Abstract
Avenanthramides (AVs) are the phytochemicals found in cereals exclusively in oats. These are widely known natural substances that have antioxidant properties. The free radical deactivation potential of the eight AVs against five reactive species has been studied in physiological pH. At physiological pH, the radical quenching processes were studied using the sequential proton loss followed by electron transfer (SPLET) from the phenolic hydroxyl groups. Using density functional theory (DFT) computations, theoretical studies have been carried out in the gas phase and aqueous solution at M06-2X/6-31 + G (d,p) level of theory. The free radical scavenging ability of the studied AVs was analyzed by using conceptual density functional theory-based parameters and electrostatic potential analysis. By examining the hydrogen atom and electron affinities of each reactive species, the relative destructive potential of each has been compared. The electron transfer capabilities between the studied compound and reactive species were identified by utilizing the ionization energy and electron affinity plots. Additionally, by calculating the redox potentials and equilibrium constants for the entire process in the aqueous solution, the viability of scavenging the free radical species by selected AVs (both in neutral and mono-deprotonated) has been investigated. From the analysis, the neutral as well as the mono-deprotonated form of AVs are found to scavenge •OH and •OOH, and •NO2 radicals effectively, while they are inefficacious toward the O2•‾ and •NO radicals.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Natural phytochemicals are regarded as a promising source of novel biologically active agents and interesting molecules for the creation of both natural and semi-synthetic medications [1,2,3]. Their antioxidant activity is the most common and well-documented. Many natural polyphenols, including phenolic acids [4,5,6,7,8], catechins [9], flavonoids [10], anthocyanins [11,12,13], coumarin [14], stilbenes[15], and terpenes [16], have been shown for intriguing radical scavenging action in vivo and in vitro. Likewise, oats, a grain of the Poaceae family, are one of the staple food crops that human beings can consume directly. Oats stick out as a notable reservoir of polyphenols, including the compound Avenanthramides (AVs) [17, 18]. Among cereals, oats hold a distinctive position as the primary source of these recognized compounds [19, 20], and they exhibit a wide range of biological activities including antioxidant, anti-inflammatory, anti-itch, anti-irritant, and antiatherogenic properties [17,18,19]. AVs have been reported to exhibit antioxidant activity, with many studies comprising 2p, 2f, and 2c in their experimental analysis using various assays. It was found that 2c dispenses comparable antioxidant activity to the standard synthetic antioxidant BHT in the β-carotene test and is more active than Trolox in the DPPH assay [20]. According to the laboratory investigation of antioxidant activity, the activity order is likewise claimed to be c ≥ s ≥ f ≥ p, and 2 ≥ 1 [21,22,23,24]. From a theoretical point of view, the radical scavenging activity and the underlying mechanisms (HAT, SET, SET-PT, and SLPET) have been documented in our prior research [25] as well as in other studies [26, 27]. However, these earlier investigations enriched our knowledge and opened new space to extend to a little bit more about the radical quenching by these compounds.
Even though free radical production is crucial to the normal functioning of metabolic systems such as cell communication, tissue homeostasis, redox regulation, immunity, defense against infectious illnesses, and many others, their overproduction causes oxidative stress, which leads to cell death, and tissue damage leads to neurodegenerative diseases, cancer, diabetes, and cardiovascular disease [28,29,30]. The major role of these was taken by the reactive oxygen species (ROS) and reactive nitrogen species (RNS). ROS are produced as a result of regular metabolic processes or in reaction to outside influences like UV radiation. The literature references reactive oxygen and nitrogen species (RONs) for their recognized harm to biological systems, primarily consisting of superoxide radical anions, hydroxyl, and hydroperoxyl radicals, as well as nitrogen monoxide and nitrogen dioxide radicals, collectively forming ROS and RNS, respectively. The most oxidizing ROS is the hydroxyl radical, which is responsible for DNA oxidative damage. The hydroperoxyl radical’s lengthy half-life permits it to reach distant cellular sites. Even though the free radical potential of the nitric oxide radical is minimal, excessive synthesis may cause damage to cells. It may react with the superoxide radical anion to form peroxynitrite, a potent oxidizing, and nitrating agent capable of causing protein, lipid, and DNA damage [31].
Although our previous work established the antioxidant activity of these compounds by three basic mechanisms, namely hydrogen atom transfer mechanism (HAT), single electron transfer followed by proton transfer mechanism (SET-PT), and sequential proton loss electron transfer mechanism (SPLET) in the gas phase and ethanol solvents [25], further investigation is required to fully uncover the feasibility of quenching reactive species by the compounds in aqueous solution at physiological pH levels.
Herein, the study extended to the feasibility of radical quenching with five reactive radical species (hydroxyl, hydroperoxyl radicals, superoxide radical anions, nitrogen monoxide, and nitrogen dioxide radicals). For this purpose, the one-electron transfer between the compound and free radical species was analyzed using redox potential and equilibrium constant. Moreover, the results are compared with the hydrogen atom affinity results to ascertain the relative free radical potential of the considered RONs. The radical quenching processes were explained both in the gas phase and the aqueous phase. The study also included a detailed surface analysis electronic structure calculation, and chemical reactivity descriptor investigation to further unravel the intrinsic properties of the compounds. Figure 1 represents the compounds included in the study.
