Abstract
Adults of both sexes of Naobranchia variabilis Brian, 1924 (Lernaeopodidae) are described based on material collected from the gill filaments of Balistes capriscus Gmelin, caught off the coast of Algeria. This is the second species of Naobranchia Hesse, 1863 to be found in the Mediterranean and the host record is new. Morphological comparisons are made with existing descriptions of N. variabilis and it is inferred, from small variations between material from different hosts and different localities, that N. variabilis may represent a species complex. The corrugated lobes on the head of Naobranchia females are interpreted as novel structures involved with temporary attachment during feeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Copepods are common and diverse parasites of marine fishes. The parasitic copepod fauna of Mediterranean marine fishes was reviewed by Raibaut et al. (1998) who recorded a total of 226 copepod species after examination of a total of 182 fish species. This list of copepod species included a few invalid species (nomina nuda) but since 1998 a significant number of additional species have been reported from Mediterranean fishes, including some new species (e.g. Hamza et al., 2007; Demirkale et al., 2014) as well as species which appear to have been brought into the Mediterranean on Red Sea invasive hosts (e.g. El-Rashidy & Boxshall, 2010, 2012). Within the Mediterranean region, sampling effort has been patchy and there are significant gaps in knowledge of the parasitic copepod fauna, especially along parts of the north coast of the African continent. The parasitic fauna of Algerian coastal fishes is relatively poorly known. Rose & Vaissière (1952, 1953) provided an early inventory for Algeria and several recent contributions have added to this list including Hamza et al. (2007), Ramdane & Trilles (2007), Hamza-Yousfi (2010) and Boualleg et al. (2011).
In this account we report the discovery of a species of Naobranchia Hesse, 1863 recovered from the gills of grey triggerfish Balistes capriscus Gmelin caught in Algerian coastal waters. To date, only a single species of this genus has been reported from the Mediterranean (Raibaut et al., 1998). The genus Naobranchia belongs to the family Lernaeopodidae (see Boxshall & Montú, 1997; Boxshall & Halsey, 2004) and is characterised by the modification of the paired maxillae of the adult female into two muscular, ribbon-like bands which secure attachment by encircling the gill filaments of the host. The genus Naobranchia currently comprises 21 valid species (Boxshall & Walter, 2014): N. alta Kabata, 1992; N. aulopi Yamaguti, 1939; N. auriculata Shiino, 1958; N. cygniformis Hesse, 1863; N. denticis Madinabeitia & Nagasawa, 2011; N. hemiconiati Nunes-Ruivo, 1963; N. kabatana Dippenaar & Jordaan, 2008; N. lizae (Krøyer, 1863); N. maxima Ho, 1975; N. microsoma Dojiri, 1981, N. occidentalis Wilson, 1915; N. pagelli Nunes-Ruivo, 1963; N. polynemi Tripathi, 1962; N. pritchardae Kensley & Grindley, 1973; N. sargi Nunes-Ruivo, 1963; N. scorpaenae Dojiri, 1981; N. smardis Nunes-Ruivo, 1963; N. spinosa Pearse, 1952; N. variabilis Brian, 1924; N. vermiformis Rangnekar, 1956; and N. wilsoni Nigrelli, 1933. Only N. cygniformis has previously been reported from the Mediterranean (Brian, 1906; Delamare Deboutteville & Nunes-Ruivo, 1952; Radujkovic & Raibaut, 1989; Raibaut et al., 1998). Here we record the discovery of N. variabilis as the second species to be reported and this is the first record of its occurrence in the Mediterranean.
Materials and methods
Host fishes, Balistes capriscus Gmelin, were purchased from a local fisherman at Tamentfoust on the Algerian coast, about 30 km to the east of Algiers, and transported to the laboratory for immediate examination. Parasites were removed from the gills under a dissection microscope and preserved in 70% ethanol. Parasites were cleared in a drop of lactic acid or lactophenol, and dissected using electrolytically-sharpened tungsten needles. Whole animals and dissected appendages were examined as temporary preparations on a Leitz Diaplan microscope equipped with differential interference contrast optics and drawings were made with the aid of a drawing tube. Morphological terminology follows Huys & Boxshall (1991). Host names follow FishBase (Froese & Pauly, 2014). All measurements are in millimetres and are presented as the range followed by the mean in parentheses.
