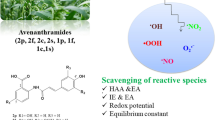
Abstract
As a derivative of cinnamic acid, ferulic acid (FA) is a bio-active ingredient of many foods and is considered to be a good natural antioxidant. A theoretical study on the reaction mechanism of FA scavenging two damaging radicals (·OH and ·NO2) was investigated through the density functional theory (DFT) method. Two most possible reaction mechanisms, hydrogen atom transfer (HAT) and radical adduct formation (RAF), were studied. All possible reaction/attack sites were examined, and the corresponding pathways in both gaseous and aqueous medium were identified by thermodynamic and kinetic calculations. It was found that the most active site of FA scavenging ·OH is the –OH group in benzene ring by HAT mechanism. While, for scavenging ·NO2, the RAF reaction on the C = C double bond is the dominant channel in the aqueous phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Free radicals are species containing one or more unpaired electrons, which gives them a high reactivity. Free radicals were initially thought to be oxygen-centered radicals, defined as reactive oxygen species (ROS), but also include a subgroup of reactive nitrogen species (RNS), all of which are products of normal cell metabolism. The overproduction of ROS and RNS in vivo results in oxidative stress and nitrosative stress, which leads to the damage to cell macromolecules including lipids, proteins, and DNA, thus increases the risk of ischemic heart disease, Alzheimer’s disease, ischemia–reperfusion injury, skin disease, kidney disease, and other diseases [1,2,3,4]. That is what prompted us to develop ways to control ROS and RNS in pathophysiological processes, and the antioxidant-based response is one of them. Both natural and synthetic antioxidants had been developed, while the toxicity found in the synthetic antioxidants led to a growing interest in cheap, non-toxic natural antioxidants [5,6,7].
Ferulic acid (4-hydroxy-3-methoxycinnamic acid, FA) is an important phenolic acid, which can be found in various natural plants such as whole grains, spinach, parsley, grapes, rhubarb, and cereal seeds [8]. FA has low toxicity and possesses a broad spectrum of biological and pharmacological properties, including anti-inflammatory, antibacterial, antiviral, antioxidant, antihypertension, antidiabetes, and anticancer effects [9,10,11,12]. One of the most important functions of phenolic acids, especially cinnamic acid derivatives, is their antioxidant activity, manifested in the ability to eliminate oxygen and free radicals (mainly superoxides, hydroxyl groups and hydroxyl peroxides). FA is considered to be a superior antioxidant, and its antioxidant properties have been confirmed by many experimental studies [13,14,15,16]. FA is more readily absorbed into the body than other phenolic acids and stays in the blood longer. The beneficial effects of FA on human health have been widely advocated, at least in part, because of its strong antioxidant activity [17, 18].
Therefore, the main objective of this work is to systematically study the reaction mechanism of FA with two typical ROS and RNS (·OH and ·NO2), and to provide thermodynamic and kinetic details of all possible reaction pathways. It is hoped that the results of this study can provide some theoretical basis for the development of natural and high-activity scavengers against ROS and RNS.
Computational methods
Full geometry optimization and frequency calculations were performed at the M05-2X/6-311 g(d,p) functional and basis set, using the GAUSSIAN 09 computational package [19]. The M05-2X functional has been successfully used in the studies on thermochemistry, kinetics, and non-covalent interactions, especially for calculating the energies of reactions involving free radicals [20,21,22,23,24,25].
Unrestricted calculations were used for open shell systems. To determine the nature of all stationary points, vibrational frequencies were obtained, at the same time, the local minima and transition states (TS) were identified by the number of imaginary frequencies (0 or 1, respectively). Intrinsic reaction coordinate (IRC) calculations have also been carried out to confirm that all TSs properly connect reactions and products.
To mimic the aqueous media in the body, the solvent effects were introduced using the solvation model of the continuum solvation model based on solute electron density (SMD) which is recommended by Gaussian Manual to compute solvation energy [26]. At the level of M05-2X/6–311 + + G(d,p), all the reactant complexes and product complexes were optimized in aqueous solution by SMD model to evaluate the reaction Gibbs energies and reaction enthalpies. Due to the complexity and expensiveness of transition states optimization, the solvation effects of TS were estimated by the single point calculations on the basis of the optimized gas-phase geometries using SMD model. Both zero-point vibrational energies and thermal corrections at 298.15 K obtained at the M05-2X/6–311 + + G(d,p) level were used to correct electron energies of single point calculations.
