Abstract
A new species of parasitic copepod of the family Lernanthropidae is described from an immigrant population of the blotchfin dragonet Callionymus filamentosus Valenciennes (family Callionymidae) in the Eastern Mediterranean. Both sexes are described on the basis of material caught in Egyptian waters off the Alexandria coast at Abuqir. The new species shares with Lernanthropus breviculus Kabata, 1979 the possession of a small dorsal plate on the trunk that is so narrow at its origin that it does not overlap the bases of the fourth legs, which are therefore visible in dorsal view. These species differ in the shape of the cephalothorax and in the extent of the dorsal plate, which is shorter in the new species, revealing the caudal rami in dorsal view. Previous records of parasitic copepods utilising callionymids as hosts are reviewed: most belong to the families Pennellidae and Chondracanthidae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family Lernanthropidae Kabata, 1979 currently comprises about 150 species belonging to eight genera (Boxshall & Halsey, 2004), which are mostly found in warmer waters, with the number of species decreasing in higher latitudes (Kabata, 1979a). The genus Lernanthropus de Blainville, 1822 is the largest of the family, containing approximately 120 valid species, all of which parasitise marine fish hosts belonging to least 31 different teleost families (Boxshall & Halsey, 2004). In the Mediterranean the family was represented by 10 species, nine of Lernanthropus and one of Lernanthropinus Do, in Ho & Do, 1985, all treated as species of Lernanthropus by Raibaut et al. (1998). The Mediterranean species of Lernanthropus are known to utilise hosts from eight families: the Belonidae, Carangidae, Gempylidae, Moronidae, Mugilidae, Sciaenidae, Serranidae and Sparidae. The Mediterranean species of Lernanthropinus, L. trachuri (Brian, 1903), parasitises members of the Carangidae (see Raibaut et al., 1998). Recently, El-Rashidy & Boxshall (2009, 2010) recorded a single species belonging to Mitrapus Song & Shen, 1976 on two clupeids, Etrumeus teres (DeKay), an immigrant species from the Red Sea, and Sardinella aurita Valenciennes, a native Mediterranean species.
A species of Lernanthropus was found to be a common parasite of the blotchfin dragonet Callionymus filamentosus Valenciennes, an immigrant callionymid fish from the Red Sea (Golani et al., 2002), in waters off the Egyptian Mediterranean coast near Alexandria. The same species was also found on C. filamentosus caught off the Mediterranean coast of Israel. This is the first record of a member of the family Lernanthropidae parasitising a dragonet host. This new species of Lernanthropus is described below from specimens of both sexes, and records of parasitic copepods utilising dragonets as hosts are reviewed.
Materials and methods
Parasites were collected from the gill filaments of Callionymus filamentosus caught off Abuqir on the Alexandria coast. Copepods were picked off the gills, cleaned of mucus and preserved in 70% ethyl alcohol. They were then dissected, mounted in lactophenol as temporary slide preparations and examined using an Olympus compound microscope. Measurements were made using an ocular micrometer and drawings were made with the aid of a drawing tube. Morphological terminology follows Boxshall (1990) and Huys & Boxshall (1991). Host names were validated against FishBase (Froese & Pauly, 2011). Type-material is stored in the collections of the Natural History Museum, London and in the first author’s personal collection.
Family Lernanthropidae Kabata, 1979
Genus Lernanthropus de Blainville, 1822
Lernanthropus callionymicola n. sp.
Type-host: Callionymus filamentosus Valenciennes (Callionymidae).
Type-locality: Eastern Mediterranean waters off Abuqir, near Alexandria, Egypt.
Other localities: Mediterranean coast of Israel.
Site on host: Gills.
Type-material: Holotype female [BMNH 2011.1179]; 123 paratype females [10 females BMNH 2011.1180-1189] and 8 paratype males [2 males BMNH 2011.1190-1191]; remaining paratype material in collection of first author. This material was collected from 54 specimens of the host C. filamentosus caught on 18 November, 2010 and 24 February, 2011: the prevalence was 63% and the mean abundance was 3.9.