Material for scanning electron microscopy (SEM) was washed in distilled water, dehydrated through a graded acetone series, critical point dried using liquid carbon dioxide as the exchange medium, mounted on aluminium stubs and sputter coated with gold-palladium. Coated material was examined on a Phillips XL30 FE SEM at 5 kV.
Voucher specimens are deposited in the collections of the Natural History Museum, London, UK (registration numbers NHMUK 2011.83–86); the remaining material is in the collection of the first author.
-
Family Lernaeopodidae H. Milne Edwards, 1840
-
Genus Naobranchia Hesse, 1863
Naobranchia variabilis Brian, 1924
Host: Balistes capriscus Gmelin (Tetraodontiformes: Balistidae).
Locality: Off Tamentfoust (east of Algiers, 36°47′N, 3°12′E) on Algerian coast.
Site on host: Gill filaments.
Material examined: 17 females and 10 males. Voucher material: 3 females (NHMUK 2011.83-85); 1 male (NHMUK 2011.86).
Description (Figs. 1–4)
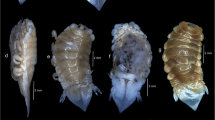
Female. Body length of adult female (Figs. 1A–C, 2F) 5.11–6.23 (5.63; n = 4), measured from tip of cephalothorax to posterior margin of abdomen. Cephalothorax 2.53–3.52 (2.94) long, cylindrical, tapering gradually towards rounded frontal margin. Head (Fig. 1C) small, bearing oral cone and mouthparts anteroventrally plus pair of corrugated lobes (Fig. 2A, B) located lateral to maxillipeds and protruding beyond margin of cephalothorax (Fig. 1B). Junction between base of cephalothorax and trunk marked by collar-like swelling, well-defined dorsally (Fig. 1A). Trunk dorsoventrally flattened, with rounded corners; wider than long, 1.60–2.00 × 1.88–2.35 (1.80 × 2.33). Egg-sacs (Fig. 1A–C) occupying entire lateral margins of trunk, extending from anterior margin of trunk posteriorly to project beyond tip of abdomen. Eggs enclosed within transparent membranous sacs, each supported by 3 paired ribbons originating as outgrowths from trunk: dorsal ribbon originating in posterior third and curving inwards towards posterior margin, ventral ribbon arising from ventrolateral margin, passing dorsally and dividing into anterodorsal and posterior tapering branches, posterior ribbon originating near abdomen. Abdomen (Fig. 1A) located mid-rear margin between egg-sacs, short, with median anal slit and minute paired processes representing digitiform and unarmed caudal rami.
Scanning electron micrographs of Naobranchia variabilis, adult female. A, Anterior part of cephalothorax, ventrolateral view; B, Corrugated lobe; C, Antennule and antenna; D, Mandible; E, Tip of mandible with corrugated lobe in background; F, Adult female, lateral view with maxillary bands extended. Scale-bars: A, F, 1 mm; B, 20 µm; C–E, 10 µm
Antennule (Figs. 1D, 2C) shorter than antenna; indistinctly 3-segmented, comprising stout cylindrical proximal segment armed with single spiniform setal element; narrower middle segment armed with sturdy process; terminal segment tapering into apical spine and bearing slightly shorter subapical spine. Antenna (Figs. 1E, 2C) biramous, indistinctly segmented, with large protopod bearing 2 unequal rami. Endopod 2-segmented, basal segment unarmed, terminal segment with 4 short spiniform setae and rounded process at tip. Exopod smaller, unsegmented, bearing 2 small setal vestiges.