Chemical structures of selected AVs, and atom labeling are also included (p = p-coumaric acid, f = ferulic acid, c = caffeic acid, and s = sinapic acid)[25]
2 Computational details
The calculations were performed, and the geometries of all the structures were fully optimized with M06-2X/6-31 + G (d, p) level of theory using the Gaussian 16W suite [32]. The optimized structures are given in Fig. S1. A detailed description of conformational search and geometry optimization is given in our previous work [25]. At the same level of theory, the selected free radical species were optimized using the unrestricted open-shell technique. The integral equation formalism of the polarized continuum model (IEF-PCM) was used to account for solvent effects [33]. The solvent water (ε = 78.35) was selected to model the aqueous environment. The analyses such as electrostatic potential and chemical reactivity descriptors were finished by Multiwfn 3.8 [34], which is a multifunctional wave function analysis program. The wavefunction files for the compounds were acquired from the Gaussian 16W, which is the input file used by the wavefunction analyzer program Multiwfn 3.8. All isosurface maps were rendered by the VMD 1.9.1 program [35] based on the outputs of Multiwfn. To obtain more accurate results, the grid spacing for the quantitative analyses of the electrostatic potential on the van der Waals (vdW) surface was set to 0.25 Bohr, which is slightly finer than the default settings. The polarizable continuum model was used to include solvent effects. The antioxidant activity results of all the studied compounds obtained from M06-2X/6–31 + G (d, p) methodology are compared with M06-2X/6–311 + + G (d, p) level of theory in aqueous solution.
3 Results and discussion
3.1 Reactivity site analysis
3.1.1 ESP
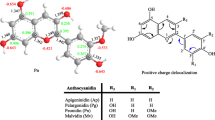
Herein, to unravel the chemical reactivity of the compounds, the electrostatic potential (ESP) map was utilized for identifying the sites of electrophilic and nucleophilic attack [36]. The electrostatic potential on a van der Waals (vdW) surface of compounds is shown in Fig. 2. The blue regions indicate positive potential, i.e., the sites for nucleophilic attack, while the red regions are prone to electrophilic attack. The green regions are neutral. It can be seen that the blue regions in all the compounds are located on the hydroxyl proton, whereas the red regions lie at the oxygen atoms. Apart from the surface color, the diagrams also represented the quantitative nature of surface local minima (small blue spheres) and maxima (small orange spheres). The largest negative value was found to be at O9ꞌ (carbonyl) in all the compounds, and it lies around 35–37 kcal/mol, which corresponds to the global minimum on the surface. The highest negative potential at the O9ꞌ reveals the high delocalizing nature of the carbonyl bond.
For 2p, the positive potential at 5-OH (59.37 kcal/mol) exceeds that of the acidic protons (58.25 kcal/mol). Conversely, when compared to the 5-OH, the phenolic hydroxyl group at the 4ꞌ position exhibits lower positive potential values and is less susceptible to nucleophilic attack. Regarding 1p, this returns to the same, the 4'OH group is more susceptible to nucleophilic attack. Compared to 2p, the compound 2f exhibits lower nucleophilic attack by the phenolic hydroxylic groups. The positive potential of 4’OH diminishes to 43.59 kcal/mol compared to 2p which will be due to intramolecular stabilizing interaction with the oxygen of OCH3. Moreover, for 2c and 1c, the highest positive potential or global maximum was found to be arising from the 4ꞌ hydroxyl proton (65.62 kcal/mol for 2p and 65.97 kcal/mol for 1c). This gives information regarding the intramolecular interactions which are likely to be displayed by the molecule. Also found that substitution of the ortho-position of 4ꞌOH decreases the positive potential value of upon methoxy group (2 s and 1 s), whereas increases with the hydroxyl group (2c and 1c).
3.1.2 Chemical descriptors
The conceptual density functional theory (CDFT)-based chemical reactivity descriptors such as electronegativity (χ), chemical hardness (ƞ), chemical softness (S), electrophilicity index (ω), and chemical potential (μ) were defined to get a deeper understanding of the chemical reactivity of the compound [37,38,39,40] ((Eqs. (1)–(8)). The chemical reactivity descriptors are tabulated in Table 1 and calculated using the energy vertical method. The capability of the compound to accept precisely one electron from a donor is measured by its electron affinity (EA), and the measure of the compound to give away an electron is given by its ionization potential (IP) (Eq. (1)-(2)).
The chemical hardness of the molecule is the rigidity of the molecule, i.e., the resistance of a molecule to undergo polarization of the atom according to the external environment [38]. It reveals the overall charge cloud and the stability of the molecule. Chemical softness is the reverse of hardness, i.e., it explains the easiness of the electron cloud to undergo polarization [38]. The chemical potential of the molecule is generally the tendency of the electron to move from the region of higher potential to lower potential until it becomes equilibrated. In DFT, chemical potential measures the escaping tendency of an electron from equilibrium and is the negative of electronegativity [41]. Electronegativity is the tendency to attract electrons toward it. The electrophilicity index is the strength of the electrophilicity of the species which is an indication of chemical reactivity and a useful tool in accessing chemical and toxicological potentials of the molecule [40].