Other material examined: 10 females from C. filamentosus collected off the Mediterranean coast of Israel on 6 July, 2008 by B. Galil, M. Goren and A. Diamant: registration numbers of vouchers BMNH 2011.1231-1240.
Etymology: The species name refers to the host family.
Description
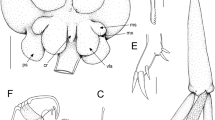
Lernanthropus callionymicola n. sp., female. A, habitus, dorsal; B, habitus, lateral; C, habitus, ventral; D, genito-abdomen and caudal rami, ventral; E, antennule; F, antenna, posterior; G, bifid process in membrane at basal articulation of subchela; H, blunt proximal process on subchela; I, dorsal plate of paratype with rounded posterior margin, dorsal; J, parabasal flagellum. Scale-bars: A-C, 0.5 mm; D,F, 100 μm; E,J, 25 μm; G,H, 10 μm; I, 0.25 mm
Lernanthropus callionymicola n. sp., female. A, tip of mandible showing marginal teeth; B, maxillule, ventral; C, maxilla, posterior; D, maxilliped, posterior; E, leg 1, anterior; F, leg 2, anterior; G, basal seta of leg 3; H, tip of exopod leg 4; I, basal seta of leg 4. Scale-bars: A,G,I, 10 μm; B,E,F,H, 25 μm; C, 50 μm; D, 100 μm
Body comprising cephalothorax and trunk (Fig. 1A–C); mean body length excluding fourth legs 2.32 ± 0.24 mm, range 1.8–2.6 mm (based on 15 specimens). Cephalothorax c.1.2 times longer than wide; lateral margins more or less parallel, expanded into ventro-laterally directed folds on either side of cephalothorax (Fig. 1C); antero-lateral corners of cephalothorax produced into slight lobes, forming weakly trilobate frontal margin (Fig. 1A). Trunk narrow, produced posteriorly into narrow dorsal plate, not overlapping bases of bilobate leg 4; dorsal plate covering entire abdomen but caudal rami visible in dorsal view; dorsal plate typically with nearly straight posterior margin (Fig. 1A), rounded in some specimens (Fig. 1I). Abdomen small, not clearly differentiated from genital complex (Fig. 1D), bearing paired, digitiform caudal rami on ventral surface. Each caudal ramus armed with 3 setae and 2 spine-like setal elements at apex (Fig. 1D).
Antennule (Fig. 1E) distinctly 6-segmented; some segments with irregular cuticular thickening; setal formula: 1: 3: 2: 2: 1: 12 + 2 aesthetascs. Parabasal flagellum (Fig. 1J) slender, located near of base of each antennule. Antenna (Fig. 1F) robust, comprising massive corpus, bearing papilliform element on medial surface, and distal subchela showing traces of suture line; subchela armed with small bifid process (Fig. 1G) in zone of arthrodial membrane in articulation between corpus and subchela, small distal seta and blunt proximal process (Fig. 1H). Mandible stylet-like, armed distally with 8 marginal teeth (Fig. 2A). Maxillule (Fig. 2B) bilobate; smaller outer lobe tipped with 1 spiniform element; larger inner lobe tipped with 3 unequal spiniform elements. Maxilla (Fig. 2C) 2-segmented, comprising proximal syncoxa (lacertus) and distal basis (brachium); basis ornamented with patch of spinules and single bifid process distally; terminal claw armed with sharp denticles on inner surface. Maxilliped (Fig. 2D) 2-segmented, comprising massive corpus, with papilliform element on medial surface, and distal subchela; subchela comprising compound endopodal segment, partly subdivided by incomplete suture line, and strongly curved terminal claw.