Reaction enthalpy in solution was computed by the difference of enthalpy values between products and reactants optimized in the presence of SMD model. Relative Gibbs energy in solutions was computed using thermodynamic cycle and Hess’ law which explicitly include solvation energy. For example, the thermodynamic cycle for the addition reaction between FA and ·OH is as follows:
With this strategy, the Gibbs energy of reaction in solution (ΔGsolv) can be determined as the sum of the Gibbs energy of reaction in the gaseous phase (ΔGgas) and the difference in solvation energies (ΔΔGs):
where ΔΔGs is calculated as:
where ΔGs is the solvation energy. The reference state is 1 M in all cases. The solvent cage effect was included with Okuno’s corrections, which take into account the free volume theory. These corrections agree well with those independently obtained by Ardura et al. [27, 28]. In this work, the expression used to correct the Gibbs energy as follows:
where n is the reaction molecularity. According to Eq. 7, the solvent cage effect causes a decrease of 2.54 kcal/mol in ΔG for a bi-molecular reaction at 298.15 K [29].
Results and discussion
It is generally believed that the reaction between phenolic compound and radical (·R) takes place mainly through three mechanisms: direct H-transfer process from the antioxidant molecule (Eq. 5), radical adduct formation (Eq. 6) and single-electron transfer process (Eqs. 7a and 7b):
Hydrogen atom transfer (HAT):
Radical adduct formation (RAF):
Single-electron transfer (SET):
Based on the previous studies on the reaction mechanism of gallic acid, caffeic acid, erucic acid, and other phenolic compounds with radicals, the mechanism of SET is not obvious [30,31,32,33]. Therefore, the reaction mechanisms of HAT and RAF are included in this paper.
The optimized structure of FA as well as the atomic numbering scheme are shown in Fig. 1. As can be seen, the FA has a nearly planar structure with a dihedral angle of about 179.93° between the benzene ring and the carbonyl group. The planar structure of the FA implies that it is completely conjugated and results in electrons having a wide range of spin delocalization, which can explain its potential scavenging activity on radicals [34].
·OH scavenging activity
To determine the activity of FA capturing ·OH, the reactions of two HAT channels (denoted as OH1 and OH2) as well as three RAF channels ( denoted as OC3, OC4, and OC5) were studied. At the level of M05-2X/6–311 + G (d,p), all reactant complexes and product complexes of FA scavenging ·OH were optimized and the geometries are shown in Figs. 2 and 3, respectively. At the same level, the reaction Gibbs free energies (ΔG) and reaction enthalpies (ΔH) for all pathways in the gaseous phase and in the aqueous phase were obtained and listed in Table 1.
From Table 1, we can find that all pathways of FA scavenging ·OH are exothermic (ΔH < 0), and except OC5, all the other four pathways are exergonic (ΔG < 0). Therefore, these four pathways (OH1, OH2, OC3, and OC4) are thermodynamic feasible in both the gaseous and aqueous phases. Among them, the HAT pathway OH1 is the most exothermic and exergonic reaction, indicating that it is the most thermodynamically advantageous pathway in both the gaseous and aqueous phases. In the aqueous phase, the absolute value of ΔGsolv of two HAT pathways is greater than that of three RAF pathways, suggesting that all HAT reactions can release more energy. So we infer that, from the thermodynamic point of view, both HAT and RAF mechanisms are feasible for FA capturing ·OH in vivo, but the HAT mechanism is more advantageous.
At the M05-2X/6-311G (d, p) level, the TSs of all pathways for FA capturing ·OH is identified and shown in Fig. 4, and the activation energy barriers (ΔG≠) in both the gaseous and aqueous phases are calculated and listed in Table 2.
For the 5 reactions of FA with ·OH, the activation energy barrier of the pathway OH1 is lowest wherever in the gaseous phase or in the aqueous phase. Therefore, the HAT reaction OH1 is the major pathway of FA scavenging ·OH. This result is consistent with the thermodynamic result discussed above. Therefore, from the thermodynamics and kinetics point of view, OH1 is the most dominant pathway in all reactions of FA with ·OH, and the HAT is the dominant mechanism of two feasible mechanism. In addition, the pathway OC4 has the lowest activation energy barrier in all RAF reactions, so it is a more dynamically advantageous pathway for the RAF mechanism.
·NO2 scavenging activity
For the reaction of FA with ·NO2, the active site is the same as for capturing ·OH. So similarly, two HAT pathways (NH1 and NH2) and three RAF pathways (NC3, NC4, and NC5) have been studied.
At the level of M05-2X/6–311 + G (d,p), all reactant complexes and product complexes of FA scavenging ·NO2 are optimized, and the geometries are shown in Figs. 5 and 6, respectively. At the same level, the reaction Gibbs free energies (ΔG) and reaction enthalpies (ΔH) for all pathways in the gaseous phase and in the aqueous phase are obtained and listed in Table 3.