The obtained ionization potential and electron affinity are in the range of 7.55–7.87 eV and 0.55–0.60 eV, respectively. These values are comparable to the values of quercetin which is a well-known antioxidant molecule, where IP and EA, respectively, are 7.86 and 1.03 eV. The investigated compound’s high ionization potential and low electron affinity demonstrate that it is more difficult to remove an electron than to accept one. The electronegativity of the studied compounds is low compared to quercetin. Hence, the chances of electron transfer from the compounds to neutralize free radical species are high. Similarly, the chemical potential values are also lower than the reference compounds. The chemical potential and electronegativity are the properties of the entire molecule, which remains constant throughout the system. Hardness is identified as the charge density, which is the ability to hold an electronic charge once it has been acquired. Hardness indicates a molecule’s difficulty to using electrons, while softness is the inverse of hardness. These characteristics have comparable values to quercetin, indicating their potential as anti-oxidants. The chemical reactivity descriptors of the quercetin were calculated by optimizing the structure with a suitable level of theory.
3.1.3 Gas phase basicity (GPB) and acidity constant (pKa) determination
Next, the order of deprotonation tendencies of each hydroxyl group in the selected AVs was analyzed. The phenolic hydroxyl groups as well as the carboxylic functional group may dissociate or get ionized depending on the pH of the medium. Hence, the step-wise proton release in aqueous solution of compounds was studied by calculating the pKa values of each OH proton using the thermodynamic cycle given in Fig. 3. The gas phase basicity (GPB) was computed as the negative of the Gibbs energy change associated with its protonation reaction in the gas phase.
Applying the thermodynamic cycle given in Fig. 3, the basicity of AV in an aqueous solution was calculated using Eq. (9), and Eq. (10) is used to calculate the pKa values of each deprotonation.
where ∆G0solv (AV (OH) n) and ∆G0solv (AV (OH) n-1) are the solvation energies of the AV and its anion, respectively. The standard hydration Gibbs energy of the proton, ∆G0solv(H+), was taken as −265.89 kcal/mol at 298.15 K temperature and 1 atm pressure [42, 43].
The computed gas and aqueous solution basicities for the ionization of each hydroxyl group present in the compounds, along with their sequential pKa values, are given in Table 2. The lower pKa value of the carboxylic hydroxyl group of all the selected compounds revealed the predominance of carboxylates of AVs in physiological pH. The previous reports also suggest that the compounds containing carboxylic functional groups exist in their monoanionic forms at physiological pH [44]. The entire studied compounds exist in their mono-deprotonated form in biological conditions.
The second deprotonation sites in all the compounds are found to be favored from 4'OH. It refers to the enhanced charge delocalization of anionic species generated from the para-phenolic group via extended conjugation. On comparing, the pKa for second deprotonation, the lower was found to be for caffeic acid derivatives, 2c and 1c, 9.92 and 8.73 kcal/ mol, respectively. The lower value of pKa of 2c and 1c will be due to stabilization by intramolecular hydrogen bonding from the nearest hydroxyl group in addition to the charge delocalization. The obtained pKa values are consistent with the experimentally reported pKa values of the caffeic acid [45, 46]. Literature reports the pKa values of the acidic proton, 4ꞌOH, and 3ꞌOH, respectively, 3.6–4.49, 8.6–9.3, and 10.3–12.7 kcal/mol [45, 46]. The methoxy substitution (electron-donating) ortho to 4ꞌ OH decreases the tendency of proton transfer due to the formation increased charge cloud near the anionic species. For 2c and 1c, the pKa values 5 OH and 3ꞌOH lie in the range of 16–18, meant for the higher basicity. The bulkiness and type of the substituents are two significant elements that influence the deprotonation sequence. Furthermore, the stability of the resultant anionic species is important in defining the subsequent deprotonation and pKa values of the phenolic groups.
The pKa values show that the compounds exist as their mono-deprotonated forms (carboxylate form) at physiological pH. With the increase in pH, the next deprotonation occurs from the 4ꞌOH phenolic hydroxyl group of the compounds which results in the formation of dianion of AV. The pH range at which each compound forms its dianion changes according to the structure. The compound 1c and 2c around pH 8.7 and 9.92, respectively, forms their AV 4ꞌ-O-monoanion carboxylates. The pKa values of other AVs 2p, 2f, 2 s, 1p, 1f, and 1 s, respectively, for the formation of its di-anionic form are 12.75, 15.63, 13.9, 12.67, 14.37, and 13.24. It means that these compounds form 4ꞌO− anion from the carboxylate forms of its corresponding AV at an increased pH value. Figure 4 represents the effect of pH on the ionization of 2c.
3.2 Radical scavenging activity
Our previous study reported the antioxidant activity of the compound by HAT, SET-PT, and SPLET mechanisms in the gas phase and ethanol medium [25]. Our study concluded the preference HAT mechanism in the gas phase and the SPLET mechanism in the ethanol medium [25]. In the HAT mechanism, the radical deactivation occurs through the donation of hydrogen atoms by the phenolic OH groups in each compound, and also obtained that the carboxylic acid proton and amide proton (N–H) are least stable for the radical quenching process. In the solution phase, phenolic OH groups scavenge free radical species by forming its anion and followed by electron transfer. (COOH and N–H proton are obtained to be less significant due to comparatively higher value of ETE.) Later on, Xue et al. [26]. and Purushothaman and co-workers[27] concluded expression of the same mechanism in both gas phase and solution phases. The HAT, SET-PT, and SPLET parameters and a detailed description of these mechanisms of the AVs in aqueous solution are explained in the supplementary information (Section S1 and Table S1, Table S2, Table S4). Herein, we compared the results of antioxidant activity with M06-2X/6–31 + G (d, p) and M06-2X/6–311 + + G (d, p) levels of theories and also the antioxidant activity of phenolic hydroxyl groups in deprotonated form (carboxylate form of each compounds) of each compounds also included.