Mandible (Figs. 1F, 2D, E) with 9 teeth; third tooth from tip markedly shorter. Maxillule (Fig. 1G) comprising tapering lobe bearing 2 long setae apically, plus shorter lateral seta at mid-length. Maxillae (Fig. 1B) ribbon-like, each maxilla separate, forming flattened band surrounding gill filament of host; each maxilla arising from and with tip fused to ovoid ventral pedestal separated from trunk surface by deep encircling groove; each maxilla containing 3 defined muscle bands.
Maxilliped (Figs. 1H, 2F, 4B) subchelate, comprising robust basal segment with myxal margin produced into spinous process opposing tip of claw and with papilla on surface of segment; subchela comprising incorporated endopod plus terminal claw; endopodal segment bearing small spinous process proximally and inner spine distally; terminal claw curved, unarmed.
Male. Adult male (Fig. 4A) attached to female dorsally, at base of egg-sacs. Body 0.42–0.48 (0.45; n = 6) long, with maximum width 0.40. Body unsegmented but comprising anterior cephalothorax covered by defined dorsal cephalothoracic shield (Fig. 4A) and posterior trunk tapering markedly to subterminal, genito-abdominal papilla; surface of trunk and papilla covered with finely wrinkled cuticle. Surface of dorsal cephalothoracic shield ornamented with marginal ring of sensory papillae (arrowed in Fig. 4C). Genito-abdomen with terminal anal slit and paired vestiges of caudal rami.
Antennule (Figs. 3A, 4C) similar to female, indistinctly 3-segmented, comprising elongate proximal segment bearing single spiniform setal element; narrower middle segment armed with sturdy process distally; terminal segment narrow, indistinctly separated from tapering apical spine. Antenna (Figs. 3B, 4C) biramous, similar to female; distal segment of endopod bearing only 3 spiniform setae and single rounded process at tip; exopod short with 2 small setal vestiges.
Mandible and maxillule as in female. Maxillae (Fig. 3E) subchelate; comprising robust bulbous proximal segment and terminal claw-like segment; tip of claw opposing spinulose lobe located on medial margin of basal segment.
Maxilliped (Fig. 3D) 3-segmented; first segment short, unarmed; middle segment robust and with medial surface produced into 2 irregular conical processes distally, third segment unarmed, bearing short terminal claw; tip of claw fitting into space between processes on second segment.
Remarks
Using the key to females of Naobranchia species provided by Madinabeitia & Nagasawa (2011) to confirm the identity of the Algerian material was problematic, since the first choice is based on relative length of cephalothorax to trunk. We consider that this character is likely to be unreliable as the cephalothorax appears to be contractile: in N. kabatana, for example, two females are figured by Dippenaar & Jordaan (2008), in one the cephalothorax is half as long as the trunk while in the other the cephalothorax is about the same length as the trunk. In the original description of N. variabilis Brian (1924) highlighted similar variability, mentioning that among the type-series was a female with a cephalothorax longer than the trunk. Indeed, Brian (1924) figured two females, one with a cephalothorax about as long as the trunk and another with a contracted cephalothorax, markedly shorter than the trunk.
Using Madinabeitia & Nagasawa’s key to species, if we regard the cephalothorax as about the same length as the trunk, then the Algerian material keys out as N. spinosa, due to the shape of the trunk (wider than long). If we treat the cephalothorax as shorter than the trunk, then the Algerian material keys out as N. variabilis. Finally, if we treat the cephalothorax as longer than the trunk, then the next choice is based on how much longer, and in the Algerian material the cephalothorax is only about 1.2 times longer, which does not correspond well to the choices offered (from 1.5 times up to 4 times). The closest species would be N. polynemi, which has a cephalothorax 1.5 times longer (and lateral egg-sacs).
