2014), with a considerable risk of generating erroneous conclusions. Further studies on other host species from the Pampas region, which constitutes the southern limit of distribution for many Neotropical fish families and whose monogenean faunas are virtually unknown, will provide interesting and valuable sources of evidence for future zoogeographical and evolutionary research on dactylogyrids in the Neotropics.
References
Braga, M. P., Araújo, S. B. L., & Boeger, W. A. (2014). Patterns of interaction between Neotropical freshwater fishes and their gill Monogenoidea (Platyhelminthes). Parasitology Research, 113, 481–490.
Cohen, S. C., Justo, M. C. N., & Kohn, A. (Eds) (2013). South American Monogenoidea parasites of fishes, amphibians and reptiles. Rio de Janeiro: Ed. Oficina de Livros, 663 pp.
Cohen, S. C., Kohn, A., & Boeger, W. A. (2012). Neotropical Monogenoidea. 57. Nine new species of Dactylogyridae (Monogenoidea) from the gill of Salminus brasiliensis (Characidae, Characiformes) from the Paraná River, State of Paraná. Brazil. Zootaxa, 3149, 57–68.
Colautti, D. C., Baigún, C. R. M., Llompart, F., Maiztegui, T., Garcia de Souza, J. R., Solimano, P., Balboni, L., & Berasain, G. E. (2015). Fish assemblage of a Pampean shallow lake, a story of instability. Hydrobiologia, 1, 175–186.
Cuglianna, A. M., Cordeiro, N. S., & Luque, J. L. (2003). Annulotrematoides bryconi sp. n. (Monogenea: Dactylogyridae) parasitic on Brycon cephalus (Osteichthyes: Characidae) from Brazil. Folia Parasitologica, 50, 272–274.
Ergens, R., & Moravec, F. (1989). Notes on Urocleidoides reticulatus Mizelle et Price, 1964 (Monogenea: Ancyrocephalinae). Folia Parasitologica, 2, 57–67.
Fannes, W., Vanhove, M. M. P., Huyse, T., & Paladini, G. (2015). A scanning electron microscope technique for studying the sclerites of Cichlidogyrus. Parasitology Research, 114, 2031–2034.
Gutierrez, P. A., & Suriano, D. M. (1992). Ancyrocephalids of the genus Dernidospermus Suriano, 1983 (Monogenea) parasites from siluriform fishes in Argentina, with descriptions of three new species. Acta Parasitologica, 37, 169–172.
Hanek, G., Molnar, K., & Fernando, C. H. (1974). Three new genera of Dactylogyridae (Monogenea) from freshwater fishes of Trinidad. Journal of Parasitology, 60, 911–913.
Kritsky, D. C., & Boeger, W. A. (1995). Neotropical Monogenoidea. 26. Annulotrematoides amazonicus a new genus and species (Dactylogyridae, Ancyrocephalinae), from the gills of Psectrogaster rutiloides (Kner) (Teleostei, Characiformes, Curimatidae) from the Brazilian Amazon. Proceedings of the Biological Society of Washington, 108, 528–532.
Kritsky, D. C., & Boeger, W. A. (2002). Neotropical Monogenoidea. 41: New and previously described species of Dactylogiridae (Platyhelminthes) from the gills of marine and freshwater perciform fishes (Teleostei) with proposal of a new genus and hypothesis on phylogeny. Zoosystema, 24, 7–40.
Kritsky, D. C., Boeger, W. A., & Thatcher, V. E. (1985). Neotropical Monogenea. 7. Parasites of the pirarucu, Arapaima gigas (Cuvier), with descriptions of two new species and redescription of Dawestrema cycloancistrium Price and Nowlin, 1967 (Dactylogyridae: Ancyrocephalinae). Proceedings of the Biological Society of Washington, 98, 321–331.
Kritsky, D. C., & Leiby, P. D. (1972). Dactylogyridae (Monogenea) from the freshwater fish, Astyanax fasciatus (Cuvier), in Costa Rica, with descriptions of Jainus hexops sp. n., Urocleidoides costaricensis, and U. heteroancistrium combs. n. Proceedings of the Helminthological Society of Washington, 39, 227–230.