From Table S1, it can be observed that for the compounds 2p and 2f, the lowest BDE is exhibited by the 5-OH followed by 4ꞌ-OH, and in other cases, the 4ꞌ-OH is followed by 5-OH. No much change in BDE was observed in the aqueous phase. However, it follows the same trend as the gas phase of each molecule under consideration. In the aqueous phase, the BDE value of 4ꞌ-OH is found to be lower than in the gas phase due to greater stabilization of the radical formed. The compound 2c and 1c possess the lowest BDE among others through 4ꞌ-OH with BDE of approximately 77–79 kcal/mol. The lowest energy revealed the stabilization of radicals formed through delocalization through the ring [25] and the stabilization of the radicals formed by intramolecular hydrogen bonds. In the aqueous solution, the BDE of the 4ꞌ-OH slightly increased. It may be due stabilization of radicals formed with hydrogen from the solution, which is less feasible than stabilization through delocalization. For 3ꞌ-OH and 5-OH, the bond becomes more potent in the aqueous phase by their lower BDE values compared to the gas phase values which will be due to the stabilization of radicals formed through the solvent cage effect. Considering 2f and 2 s, the presence of a methoxy group ortho to 4ꞌ-OH decreased the BDE value of 4ꞌ-OH but was still greater than 5-OH, and the addition of second methoxy group, a decrease in BDE much less than 5-OH was observed. So for 2 s hydrogen donation, capability of 4ꞌ-OH becomes more favorable than 2f and 2 s and hence has a higher H-donating ability. So based on the BDE, the hydrogen donating capability obeys the order 2c > 1c > 2 s > 1 s > 2f > 1f > 2p > 1p in the gas phase and aqueous phase. The order obtained is consistent with the order of radical-trapping activity in the DPPH assay [22]. The results of BDE values are very close to each other in both levels of theories and follows the same trends among compounds. The change BDE value of 0.27 kcal/mol is obtained for 4ꞌ-OH of 2c in M06-2X/6–311 + + G (d, p) compared to M06-2X/6–31 + G (d, p). The differences between both levels of theories values are found to be not greater than 10 kcal/mol [7].
The ionization potential (IP) of AVs becomes lesser in the aqueous phase revealing the high electron transferability in the aqueous phase than gas phase (Table S2). The sequence of IP values of compounds in the gas phase is observed to be 2 s < 2f < 2p < 2c < 1 s < 1f < 1c < 1p in the gas phase, whereas in the aqueous phase, it follows 1f < 2p < 2 s < 2p < 1 s < 1c < 1p < 2f < 2c. The order of radical scavenging activity is much different from the HAT mechanism because IP value influences the whole structure of the molecule, whereas HAT is affected by the local phenomena induced by the substituents. Even though the IP values in gas phase not much deviates in both theories and follows same trend, an observable deviation is found in aqueous phase, approximately of 23 kcal/mol. But still both theories follows same trend in activity. The higher IP values in M06-2X/6–31 + G (d, p) are found to be due to the very low solvation enthalpies of electron in aqueous phase. Solvation enthalpies of electron and proton in both levels of theories in both solvents are given in Table S3. The solvation enthalpies of electron in aqueous phase both M06-2X/6–311 + + G (d, p) and M06-2X/6–31 + G (d, p) levels of theories are −0.041879 and −0.002978 Hartree, respectively, which are calculated by methodology suggested by Marković et al.[47].
The proton dissociation enthalpy values obtained by the deprotonation of radical cation formed in the aqueous phase are more feasible than the gas phase of approximately 200 kcal/mol. The 4ꞌOH of 1c and 2c has the lowest value of PDE in both phases. The 4ꞌOH of each compounds possesses lower PDE value among other OH groups in the respective molecules except for 2p and 2f. For 2p and 2f, the 5OH has lower PDE value. The PDE values of each compounds differ only approximately of 1 kcal/mol with M06-2X/6–311 + + G (d, p) and M06-2X/6–31 + G (d, p) levels of theories, and both follows same trend. The lower PDE value in aqueous phase than the BDE value does not conclude the feasibility of the mechanism toward radical scavenging activity. Because, to reach the second step, (i.e., PDE), the compounds should overcome the energy barrier of first step (IP). So when compared to BDE values, IP values are very high. Hence, SET-PT mechanism can be excluded from the antioxidant activity of selected compounds in aqueous phase too.
The parameters of SPLET mechanism in aqueous phase, the PA, and ETE value both M06-2X/6–311 + + G (d, p) and M06-2X/6–31 + G (d, p) levels of theories are given in Table S4. As expected, PA values in the aqueous medium are lower than the gas phase and also lower than the BDE value corresponding in the aqueous medium. Hence in polar solvent, herein in water, the AVs favor deprotonation of the hydroxyl group. The PA values are very close in both the level of theories, and the differences in enthalpies are less than one kcal/mol. In aqueous medium, the PA values are around 280 kcal/mol lower than the gas phase. Among the selected compounds, the c-series compounds (2c and 1c) possess lower PA values and which is by the group 4ꞌOH. The second step of SPLET mechanism is the electron movement from the anionic form of the compound. The ETE is the corresponding descriptor. Due to low solvation enthalpy value of electron, the ETE value calculated using M06-2X/6–311 + + G (d, p) are found to be lower than M06-2X/6–31 + G (d, p) as explained in case of IP values. The difference is around 20–24 kcal/mol. Hence, the M06-2X/6–311 + + G (d, p) level of theory is better for explaining the SPLET mechanism in aqueous phase. Both the results of M06-2X/6–31 + G (d, p) and M06-2X/6–311 + + G (d, p) follow same trend in reactivity.