Madinabeitia & Nagasawa (2011) recognised that three different egg-sac positions relative to the trunk were found within Naobranchia. The Algerian material has egg-sacs that lie along the entire lateral margins of the trunk, a character shared by eight species: N. variabilis, N. lizae, N. wilsoni, N. spinosa, N. vermiformis, N. polynemi, N. pritchardae and N. kabatana. The Algerian material can be readily distinguished from N. lizae, N. vermiformis, N. kabatana and N. polynemi, all of which have a trunk which is markedly longer than wide. Naobranchia pritchardae has a very distinctive shape, with a relatively short trunk bearing egg-sacs that are almost twice the length of the trunk, extending posteriorly well beyond the end of the trunk. The Algerian material has a broad (wider than long) trunk and it shares this with N. variabilis, N. wilsoni and N. spinosa.
Naobranchia spinosa is a small species, with a total length of only 2.8 mm for the adult female (Pearse, 1952a). The cephalothorax (length 1.2 mm) is shorter than the trunk (length 1.6 mm). The trunk is sub-circular in dorsal view, with egg-sacs that follow the curve of the trunk, and the abdomen is very small. No supporting ribbons on the egg-sacs are illustrated by Pearse (1952a) or mentioned in the text. This species differs from the Algerian material in its small body size, proportional length of cephalothorax and trunk, and the diminutive size of the abdomen.
The total body length of female N. wilsoni ranges from 3.5 to 6.2 mm (Nigrelli, 1935), very similar to that of the Algerian material (5.11–6.23 mm). The egg-sacs extend along the trunk forming rounded margins, and they are divided by what Nigrelli terms a “muscular ring”, although judging by the illustration, this structure probably represents a dorsal supporting ribbon even though Nigrelli states that the egg-sacs lack such “supporting ribs”. According to Nigrelli (1935), the maxillae are long and narrow and contain one longitudinal muscle strand. There are several differences between the Algerian material and N. wilsoni, the major one being the presence of the ovoid pedestal serving as the base for the maxillary bands in the former. In N. wilsoni the maxillae arise directly from the ventral surface of the trunk, with no trace of a pedestal. The shape of the trunk and the form of the supporting ribbons on the egg-sacs also differ.
The remaining species to compare in detail with the Algerian material is N. variabilis. The original description of N. variabilis was based on material collected from the gills of Lagocephalus laevigatus (L.) (Tetraodontidae) taken off the coast of Mauritania in the eastern Atlantic (Brian, 1924). It was subsequently reported from the western Atlantic (Bere, 1936; Pearse, 1952a, b; Causey, 1955), the Indian Ocean (Pillai, 1962) and Australia (Kabata, 1968; Roubal, 1999). The original description by Brian (1924) showed lateral and ventral views of the adult female habitus, the oral region of the female and the entire male (in ventral view) but the level of detail provided was rather generic. Bere (1936) provided a brief supplementary description based on material collected from several different hosts in the Gulf of Mexico. Little detail is available in Bere (1936) but she did illustrate a branching ventral ribbon supporting the egg-sacs, as present in the Algerian material. Pillai (1962) provided a detailed redescription based on Indian material from Lagocephalus inermis (Temminck & Schlegel). Kabata (1968) redescribed female N. variabilis, based on material from another tetraodontid host, Marilyna pleurosticus (Günther) (as Sphaeroides pleurosticus), taken off Queensland, Australia.
Using Kabata’s (1968) description as the basis for comparison reveals many similarities between the Algerian material and the Australian material of N. variabilis. The egg-sacs lie along the margins of the broad trunk and are supported by a similar pattern of ribbons in both species: a dorsal ribbon originating in posterior third, a ventral ribbon arising from ventrolateral margin, passing dorsally and dividing into anterodorsal and posterior branches, and a posterior ribbon. The maxillae arise on a broad pedestal-like structure illustrated by Kabata (1968, figure 5C). However, there are minor differences in mouthpart structure and armature: the tip of the antennule carries an accessory setal element in the Algerian material but not in the Australian material, the antennal rami have slightly different armature, the dentition of the mandible is slightly different, and the lateral seta is missing from the maxillule in Kabata’s Australian material. There are more striking differences between the Algerian material and the Indian specimens described by Pillai (1962), which may represent a distinct but related species. We tentatively identify the material from Algeria as N. variabilis and this is the first record of N. variabilis from the Mediterranean. However, we consider it likely that N. variabilis, as currently constituted, represents a complex of closely related species.