Leg 1 biramous (Fig. 2E), with outer seta and inner spine on inflated protopod; exopod 1-segmented, armed with 5 robust terminal spines with minutely serrate margins; endopod 1-segmented, tapering distally, armed with terminal seta and ornamented with patch of spinules distally. Leg 2 (Fig. 2F) mounted on inflated subspherical prominence derived from incorporated protopod and armed with outer seta: both rami 1-segmented; exopod armed with 5 robust terminal spines; endopod armed with small apical seta and ornamented with patch of spinules distally. Leg 3 uniramous, lacking exopod; endopodal lobe forming lamella protruding postero-ventrally; endopodal lamellae of leg pair fused along distal part of inner margins (Fig. 1B,C); leg 3 armed with dorsal outer basal seta (Fig. 2G). Leg 4 (Fig. 1A–C) modified, biramous but with rami forming elongate processes armed with basal seta (Fig. 2I); outer (exopodal) lobe elongate, tipped with small spine (Fig. 2H); inner (endopodal) lobe slightly shorter than exopodal lobe. Leg 5 absent.
Lernanthropus callionymicola n. sp., male. A, tip of mandible; B, maxillule, ventral; C, maxilla, posterior; D, maxilliped, posterior; E, leg 1, anterior; F, leg 2, anterior; G, tip of modified leg 3; H, tip of modified leg 4; I, basal seta of leg 3; J, basal seta of leg 4. Scale-bars: A,B,G,H, 25 μm; C,E,F, 50 μm; D, 100 μm; I,J, 10 μm
Body smaller than female (Fig. 3A), with total length ranging from 0.76–1.19 mm (based on 10 specimens). Cephalothorax large, nearly as long as trunk, broadest at middle, with evenly convex lateral margins (Fig. 3A,B). Frontal area of cephalothorax carrying antennules and antennae, defined by slight indentation. Trunk showing traces of boundaries between third and fourth pedigerous somites (Fig. 3A,B). Genito-abdomen small (Fig. 3C), fused to trunk; caudal rami bearing 2 long setae proximally, 2 shorter setae and robust apical spine (Fig. 3D).
Antennule 6-segmented (Fig. 3E); setal formula: 1: 3: 2: 2: 1: 12 + 2 aesthetascs. Parabasal flagellum as in female, located close to base of antennule (Fig. 3E). Antenna (Fig. 3F) comprising massive corpus and distal subchela consisting of distinct endopodal segment and terminal claw; corpus armed with spine medially; subchela armed with small seta and blunt element; small bifid process present in articulation between corpus and subchela. Mandible, maxillule, maxilla and maxilliped similar to those of female (Fig. 4A–D).
Leg 1 biramous (Fig. 4E), with outer seta and inner spine on basis; surface of both rami ornamented with spinules; exopod 1-segmented, armed with 5 terminal, minutely spinulose spines and ornamented with patch of spinules distally; endopod 1-segmented, armed with spinulose apical seta nearly twice as long as segment; segment extensively ornamented with spinules. Leg 2 (Fig. 4F) with outer seta on basis; both rami 1-segmented; exopod broadening distally, armed with 5 small distal spines and with ornamentation of 4 spinules; endopod tapering slightly distally, with ventral surface ornamented with spinules, armed with long spinulose seta apically, nearly as long as segment. Leg 3 (Fig. 3A,B) uniramous, forming long cylindrical process protruding ventrally from trunk, armed with dorsal basal seta (Fig. 4I); surface of leg 3 covered with papillae and tipped with 2 small spines (Fig. 4G). Leg 4 (Fig. 4A,B) uniramous, forming long cylindrical process, with small basal seta (Fig. 4J); leg 4 longer than leg 3 and covered with papillae similar to those on leg 3, tipped with small spine (Fig. 4H).
Remarks
The new species is unusual in possessing a small dorsal plate on the trunk which is so narrow at its origin that it does not overlap the bases of the elongate, bilobed fourth legs; these can therefore be seen in dorsal view. This remarkable character state serves to distinguish the new species from all other species of the genus except L. breviculus Kabata, 1979, which was originally recorded on the labrid Cheilinus chlorourus (Bloch) [as Cheilinus chlorurus], collected from off Queensland, Australia (Kabata 1979b). The new species and L. breviculus also both share an unusual type of third leg. The third leg is uniramous and the paired lamellate rami (endopodal lobes) are fused distally to form a transverse ventral plate, although they remain separate proximally in the ventral mid-line. A few other Lernanthropus species, such as L. brevicephalus Rangnekar, 1957, have uniramous third legs lacking exopodal lobes, and in species of Lernanthropinus Do, in Ho & Do, 1985, such as L. decapteri (Pillai, 1964), the endopodal lobes of the third legs are fused. However, all these other lernanthropid taxa possess a large dorsal plate covering the bases of leg 4 and extending posteriorly to cover most of the rami of leg 4.