Considering PA + ETE values, the 4ꞌ-OH of 2c, 2 s, 1p, 1f, 1c, and 1 s are found to be less energy demanding toward radical scavenging mechanism. For 2p and 2f, the 5-OH is found to be more reactive due to its comparatively lower energy. These are the active groups in the selected compounds, where the SPLET process is most likely to occur in polar liquids. The order of radical scavenging ability in polar solvent follows as 2c > 1c > 2 s > 1 s > 2f > 1f > 2p > 1p. The order was in good agreement with the experimental results [22]. The order is consistent with that of the HAT mechanism. Considering 2 series compounds, the PA values of 5-OH do not change appreciably upon changing the substitution on the B-ring which means that substitution on the B-ring does not affect the A ring. Moving from 2f to 2c and 2 s, the deprotonation from the B-ring becomes more feasible due to the stabilization of the ortho-substituent. The ETE values are very close to each other, indicating that the introduction of substituent on the B-ring has a minor effect on the electron-donating ability of 5-O− anionic forms. Moreover, as given 2 series and 1 series compound, the OH substituent on the 5th position has little effect on the B-ring.
Hence in an aqueous solution, the SPLET pathway is the preferred mechanism, in which the OH group first forms its anion and further electron transfer leads to radical deactivation and the compound forms its radical. The generated radical further stabilized through the resonance.
The selected compounds in all cases contain a –COOH functional which gets ionized and forms its carboxylates in solution phase, which is evident from pKa calculations. Hence, the antioxidant activity of carboxylate forms of all compounds is calculated in aqueous phase and tabulated in Table S5. The descriptors BDE, IP, PDE, PA, and ETE values are reported in Table S5. From the descriptors, the PDE values are found to be lower than other descriptors but in higher value of IP, the SET-PT mechanism can be excluded from the explaining antioxidant potential of the compounds as discussed above. The PA values are found to be lower than the BDE values. Hence, SPLET will be the thermodynamically preferred pathway in carboxylate form of compounds also. The lowest PA value is exhibited by the 2c by the 4ꞌ-OH group followed by 1c by the same group. These values are found to be slightly higher than the non-dissociated or unionized form of the compound (2c in non-dissociated form PA = 45.58 kcal/mol, 2c carboxylate form PA = 46.87 kcal/mol). The order of PA + ETE follows as 2c > 1c > 2 s > 1 s > 2f > 1f > 2p > 1p. The order is consistent with the non-dissociated form of compounds.
In conclusion, HAT and SPLET is the thermodynamically preferable mechanism in gas phase and aqueous phase, respectively. The results of the antioxidant descriptors, BDE, PA, and PDE, produces close results with M06-2X/6–311 + + G (d, p) and M06-2X/6–31 + G (d, p) methodologies, and deviations were observed with IP and ETE values. It was observed that the large difference in the solvation enthalpies of electron in aqueous media is the reason for deviation. Hence, calculations involving electron solvation enthalpies in water media can be better explained by higher levels of theories, and otherwise, M06-2X/6–31 + G (d, p) was found to be sufficient.
3.2.1 Quenching of reactive oxygen species and nitrogen species (RONs)
Reactive oxygen and nitrogen species (RONs) are vital in cell signaling, immunity, and tissue homeostasis and are required for proper physiological activities [48, 49]. Excess radical species, on the other hand, are implicated in the development and accelerated pathogenesis of a variety of disorders [48]. The common radical species ROS include oxygen-based free radicals, such as the superoxide radical anion (O2•−), hydroxyl (HO•), alkoxyl (RO•), organic peroxyl (ROO•), and hydroperoxyl (HOO•) radicals. RNS comprises peroxynitrite (ONOO−), nitric oxide (NO•), and nitrogen dioxide (NO2•) [50]. We considered five RONs that are significant for biological systems among reactive free radicals. The hydroxyl, hydroperoxyl radicals, and superoxide radical anions are selected under ROS and nitric oxide and nitrogen dioxide radicals among RNS.
3.2.2 Hydrogen atom affinity (HAA) and electron affinity (EA)
The energy needs of AVs and selected radical species are essential in predicting probable radical scavenging processes. So for this purpose, the hydrogen atom affinity (HAA) and electron affinity (EA) of each radical species were calculated both in the gas phase and aqueous medium with the help of Eqs. (11) and (12), respectively, for HAA and EA.
where R• represents the radical species, R− is the radical after one electron acceptance and RH is the protonated radical species. The triplet state of NO‾ was considered for calculating the EA. The values of H• and e− were calculated using the method. The frequently recognized value of 0.7530 kcal/mol for the electron enthalpy can be used to calculate the standard values of electron enthalpies in the gas phase [51]. The enthalpies of hydrogen radicals and electrons in the gas phase and water phase are calculated by the methodology proposed by Marković et al. [47]. The enthalpy values for the hydrogen atom and electron are tabulated in Table S3 both in gas phase and aqueous phase using M06-2X/6–311 + + G (d, p) and M06-2X/6–31 + G (d, p) levels of theories.