Most reported hosts of N. variabilis are tetraodontiform fishes: Lagocephalus laevigatus (see Brian, 1924), L. inermis (see Pillai, 1962), Marilyna pleurosticus (see Kabata, 1968), Chilomycterus spinosus (L.), C. shoepfii (Walbaum) (see Bere, 1936), and Tetractenos hamiltoni (Richardson) (see Roubal, 1999). The host in Algerian waters is also a tetraodontiform fish, Balistes capriscus (Balistidae), and this is a new host record. However, the range of hosts reported for N. variabilis is very broad and includes hosts from the Lophiiformes, Perciformes, and Clupeiformes: Ogcocephalus sp. (Ogcocephalidae) (Bere, 1936), Haemulon plumierii (Lacépède) (Haemulidae) (Pearse, 1952b), Diplectrum formosum (L.) (Bere, 1936; Pearse, 1952b) and Centropristis striata (L.) (Serranidae) (Pearse, 1952b), and Brevoortia patronus Goode (Clupeidae) (Causey, 1955). Such a wide range of hosts requires confirmation, especially given the rudimentary nature of the early descriptions. We recommend that new material collected from different geographical regions and from different hosts should be examined in order to further explore this apparently variable taxon.
Discussion
The origin and function of the paired cephalothoracic structures referred to here as corrugated lobes (Fig. 2A–B) are poorly understood. They are relatively large structures that appear almost spherical in life (Fig. 2B) and in N. variabilis they have a finely corrugated surface. The corrugations are better defined proximally and can been seen clearly in the background of Fig. 2E. In descriptive accounts of other species of Naobranchia these structures have been described as corrugated spheres (Dojiri, 1981; Ho & Kim, 1996), lateral swellings (Rangnekar, 1956), striated pads (Shiino, 1958), ear-like expansions (Shiino, 1958), striated processes (Kensley & Grindley, 1973), arcuate thickenings (Pillai, 1962), semi-circular cup-like structures (Lewis, 1967), semi-lunar thickenings with groove (Tripathi, 1962), oval sucker-like structure (Yamaguti, 1939), and adhesive lobes (Ho, 1975; Kabata, 1992; Dippenaar & Jordaan, 2008). These structures vary in size and can be very conspicuous, as in N. auriculata (see Shiino, 1958). Lewis (1967) compared the structures in his material with those found by Yamaguti (1939) and noted that the orientation of the cup-like structure was longitudinal in the Japanese N. aulopi but transverse in his Hawaiian Naobranchia sp.
We consider it likely that these structures are inflated and spherical in life, but are often collapsed or deflated in fixed material, or in dissections viewed under the pressure of a coverslip. When deflated they would appear cup-like or sucker-like and the orientation would vary according to the way they folded inwards. These paired structures do not appear to be derived from any existing paired cephalothoracic appendages, all of which are present. They are novel structures, unique to Naobranchia, and we know of no similar structures in other lernaeopodid genera. Corrugated lobe is an adequate descriptive term and we infer that these lobes serve to assist in securing the tip of the cephalothorax to the host surface during periods of feeding.
References
Bere, R. (1936). Parasitic copepods from Gulf of Mexico fish. American Midland Naturalist, 17, 577–625.
Boualleg, C., Kaouachi, N., Seridi, M., Ternango, S., & Bensouilah, M. A. (2011). Copepod parasites of gills of 14 teleost fish species caught in the gulf of Annaba (Algeria). African Journal of Microbiology Research, 5, 4253–4259.
Boxshall, G. A., & Halsey, S. H. (2004). An Introduction to Copepod Diversity. London: The Ray Society, 966 pp.