Lernanthropus breviculus was originally described from a single female only, so Kabata (1979b) was unable to describe the cephalothoracic limbs in any detail. However, the three habitus drawings published in Kabata (1979b) provide sufficient characters to allow us to distinguish between these two species. L. breviculus has a somewhat hexagonal-shaped dorsal cephalothoracic shield with the widest point just posterior to its mid-level. In contrast, the cephalothorax of the new species has more or less linear lateral margins and is slightly produced at each antero-lateral corner. The dorsal plate on the trunk of L. breviculus covers the entire abdomen and caudal rami, whereas that of the new species is shorter and the caudal rami are visible in dorsal view. The relative lengths of the lobate rami of leg 4 also differ slightly; the exopod of leg 4 in L. breviculus is slightly shorter than the endopod, whereas the endopod is slightly shorter than the exopod in the new species.
Parasitic copepods on fishes of the family Callionymidae (dragonets)
Dragonets are infrequently reported as hosts for parasitic copepods, and Raibaut et al. (1998), in their analysis of parasitic copepod richness on Mediterranean fishes, categorised the Callionymidae as having a low level of species richness, together with families such as the Blenniidae (blennies) and Gobiidae (gobies). It is, however, difficult to be sure whether or not this is due to inadequate sampling of a fish family of low importance to commercial fisheries. FishBase (Froese & Pauly, 2011) lists 187 valid species in the Callionymidae, currently classified in 18 genera, of which Callionymus Linnaeus (101 species) and Synchiropus Gill (40 species) are the largest. Published records of copepods from all dragonets are collated here to ascertain whether this situation is local to the Mediterranean or is reflected globally:
-
Four species of the Chondracanthidae have been reported from dragonets: Acanthochondria fissicauda Shiino, 1955 was described from Repomucenus richardsonii (Bleeker) [as Callionymus richardsoni] taken in Japanese waters (Shiino, 1955); Chondracanthus ornatus T. Scott, 1909 was reported from Callionymus lyra Linnaeus and C. maculatus Rafinesque in European waters (Kabata, 1979a); Lagochondria nana Ho & Dojiri, 1989 was described from Callionymus sp. in Australian waters (Ho & Dojiri, 1988); and Reimer (1986) reported an unidentified species of Pseudacanthocanthopsis Yamaguti & Yamasu, 1959 from Synchiropus monacanthus Smith caught off the coast of Mozambique.
-
One species of the Taeniacanthidae, Irodes callionymi (Yamaguti, 1939), was described from Synchiropus altivelis (Temminck & Schlegel) [as Callionymus altivelis] taken in Japanese waters (Yamaguti, 1939). Reimer (1986) reported the same species, under its original combination of Anchistrotos callionymi Yamaguti, 1939, from an unidentified species of Callionymus caught off Mozambique. No description was provided and, given the wide separation of these two localities, the identity of this taeniacanthid should be confirmed.