The computed HAA and EA values are tabulated in Table 3. The highest HAA value of hydroxyl radical among all radicals in the gas phase and aqueous phase describes the highly destructive nature of the OH radical. The O2•‾ and •NO radicals are in the lower range, suggesting that they are fruitless at accepting a hydrogen atom either in the gas phase or in an aqueous solution. Further, the HAA of the majority of the species under examination increases upon solvation. The HAA values calculated both in gas phase and aqueous phase are very close with two levels of theories and hence both are acceptable.
The tendency of electron acceptance and formation of its anion is higher for •NO2 followed by •OH and •OOH in the gas phase. The electron acceptance and formation of corresponding anion O22− is found to be quite energetically unfavorable in the gas phase due to its positive electron affinity but favorable upon solvation. The negative value of •NO radical in the gas phase is also meant for the lower free radical potential. However, the EA values of each reactive species are found to be much higher in the solution phase as compared to the gas phase. The EA values in aqueous solution with M06-2X/6–311 + + G (d, p) are found to be much lower than M06-2X/6–31 + G (d, p) methodologies, which will be due to the very low solvation enthalpy of electron in aqueous phase in M06-2X/6–31 + G (d, p) method (Table S3). The trends in activity concluded are same in both methodologies.
In conclusion, according to HAA and EA values, hydrogen atom acceptance and detoxification through the HAT (hydrogen atom transfer) mechanism will be highly favorable for the radicals, hydroxyl, hydroperoxyl, and nitrogen dioxide radicals among selected free radical species in the gas phase and aqueous phase. The SET or SET-PT mechanism, which includes the transfer of a single electron (connected with EA value in Table 3), is unfavorable for superoxide radical anions and nitric oxide in the gas phase, and it is higher for radicals •OH, •OOH, and •NO2 in both solutions. In the SPLET mechanism, the second step is electron acceptance (by radical species from antioxidant species) also favorable for •OH, •OOH, and •NO2 radicals due to their feasible EA values.
3.2.3 Electron Affinity (EA) versus Ionization Energy (IE) map
To assess the electron transfer power between the radical and the selected compounds, vertical ionization energy (IE) and vertical electron affinity (EA) were employed. It has previously been observed that antioxidant species could either give or take up an electron to scavenge free radicals [52, 53]. This demonstrates that the electron transfer reaction is governed by the ionization energy and the electron affinity of the antioxidant and the free radical. Low ionization energy indicates that the molecule will donate an electron at a low energetic cost, but high electron affinity indicates that the molecule will readily take an extra electron. Here, the single electron transfer tendencies of the compounds are considered.
For this purpose, the IE versus EA map includes selected free radicals, selected AVs, and their mono-deprotonated forms (carboxylates) (Fig. 5 and Table 4). The electron flow will occur from molecules in the bottom left area of the map to molecules in the top right section of the map. Hence, a radical scavenging action will take place. All the AVs and their mono-deprotonated forms lie in the bottom left area of the map and are better electron donors and worse electron acceptors than the •OH, •OOH, and •NO2 radicals. Hence, they are predicted to act as electron donors to deactivate these free radicals.
Map allows a straightforward comparison of the electron donor–acceptor capability of AVs (squares), mono-deprotonated AVs (squares with filled colors), and radicals •OH (circle-pink), •OOH (circle-light green), O2•‾ (circle-royal blue), •NO (circle-orange), and •NO2 (circle-purple) in water (values are given in kcal/mol)
Selected AVs and their mono-deprotonated equivalents, as well as the free radicals O2•‾and •NO, are localized more or less at the same position in the map. As a result, no electron transfer between AVs or mono-deprotonated forms and the radicals O2•‾and •NO is expected. As can be seen from Table 4 and Fig. 5, deprotonated forms are better electron donors than the neutral form of the molecule. The electron affinities are lower in mono-deprotonated molecules than the neutral molecules. According to these results, deprotonated AVs will be better scavengers for radicals •OH, •OOH, and •NO2 than AVs.
3.2.4 Redox potentials and equilibrium constant calculations of AVs with reactive species
From our previous calculations, it was found that the compounds in the aqueous solution preferred the SPLET pathway (proton dissociation and electron transfer) to scavenge the free radical species. Moreover, Xue and colleagues [26] reported the presence of a monoanionic form of the AVs at physiological pH and also confirmed from previous pKa calculations (formation of carboxylates at low pH). Thus, we have considered the viability of various reactive species under study by AVs through a SPLET pathway in the aqueous solution at physiological pH. The redox potential and equilibrium constant for the overall reaction were calculated by employing the previously reported methodologies [54]. Let us now explore if neutral and mono-deprotonated versions of compounds in an aqueous solution could effectively scavenge various RONs. The overall redox reaction of neutral and mono-deprotonated AV with RONs can be represented by Eqs. (13) and (14), respectively. In Eq. 13, the overall route of radical deactivation by the neutral form of the compound (non-dissociated) of AV through the SPLET pathway is included, whereas in Eq. 14, the mono-deprotonated form or carboxylate form of AVs is considered. The feasibility of scavenging radicals by the pathway is analyzed by calculating the redox potential and equilibrium constant values.