As can be seen from Table 3, only the pathway NC4 is exothermic reaction in the gaseous phase, indicating that it is thermodynamically difficult for FA capturing ·NO2 in the gaseous phase. While in the aqueous phase, the pathways NH1 and NC4 are exothermic reaction, and the pathway NH1 is an exergonic reaction. Therefore, the HAT pathway NH1 and the RAF pathway NC4 are thermodynamically feasible for FA to remove ·NO2 in the aqueous phase.
At the M05-2X/6-311G(d, p) level, the TSs of all pathways for FA capturing ·NO2 are identified and shown in Fig. 7, the activation energy barriers (ΔG≠) in both the gaseous and aqueous phases are calculated and listed in Table 4.
From Table 4, we can find that the HAT pathway NH1 has the lowest activation energy barrier of all gaseous-phase reactions, so it is the major pathway of FA scavenging ·NO2 in the gaseous phase. In the aqueous phase, the pathway NC4 has the lowest activation energy barrier of any reaction. According to the previous discussion, the pathway NC4 is thermodynamically feasible, so it can be inferred that the RAF pathway NC4 is the most advantageous pathway for FA capturing ·NO2 in vivo.
Conclusions
In this paper, a theoretical calculation was carried out to investigate the activity of ferulic acid in scavenging the damaging radicals ·OH and ·NO2.
For FA scavenging ·OH, both HAT and RAF mechanisms are very feasible thermodynamically and kinetically. In the gaseous phase and in the aqueous phase, the most active site is H1 site in the benzene ring. The RAF mechanism is weaker than HAT, and the activities of the pathway OC4 is better than other two RAF pathways.
For FA scavenging ·NO2, the reactions NH1 and NC4 in the aqueous phase are thermodynamically feasible. The RAF reaction from site C4 is the major channel in vivo, while the activity of the HAT reaction NH1 is weaker.
Availability of data and material
All relevant data are within the paper and its Supporting Information files.
References
Aruoma O (1998) Free radicals, oxidative stress, and antioxidants in human health and disease. J Amer Oil Chem Soc 75(2):199–212
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82(1):47–95
Kukreja RC, Hess ML (1992) The oxygen free radical system: from equations through membrane-protein interactions to cardiovascular injury and protection. Cardiovasc Res 26:641–655
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell B 39(1):44–84
Takagi A, Kawasaki N, Momma J, Aida Y, Ohno Y, Hasegawa R, Kurokawa Y (1993) Toxicity studies of a synthetic antioxidant, 2,2-methylenebis (4-ethyl-6-tertbutylphenol) in rats. 2. Uncoupling effect on oxidative phosphorylation of liver mitochondria. J Toxicol Sci 18:49–55
Takagi A, Takada K, Sai K, Momma J, Aida Y, Suzuki S, Naitoh K, Tobe M, Hasegawa R, Kurokawa Y (1996) Chronic oral toxicity of a synthetic antioxidant, 2,20-methylenebis (4-ethyl-6-tert-butylphenol), in rats. J Appl Toxicol 16:15–23
Takagi A, Sekita K, Saitoh M, Kanno J (2005) Acute, subchronic and chronic toxicity studies of a synthetic antioxidant, 2,20-isobutylidenebis (4,6-dimethylphenol) in rats. J Toxicol Sci 30:275–285
Mattila P, Kumpulainen J (2002) Determination of free and total phenolic acids in plant-derived foods by HPLC with diode-array detection. J Agric Food Chem 50:3660–3667
Ardiansyah OY, Shirakawa H, Koseki T, Komai M (2008) Novel effects of a single administration of ferulic acid on the regulation of blood pressure and the hepatic lipid metabolic profile in stroke-prone spontaneously hypertensive rats. J Agric Food Chem 56:2825–2830
Jung EH, Kim SR, Hwang IK, Ha TY (2007) Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J Agric Food Chem 55:9800–9804
Ramar M, Manikandan B, Raman T, Priyadarsini A, Palanisamy S, Velayudam M, Munusamy A, Marimuthu Prabhu N, Vaseeharan B (2012) Protective effect of ferulic acid and resveratrol against alloxan-induced diabetes in mice. Eur J Pharmacol 690:226–235
Huang WY, Cai YZ, Zhang Y (2010) Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr Cancer 62:1–20
Niwa T, Doi U, Kato Y, Osawa T (2001) Antioxidative properties of phenolic antioxidants isolated from corn steep liquor. J Agric Food Chem 49:177–182
Ogiwara T, Satoh K, Kadoma Y, Murakami Y, Unten S, Atsumi T, Sakagami H, Fujisawa S (2002) Radical scavenging activity and cytotoxicity of ferulic acid. Anticancer Res 22:2711–2717