Boxshall, G. A., & Montú, M. A. (1997). Copepods parasitic on Brazilian coastal fishes: a handbook. Nauplius, 5, 1–225.
Boxshall, G. A., & Walter, T. C. (2014). Naobranchia Hesse, 1863. In: Walter, T. C. & Boxshall, G. A. (2014). World of Copepods database. Available through: World Register of Marine Species at http://www.marinespecies.org/ [accessed on 2014-11-25].
Brian, A. (1906). Copepodi parassiti dei Pesci d’Italia. Genova, 191 pp.
Brian, A. (1924). Parasitologia Mauritanica; Arthropoda (1ère partie), Copepoda. Copépodes commensaux et parasites des côtes mauritaniennes. Bulletin du comité d’Etudes Historiques et Scientifiques de l’Afrique occidentale française, 7, 364–427.
Causey, D. L. (1955). Parasitic copepoda from Gulf of Mexico fish. Occasional Papers of the Marine Laboratory of Louisiana State University, 9, 1–19.
Delamare Deboutteville, C., & Nunes-Ruivo, L. (1952). Copépodes parasites des poissons de Banyuls (2 série). Vie et Milieu, 3, 292–300.
Demirkale, I., Özak, A. A., Yanar, A., & Boxshall, G. A. (2014). Caligus solea n. sp. (Copepoda: Caligidae) parasitic on common sole, Solea solea (Linnaeus) from the north-eastern Mediterranean off the Turkish coast. Systematic Parasitology, 89, 23–32.
Dippenaar, S. M., & Jordaan, B. P. (2008). Description of the adult female and male of Naobranchia kabatana n.sp. (Copepoda: Lernaeopodidae) from Muraenesox bagio (Hamilton) (Muraenesocidae) caught in the Indian Ocean off South Africa. Systematic Parasitology, 70, 27–34.
Dojiri, M. (1981). Copepods of the families Lernaeopodidae and Naobranchiidae parasitic on fishes from Southern California inshore waters. Journal of Crustacean Biology, 1, 251–264.
El-Rashidy, H. H., & Boxshall, G. A. (2010). Parasitic copepods on immigrant and native clupeid fishes caught in Egyptian coastal waters off Alexandria. Systematic Parasitology, 76, 19–38.
El-Rashidy, H. H., & Boxshall, G. A. (2012). A new copepod (Siphonostomatoida: Lernanthropidae) parasitic on a Red Sea immigrant dragonet (Family Callionymidae), with a review of records of parasitic copepods from dragonets. Systematic Parasitology, 81, 87–96.
Froese, R., & Pauly, D. (Eds) (2014). FishBase. World Wide Web electronic publication. Available from http://www.fishbase.org (accessed on 26 November 2014).
Hamza, F., Boxshall, G. A., & Kechemir-Issad, N. (2007). A new species of Prohatschekia Nunes-Ruivo, 1954 (Copepoda: Hatschekiidae) parasitic on Scorpaena elongata (Cadenat) off Algeria. Systematic Parasitology, 67, 119–124.
Hamza-Yousfi, F. (2010). Les Copépodes parasites de poissons Téléostéens du littoral Algérien: biodiversité et aspects bioécologiques du parasitisme. Thèse de Doctorat d’état. Université des Sciences et de la Technologie Houari Boumediene, Alger, 368 pp.
Ho, J.-S. (1975). Copepod parasites of deep-sea fish off the Galápagos Islands. Parasitology, 70, 359–375.
Ho, J.-S., & Kim, I.-H. (1996). Copepod parasitic of Western North Pacific. Publications of the Seto Marine Biological Laboratory, 37, 275–303.
Huys, R., & Boxshall, G. A. (1991). Copepod Evolution. London: The Ray Society, 468 pp.
Kabata, Z. (1968). Copepoda parasitic on Australian fishes VIII. Families Lernaeopodidae and Naobranchiidae. Journal of Natural History, 2, 505–523.