Using the three thermodynamic cycles given in Fig. 6, the redox potential was calculated. The first step of the SPLET mechanism, i.e., loss of a proton (Eq. (15)), and the second step, scavenging of the free radical species (Eq. (16)) are shown as
The reaction (16) may split up into two half-cell reactions represented as (Eqs. (17) & (18))
The standard Gibbs free energy change for the redox reaction (∆G0rxn) can be calculated using Eq. (19),
where ∆G0 (I) is the Gibbs energy change for the step of deprotonation (first step) and ∆G0 (II) for the second step.
Then, the corresponding redox potentials and the equilibrium constants were calculated using Eqs. (24) and (25), respectively, with different radical species. Here, F represents the Faraday constant (23.06 kcal mol−1 V−1).
The equilibrium constant and redox potential for the radical reaction are tabulated in Table 5 in M06-2X/6–31+G(d, p) methodology. From the table, it is observed that among the considered reactive species, the hydroxyl group has the highest value of equilibrium constant and redox potential values for both neutral and mono-deprotonated forms of all compounds. Therefore, we can say that the scavenging of hydroxyl radicals by the compounds at the physiological pH is highly advantageous and spontaneous. The Gibbs free energies corresponding to the scavenging process via the SPLET mechanism are also found to be highly negative for hydroxyl radicals in all cases (both non-deprotonated and deprotonated forms) indicating the feasibility of radical deactivation aqueous solution (Table S6).
Among the species, c-series compounds have a higher equilibrium constant than others for all reactive species followed by s-series compounds. In c-series compounds, 2c possesses higher radical quenching in the non-dissociated form and 1c in the mono-deprotonated form. The order of radical scavenging activity follows caffeic acid (c-series) > sinapic acid (s-series) > ferulic acid (f-series) > p-coumaric acid (p-series). These results are in line with the previously reported studies on radical scavenging activity [22, 26]. The higher scavenging activity of the c-series and s-series of compounds was due to the presence of stabilizing hydroxyl and methoxy groups. The equilibrium constant and redox potential of all the compounds including both forms are negative for O2.- and. NO radicals. Hence, these radicals are not scavenged by the selected AVs. The .OOH radicals are scavenged by all the compounds except 1p. Likewise, NO2 also exhibits a positive equilibrium constant in most of the cases (2f neutral state and 1p in both forms are exceptions) and shows that it also can scavenge the selected compounds.
The higher values of the equilibrium constants in the case of the mono-deprotonated forms indicate their greater potential for scavenging the reactive species. The ease of scavenging of the considered RONs by both the neutral and mono-deprotonated forms of compounds follows the order: •OH > •OOH > •NO2 > O2 •‾ > •NO, which is consistent with the order of the hydrogen atom affinity (HAA) in aqueous solution.
4 Conclusions
In the present work, we have explored the feasibility of quenching five free radical species by eight main oat phenolic antioxidant Avenanthramides; 2p, 2f, 2c, 2 s, 1p, 1f, 1c, and 1 s using M06-2X/6–31 + G (d, p) as well as M06-2X/6–311 + + G (d, p) level of theories. Theoretical elucidation of quenching of free radical species by selected variants of compounds is addressed for the first time, even though various theoretical and experimental examinations of the antioxidant activity of these molecules have previously been conducted. The investigation of radical scavenging activity of the selected compounds in an aqueous solution utilizing the mechanism HAT, SET-PT, and SPLET employing the thermochemical parameters like bond dissociation enthalpy, ionization potential, proton dissociation enthalpy, proton affinity, and electron transfer enthalpies signifies the preference of SPLET pathway at the physiological pH. This is also proved by the pKa calculations. We already proved the choice of the HAT mechanism in the gas phase in our previous work. The order of free radical scavenging activity of selected compounds in aqueous solution at the physiological pH is found to be 2c > 1c > 2 s > 1 s > 2f > 1f > 2p > 1p. The obtained results are in accordance with the experimental findings.
Among the studied RONs, •OH, •OOH, •NO2, O2 •‾, and •NO, using the redox potential and equilibrium constant for one-electron transfer between the Avenanthramides and free radical species, the hydroxyl radical is shown to display the best affinity for hydrogen and electrons. The high equilibrium constants for the hydroxyl radical indicate that it is easily scavenged by AVs at physiological pH levels. We may also conclude that the neutral and mono-deprotonated forms of the AVs are effective at scavenging the hydroxyl and hydroperoxyl radicals as well as the nitrogen dioxide radical based on the computed equilibrium constant values. The superoxide radical anion and nitrogen monoxide radical are shown to be ineffective by the selected compounds. Among AVs, the free radical quenching of caffeic acid derivatives (c-series compounds; 2c & 1c) is found to be more reactive followed by sinapic acid derivatives (s-series compounds; 2 s & 1 s). Compared to c-series and s-series compounds, f-series (2f & 1f) and p-series (2p & 1p) compounds are found to be less in radical quenching processes. The results obtained are strictly coinciding with reported experimental antioxidant activity. The ease of scavenging of the considered RONs by both the neutral and mono-deprotonated forms of compounds follows the order: •OH > •OOH > •NO2 > O2 •‾ > •NO, which is consistent with the order of the hydrogen atom affinity (HAA) in aqueous solution. The results conclude the higher free radical quenching ability of c-series and s-series compounds, respectively, due to ortho-dihydroxy and guaiacyl moieties on the aromatic ring, which delivers higher radical stability through internal H bonds, as the superior radical scavenger.