Kritsky, D. C., & Thatcher, V. E. (1974). Monogenetic trematodes (Monopisthocotylea: Dactylogyridae) from freshwater fishes of Colombia, South America. Journal Helminthology, 48, 59–66.
Kritsky, D. C., Thatcher, V. E., & Boeger, W. A. (1986). Neotropical Monogenea. 8. Revision of Urocleidoides (Dactylogyridae, Ancyrocephalinae). Proceedings of the Helminthological Society of Washington, 53, 1–37.
Mendoza-Franco, E. F., Aguirre-Macedo, M. L., & Vidal-Martínez, V. M. (2007). New and previously described species of dactylogyridae (monogenoidea) from the gills of Panamanian freshwater fishes (teleostei). Journal of Parasitology, 93, 761–771.
Mendoza-Franco, E. F., Posel, P., & Dumailo, S. (2003). Monogeneans (Dactylogyridae, Ancyrocephalinae) of freshwater fishes from the Caribbean coast of Nicaragua. Comparative Parasitology, 70, 1–11.
Mendoza-Franco, E. F., & Reina, R. G. (2008). Five new species of Urocleidoides (monogenoidea) (Mizelle and Price 1964) Kritsky, Thatcher, and Boeger, 1986, parasitizing the gills of panamanian freshwater fishes. Journal of Parasitology, 94, 793–802.
Mendoza-Franco, E. F., Reina, R. G., & Torchin, M. E. (2009). Dactylogyrids (Monogenoidea) parasitizing the gills of Astyanax spp. (Characidae) from Panama and Southeast Mexico, a new species of Diaphorocleidus and proposal for Characithecium n. gen. Journal of Parasitology, 95, 45–55.
Mendoza-Franco, E. F., Scholz, T., Vivas-Rodríguez, C., & Vargas-Vázques, J. (1999). Monogeneans of freshwater fishes from cenotes (sinkholes) of the Yucatan Peninsula, Mexico. Folia Parasitologica, 46, 267–273.
Mendoza-Palmero, C. A., Scholz, T., Mendoza-Franco, E. F., & Kuchta, R. (2012). New species and geographical records of dactylogyrids (monogenea) of catfish (Siluriformes) from the Peruvian Amazonia. Journal of Parasitology, 98, 484–497.
Mizelle, J. D. (1936). New species of trematodes from the gills of Illinois fishes. American Midland Naturalist, 17, 785–806.
Mizelle, J. D., & Klucka, A. R. (1953). Studies on monogenetic trematodes. XIV. Dactylogiridae from Wisconsin fishes. American Midland Naturalist, 49, 720–733.
Mizelle, J. D., & Price, C. E. (1963). Additional haptoral hooks in the genus Dactylogyrus. Journal of Parasitology, 49, 1028–1029.
Molnar, K., Hanek, G., & Fernando, C. H. (1974). Ancyrocephalids (Monogenea) from freshwater fishes of Trinidad. Journal of Parasitology, 60, 914–920.
Moreira, J., Scholz, T., & Luque, J. L. (2015). First data on the parasites of Hoplias aimara (Characiformes): description of two new species of gill monogeneans (Dactylogyridae). Acta Parasitologica, 60, 254–260.
Price, C. E., & Bussing, W. A. (1968). Monogenean parasites of Costa Rican fishes. II. Proposal of Palombitrema heteroancistrium n. gen., n. sp. Proceedings of the Helminthological Society of Washington, 35, 54–57.
Rosim, D. F., Mendoza-Franco, E. F., & Luque, J. L. (2011). New and previously described species of Urocleidoides (Monogenoidea: Dactylogyridae) infecting the gills and nasal cavities of Hoplias malabaricus (Characiformes: Erythrinidae) from Brazil. Journal of Parasitology, 97, 406–417.
Rossin, M. A., & Timi, J. T. (2014). Dactylogyrid monogeneans parasitic on the Neotropical fish Oligosarcus jenynsii (Pisces: Characidae) from the Pampasic region, Argentina; with the emendation of the genus Characithecium. Zootaxa, 3893, 382–396.
Suriano, D. M. (1980). Notodiplocerus singularis gen. et sp. nov. (Monogenea Ancyrocephalinae) parasita de las branquias de Pseudocurimata gilberti (Pisces Tetragonopteridae) de la laguna de Chascomus, República Argentina. Neotropica, 26, 131–142.