Kabata, Z. (1992). Copepoda parasitic on Australian fishes, XIV. An assemblage of bathypelagic species. Journal of Natural History, 26, 9–45.
Kensley, B., & Grindley, J. R. (1973). South African parasitic Copepoda. Annals of the South African Museum, 62, 117–118.
Lewis, A. G. (1967). Copepod crustaceans parasitic on teleost fishes of the Hawaiian Islands. Proceedings of the United States National Museum, 121, 1–204.
Madinabeitia, I., & Nagasawa, K. (2011). Description of Naobranchia denticis n. sp. (Copepoda, Siphonostomatoida, Lernaeopodidae) parasitic on Dentex hypselomus (Teleostei, Sparidae) from Japanese waters, with a key to the species of Naobranchia. Crustaceana, 84, 461–476.
Nigrelli, R. F. (1935). Naobranchia wilsoni, a parasitic copepod from the gills of the porcupine fish, Diodon hystrix Linn. Transactions of the American Microscopical Society, 54, 52–56.
Pearse, A. S. (1952a). Parasitic Crustacea from the Texas Coast. Publications of the Institute of Marine Science, The University of Texas Port Aransas, Texas, 2, 5–42.
Pearse, A. S. (1952b). Parasitic crustaceans from Alligator harbor, Florida. The Quarterly Journal of the Florida Academy of Sciences, 15, 187–243.
Pillai, N. K. (1962). Copepods parasitic on south Indian fishes: families Lernaeopodidae and Naobranchiidae. Journal of the Marine Biological Association of India, 4, 58–94.
Radujkovic, B. M., & Raibaut, A. (1989). Parasites des poissons marins du Monténégro: copépodes. Acta Adriatica, 30, 237–278.
Raibaut, A., Combes, C., & Benoit, F. (1998). Analysis of the parasitic copepod species richness among Mediterranean fish. Journal of Marine Systems, 15, 185–206.
Ramdane, Z., & Trilles, J. P. (2007). Parasitic Copepods (Crustacea: Copepoda) from Algerian marine fishes. Zootaxa, 1574, 49–68.
Rangnekar, M. P. (1956). Parasitic copepods from the marine fishes of Bombay. Journal of the University of Bombay (B), 24, 42–65.
Rose, M., & Vaissière, R. (1952). Catalogue préliminaire des Copépodes de l’Afrique du Nord. Bulletin de la Société d’Histoire Naturelle de l’Afrique nord, 43, 83–99.
Rose, M., & Vaissière, R. (1953). Catalogue préliminaire des Copépodes de l’Afrique du Nord (II). Bulletin de la Société d’Histoire Naturelle de l’Afrique nord, 43, 164–176.
Roubal, F. R. (1999). Extent of gill pathology in the toadfish Tetractenos hamiltoni caused by Naobranchia variabilis (Copepoda: Naobranchiidae). Diseases of Aquatic Organisms, 35, 203–211.
Shiino, S. M. (1958). Two copepods of the Family Naobranchiidae, parasitic on Japanese fishes. Annual Report of the Prefectural University of Mie, Natural Sciences, 2, 114–119.
Tripathi, Y. R. (1962). Parasitic copepods from Indian fishes. IV. Achtheriformes. Proceedings of the First All-India Congress of Zoology, 2, 218–233.
Yamaguti, S. (1939). Parasitic copepods from fishes of Japan. Part 6. Lernaeopodoida, I. Volumen Jubilare Professor Sadao Yoshida, 2, 529–603.
Acknowledgements
The first author would like to thank the Natural History Museum for use of facilities during her study visit in November 2014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamza, F., Kechemir-Issad, N. & Boxshall, G.A. Redescription of Naobranchia variabilis Brian, 1924 (Siphonostomatoida: Lernaeopodidae), parasitic on the grey triggerfish Balistes capriscus Gmelin in Algerian coastal waters. Syst Parasitol 91, 157–165 (2015). https://doi.org/10.1007/s11230-015-9564-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-015-9564-1