Xiaokun W, Xin G, Yukari E, Hiroo S (2004) Purification and characterization of a feruloyl esterase from the intestinal bacterium Lactobacillus acidophilus. Appl Environ Microbiol 70(4):2367–2372
Nabi G, Liu ZQ (2012) Ferulic and coumaric acids: application to release oxidative stress of DNA and methyl linoleate. J Food Biochem 36:38–45
Srinivasan M, Sudheer AR, Menon VP (2007) Ferulic acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr 40(2):92–100
Sultana R (2012) Ferulic acid ethyl ester as a potential therapy in neurodegenerative disorders. BBA-mol Basis Dis 1822(5):748–752
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Fox DJ et al (2009) Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford
Zavala-Oseguera C, Alvarez-Idaboy JR, Merino G, Galano A (2009) OH radical gas phase reactions with aliphatic ethers: a variational transition state theory study. J Phys Chem A 113:13913–13920
Vega-Rodriguez A, Alvarez-Idaboy JR (2009) Quantum chemistry and TST study of the mechanisms and branching ratios for the reactions of OH with unsaturated aldehydes. Phys Chem Chem Phys 11:7649–7658
Galano A, Alvarez-Idaboy JR (2009) Guanosine + OH radical reaction in aqueous solution: a reinterpretation of the UV-vis data based on thermodynamic and kinetic calculations. Org Lett 11:5114–5117
Black G, Simmie JM (2010) Barrier heights for H-atom abstraction by HO2 from n-butanol—a simple yet exacting test for model chemistries? J Comput Chem 31:1236–1248
Galano A, Macías-Ruvalcaba NA, Campos ONM, Pedraza-Chaverri J (2010) Mechanism of the OH radical scavenging activity of nordihydroguaiaretic acid: a combined theoretical and experimental study. J Phys Chem B 114:6625–6635
Zhao Y, Truhlar DG (2008) How well can new-generation density functionals describe the energetics of bond-dissociation reactions producing radicals? J Phys Chem A 112:1095–1099
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem A 113(18):6378–6396
Okuno Y (1997) Theoretical investigation of the mechanism of the Baeyer-Villiger reaction in nonpolar solvents. Chem-Eur J 3:212–218
Ardura D, Lopez R, Sordo TL (2005) Relative Gibbs energies in solution through continuum models: effect of the loss of translational degrees of freedom in bimolecular reactions on Gibbs energy barriers. J Phys Chem B 109:23618–23623
Galano A, Alvarez-Idaboy JR (2013) A computational methodology for accurate predictions of rate constants in solution: application to the assessment of primary antioxidant activity. J Comput Chem 34:2430–2445
Leopoldini M, Chiodo SG, Russo N, Toscano M (2011) Detailed investigation of the OH radical quenching by natural antioxidant caffeic acid studied by quantum mechanical models. J Chem Theor Comput 7:4218–4233
Leopoldini M, Pitarch IP, Russo N, Toscano M (2004) Structure, conformation, and electronic properties of apigenin, luteolin, and taxifolin antioxidants. A first principle theoretical study. J Phys Chem A 108(1):92–96
Wright JS, Johnson ER, Dilabio GA (2001) Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc 123:1173–1183
Alicja U, Marcin M, Małgorzata S (2012) Quantum-chemical calculations of the antioxidant properties of trans-p-coumaric acid and trans-sinapinic acid. CMST 18(2):117–128
Acker SV, Groot MD, Dirk-Jan V, Tromp M, Gabrielle D, Van D et al (1996) A quantum chemical explanation of the antioxidant activity of flavonoids. Chem Res Toxicol 9:1305–1312
Acknowledgements
We thank the Chinese Academy of Sciences for the grid computing server provided.
Funding
This work is financially supported by the Doctorial Funding Program of Heilongjiang Institute of Technology (2017BJ19), the Heilongjiang Province Fundamental Scientific Research Expenses of Undergraduate Universities (2020CX05), the Natural Science Foundation of Heilongjiang Province (LH2019B028), and the National Natural Science Foundation of China (Grant No. 51803050).
Author information
Authors and Affiliations
Contributions
Conceptualization: Y. L., P. S. Data curation: W. W., D. D. W. Formal analysis: Y. L., W. W. Investigation: Y. L., D. D. W., X. J. B. Methodology: H. Z., P. S. Resources: Y. L., P. S., W. W. Supervision: H. Z. Validation: D. D. W., X. J. B.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lu, Y., Wang, W., Wang, D. et al. Reaction mechanism of ferulic acid scavenging OH and NO2 radicals: a theoretical study. Struct Chem 33, 641–647 (2022). https://doi.org/10.1007/s11224-021-01855-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-021-01855-2