-
At least six pennellid species, both larval stages and adult metamorphic females, have been reported from dragonets. Lernaeocera lusci (Basset-Smith, 1896) has been found repeatedly on the gills of C. lyra and C. reticulosus Valenciennes in European waters (e.g. Basset-Smith, 1896; Hansen, 1923; Scott, 1929; Schuurmans Stekhoven, 1936; Kabata, 1958; Boxshall, 1974), and Van Damme et al. (1993) concluded from quantitative analysis that these two hosts played a significant role in the life-cycle of L. lusci in the North Sea. A congener, L. branchialis Linnaeus, 1767, has also been reported from C. lyra (see Hamond, 1969; Kabata, 1979a) and from C. maculatus (see Palm et al., 1999) in northwestern European waters. Haemobaphes ambiguus T. Scott, 1900 occurs on C. maculatus, C. pusillus Delaroche [as C. festivus] and C. risso Lesueur [as C. belenus] (T. Scott, 1900; Delamare Deboutteville, 1950; Delamare Deboutteville & Nunes-Ruivo, 1955). Three species of Phrixocephalus C.B. Wilson, 1908 were described from Japanese waters: P. longicollum Shiino, 1956 and P. umbellatus Shiino, 1956 from the eyes of Repomucenus richardsonii [as C. richardsoni] and P. reniformis Shiino, 1956 from the eyes of C. japonicus Houttuyn [as Calliurichthys japonicus] (see Shiino, 1956).
-
There are isolated records of other families: the lernaeopodid Clavella quadrata (Basset-Smith, 1896) (as Anchorella quadrata) was described from Callionymus lyra (see Basset-Smith, 1896). Hamond (1969) reported this same parasite, under the binomen Clavellopsis quadrata (Bassett-Smith), from Callionymus lyra in the North Sea. This taxon was subsequently synonymised with Clavella adunca (Strøm, 1762) by Kabata (1979a). Kabata (1979a) considered that the single report of a caligid, Lepeophtheirus pectoralis (Müller, 1776), from Callionymus lyra (see Baird, 1850) could possibly have been the accidental result of host transfer within the trawl.
Relatively few parasitic copepod species have as yet been reported from callionymid hosts, but we consider that this may reflect the paucity of sampling. Members of the family Pennellidae have been the most commonly reported copepods from dragonets, and it is interesting to note that Uyeno & Nagasawa (2010) have recently described three new pennellid species from gobiid hosts, i.e. another family of similarly non-commercial, small and largely shallow-water fishes, just like the Callionymidae. It seems possible that there is a significant but under-sampled parasite fauna utilising such families as hosts.
References
Baird, W. (1850). The Natural History of the British Entomostraca. London: The Ray Society, 364 pp.
Basset-Smith, P. W. (1896). Notes on the parasitic Copepoda of fish obtained at Plymouth, with descriptions of new species. Annals and Magazine of Natural History, 18, 8–16.
Boxshall, G. A. (1974). Infections with parasitic copepods in North Sea marine fishes. Journal of the Marine Biological Association of the United Kingdom, 54, 355–372.
Boxshall, G. A. (1990). The skeletomusculature of siphonostomatoid copepods, with an analysis of adaptive radiation in structure of the oral cone. Philosophical Transactions of the Royal Society, London, series B, 328, 167–212.
Boxshall, G. A., & Halsey, S. H. (2004). An introduction to copepod diversity. London: The Ray Society, 966 pp.
Delamare Deboutteville, C. (1950). Copépodes parasites de poisons de Banyuls (1re série). Vie et Milieu, 1, 305–309.
Delamare Deboutteville, C., & Nunes-Ruivo, L. (1955). Remarques sur le développement de la femelle d’Haemobaphoides (T. Scott) et analyse critique des genres Haemobaphes Steenstrup et Lütken, Haemobaphoides T. et A. Scott et Collipravus Wilson (Crustacea Copepoda). Bulletin de la Société Zoologique de France, 80, 27–37.
El-Rashidy, H. H., & Boxshall, G. A. (2009). Parasites gained: alien parasites switching to native hosts. Journal of Parasitology, 95, 1326–1329.
El-Rashidy, H. H., & Boxshall, G. A. (2010). Parasitic copepods on immigrant and native clupeid fishes caught in Egyptian coastal waters off Alexandria. Systematic Parasitology, 76, 19–38.
Froese, R., & Pauly, D. (2011). FishBase. World Wide Web Electronic Publication. [Consulted on 15 May 2011].
Golani, D., Orsi-Relini, L., Massuti, E., & Quignard, J.-P. (2002). Fishes. CIESM Atlas of exotic species in the Mediterranean (ed. F. Briand). Monaco: CIESM Publishers, 256 pp.