Suriano, D. M. (1981). Androspira gen. nov. (Monogenea Ancyrocephalinae) parasito branquial de Pseudocurimata gilberti (Quoy & Gaimard, 1824) Fernández-Yepes, 1948 (Pisces Tetragonopteridae) de la Laguna de Chascomús, República Argentina. Neotropica, 27, 67–78.
Suriano, D. M. (1983). Dernidospermus anus gen. nov. sp. nov. (Monogenea: Ancyrocephalinae) parasita branquial de Loricaria (L) anus Valenciennes, 1840 (Pisces: Loricariidae) de la Laguna de Chascomus-Provincia de Buenos Aires-República Argentina. Neotropica, 29, 111–119.
Suriano, D. M. (1985). El género Unilatus Mizelle y Kritsky, 1967 (Monogenea: Ancyrocephalidae) parásito de Siluriformes (Pisces: Loricariidae) del Rio Negro, Manaus, Brasil. Neotropica, 31, 163–175.
Suriano, D. M. (1986a). Philocorydoras platensis gen. n. et. sp. n. (Monogenea: Ancyrocephalidae) from Corydoras paleatus (Jenyns) (Pisces: Callichthyidae) in Laguna Chascomús - República Argentina. Helminthologia, 23, 249–256.
Suriano, D. M. (1986b). The genus Urocleidoides Mizelle and Price, 1964 (Monogenea: Ancyrocephalidae). Anatomy and systematic position. Urocleidoides mastigatus sp. n. and U. travassosi (Price, 1934) Molnar, Hanek and Fernando, 1974 from Rhamdia sapo (Valenciennes, 1840) Eigenmann, 1888 and Pimelodella laticeps Eigenmann, 1917 (Pisces: Siluriformes) in Laguna Chascomus, República Argentina. Physis Secc. B, 44, 73–80.
Suriano, D. M. (1997a). The genus Urocleidoides Mizelle and Price, 1964 (Monogenea: Ancyrocephalidae) parasitizing characoidei fishes in Argentina. Physis, 53, 1–6.
Suriano, D. M. (1997b). Palombitrema heteroancistrium Price and Bussing, 1968 (Monogenea: Ancyrocephalidae) from Astyanax (A.) fasciatus fasciatus (Cuvier, 1819) (Pisces: Characidae) in Chascomu’s Lake, Argentina: anatomy and systematic position. Physis, 53, 7–10.
Suriano, D. M., & Incorvaia, I. S. (1995). Ancyrocephalid (Monogenea) parasites from siluriform fishes from the Paranean-Platean ichthyogcographical province in Argentina. Acta Parasitologica, 40, 113–124.
Thatcher, V. E. (2006). Amazon fish parasites, 2nd edn. Sofia, Bulgaria: Pensoft Publishers.
Vari, R. P. (1992). Systematics of the Neotropical Characiform genus Cyphocharax Fowler (Pisces: Ostariophysi). Smithsonian Contributions to Zoology, 529, 1–137.
Wong, W. L., Tan, W. B., & Lim, L. H. S. (2006). Sodium dodecyl sulphate as a rapid clearing agent for studying the hard parts of monogeneans and nematodes. Journal of Helminthology, 80, 87–90.
Acknowledgements
The authors wish to thank to Dr. Darío Colautti and his research team (Instituto de Limnología Raúl Ringuelet - CONICET) for providing fish samples from Lake Chascomús. We also thank Dr. Walter Boeger for his valuable comments and suggestions.
Funding
Financial support was provided by grants from the Agencia Nacional de Promoción Científica y Tecnológica, Argentina (ANPCYT- PICT # 2131-2010; CONICET-PIP # 112-201501-00973).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed. Permit for fishing provided by Ministerio de Asuntos Agrarios de la Provincia de Buenos Aires, Argentina (Disposición 164, August 23th, 2012).
Rights and permissions
About this article
Cite this article
Rossin, M.A., Timi, J.T. Dactylogyrid monogeneans parasitising Cyphocharax voga (Hensel) (Teleostei: Curimatidae) from the Pampas region, Argentina: new and previously described species. Syst Parasitol 93, 701–714 (2016). https://doi.org/10.1007/s11230-016-9654-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-016-9654-8