Hamond, R. (1969). The copepods parasitic on Norfolk marine fishes. Transactions of the Norfolk and Norwich Naturalists’ Society, 21, 229–234.
Hansen, H.J. (1923). Crustacea Copepoda. II. Copepoda parasita and hemiparasita. Danish Ingolf Expedition, 3(7), 1–92, pls 1–5.
Ho, J.-S., & Dojiri, M. (1988). Copepods of the family Chondracanthidae parasitic on Australian marine fishes. Australian Journal of Zoology, 36, 273–291.
Huys, R., & Boxshall, G. A. (1991). Copepod evolution. London: The Ray Society, 468 pp.
Kabata, Z. (1958). Lernaeocera obtusa n. sp.; its biology and its effects on the haddock. Marine Research Edinburgh, 1958 (No. 3), 1–26.
Kabata, Z. (1979a). Parasitic copepods of British fishes. London: The Ray Society, 468 pp.
Kabata, Z. (1979b). Parasitic copepods of Australian fishes, XII. Family Lernanthropidae. Crustaceana, 37, 198–213.
Palm, H. W., Klimpel, S., & Bucher, C. (1999). Checklist of metazoan fish parasites of German coastal waters. Berichte aus dem Institut fur Meereskunde an der Christian-Albrechts-Universität Kiel, 307, 1–148.
Raibaut, A., Combes, C., & Benoit, F. (1998). Analysis of the parasitic copepod species richness among Mediterranean fish. Journal of Marine Systems, 15, 185–206.
Reimer, L. W. (1986). Parasitic copepods of fishes from the coast of Mozambique. Wiadomości Parazytologiczne, 32, 505–506.
Schuurmans Stekhoven, J. H. (1936). Beobachtungen zur Morphologie und Physiologie der Lernaeocera branchialis und L. lusci. Zeitschrift für Parasitenkunde, 8, 659–696.
Scott, T. (1900). Notes on some parasites of fishes. 18th Annual Report of the Fishery Board for Scotland, 144–188, pls V–VIII.
Scott, A. (1929). The copepod parasites of Irish Sea fishes. Proceedings and Transactions of the Liverpool Biological Society, 43, 81–119.
Shiino, S. M. (1955). Copepods parasitic on Japanese fishes. 9. Family Chondracanthidae, Subfamily Chondracanthinae. Report of the Faculty of Fisheries, Prefectural University of Mie, 2, 70–111.
Shiino, S. M. (1956). Copepods parasitic on Japanese fishes. 11. Genus Phrixocephalus. Report of the Faculty of Fisheries, Prefectural University of Mie, 2, 242–267.
Uyeno, D., & Nagasawa, K. (2010). Three new species of the family Pennellidae (Copepoda: Siphonostomatoida) from gobiid fishes (Actinopterygii: Perciformes) in coastal waters of the western Pacific Ocean. Zootaxa, 2687, 29–44.
Van Damme, P. A., Maertens, D., Arrum, A., Hamerlynck, O., & Ollevier, F. (1993). The role of Callionymus lyra and C. reticulatus in the life cycle of Lernaeocera lusci in Belgian coastal waters (Southern Bight of the North Sea). Journal of Fish Biology, 42, 395–401.
Yamaguti, S. (1939). Parasitic copepods from fishes of Japan. Part 4. Cyclopoida, II. In: Volumen Jubilare pro Prof. Sadao Yoshida, 2, 391–415, pls 1–13.
Acknowledgement
The second author would like to thank Prof. B. Galil for alerting him to the presence of this copepod in the Eastern Mediterranean and for allowing him to examine the material collected by B. Galil, M. Goren and A. Diamant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Rashidy, H.H., Boxshall, G.A. A new copepod (Siphonostomatoida: Lernanthropidae) parasitic on a Red Sea immigrant dragonet (Actinopterygii: Callionymidae), with a review of records of parasitic copepods from dragonets. Syst Parasitol 81, 87–96 (2012). https://doi.org/10.1007/s11230-011-9326-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-011-9326-7