Data availability
Not applicable.
References
Harvey AL, Edrada-Ebel R, Quinn RJ (2015) The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov 14:111–129. https://doi.org/10.1038/nrd4510
Newman DJ, Cragg GM (2020) Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 83:770–803. https://doi.org/10.1021/acs.jnatprod.9b01285
Dehelean CA, Marcovici I, Soica C, et al (2021) Plant-derived anticancer compounds as new perspectives in drug discovery and alternative therapy. Molecules 26
Rajan VK, Shameera Ahamed TK, Muraleedharan K (2018) Studies on the UV filtering and radical scavenging capacity of the bitter masking flavanone Eriodictyol. J Photochem Photobiol B Biol 185:254–261. https://doi.org/10.1016/j.jphotobiol.2018.06.017
Rajan VK, Muraleedharan K (2017) A computational investigation on the structure, global parameters and antioxidant capacity of a polyphenol, Gallic acid. Food Chem 220:93–99. https://doi.org/10.1016/j.foodchem.2016.09.178
Sumayya PC, Mujeeb VMA, Muraleedharan K (2022) Radical scavenging capacity, UV activity, and molecular docking studies of 2ʹ, 5ʹ, 3, 4-Tetrahydroxychalcone: An insight into the photoprotection. Chem Phys Impact 5:100126. https://doi.org/10.1016/j.chphi.2022.100126
Shameera Ahamed TK, Rajan VK, Sabira K, Muraleedharan K (2019) DFT and QTAIM based investigation on the structure and antioxidant behavior of lichen substances Atranorin, Evernic acid and Diffractaic acid. Comput Biol Chem 80:66–78. https://doi.org/10.1016/j.compbiolchem.2019.03.009
Ragi C, Muraleedharan K (2023) Antioxidant activity of Hibiscetin and Hibiscitrin: insight from DFT, NCI, and QTAIM. Theor Chem Acc 142:30. https://doi.org/10.1007/s00214-023-02970-5
Grzesik M, Naparło K, Bartosz G, Sadowska-Bartosz I (2018) Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem 241:480–492. https://doi.org/10.1016/j.foodchem.2017.08.117
Pietta P-G (2000) Flavonoids as Antioxidants. J Nat Prod 63:1035–1042. https://doi.org/10.1021/np9904509
Rajan VK, Ragi C, Muraleedharan K (2019) A computational exploration into the structure, antioxidant capacity, toxicity and drug-like activity of the anthocyanidin “Petunidin.” Heliyon 5:e02115. https://doi.org/10.1016/j.heliyon.2019.e02115
Rajan VK, Hasna CK, Muraleedharan K (2018) The natural food colorant Peonidin from cranberries as a potential radical scavenger—A DFT based mechanistic analysis. Food Chem 262:184–190. https://doi.org/10.1016/j.foodchem.2018.04.074
Pottachola S, Kaniyantavida A, Karuvanthodiyil M (2021) DFT study of structure and radical scavenging activity of natural pigment delphinidin and derivatives. In: Glossman-Mitnik D (ed). IntechOpen, Rijeka, p Ch. 16
Borges Bubols G, da Rocha VD, Medina-Remon A et al (2013) The Antioxidant activity of coumarins and flavonoids. Mini-Reviews Med Chem 13:318–334
Hamadouche S, Ounissi A, Baira K et al (2021) Theoretical evaluation of the antioxidant activity of some stilbenes using the Density Functional Theory. J Mol Struct 1229:129496. https://doi.org/10.1016/j.molstruc.2020.129496
Gonzalez-Burgos E, Gomez-Serranillos MP (2012) Terpene compounds in nature: a review of their potential antioxidant activity. Curr Med Chem 19:5319–5341
Perrelli A, Goitre L, Salzano AM et al (2018) Biological activities, health benefits, and therapeutic properties of Avenanthramides: From skin protection to prevention and treatment of cerebrovascular diseases. Oxid Med Cell Longev 2018:6015351. https://doi.org/10.1155/2018/6015351
Li X, Zhou L, Yu Y et al (2022) The potential functions and mechanisms of oat on cancer prevention: A review. J Agric Food Chem 70:14588–14599. https://doi.org/10.1021/acs.jafc.2c06518
Yu Y, Zhou L, Li X et al (2022) The progress of nomenclature, structure, metabolism, and bioactivities of oat novel phytochemical: Avenanthramides. J Agric Food Chem 70:446–457. https://doi.org/10.1021/acs.jafc.1c05704
Peterson DM, Hahn MJ, Emmons CL (2002) Oat Avenanthramides exhibit antioxidant activities in vitro. Food Chem 79:473–478. https://doi.org/10.1016/S0308-8146(02)00219-4
Bratt K, Sunnerheim K, Bryngelsson S et al (2003) Avenanthramides in Oats (Avena sativa L.) and structure−antioxidant activity relationships. J Agric Food Chem 51:594–600. https://doi.org/10.1021/jf020544f
Fagerlund A, Sunnerheim K, Dimberg LH (2009) Radical-scavenging and antioxidant activity of Avenanthramides. Food Chem 113:550–556. https://doi.org/10.1016/j.foodchem.2008.07.101
Zhang T, Shao J, Gao Y et al (2017) Absorption and elimination of oat Avenanthramides in humans after acute consumption of oat cookies. Oxid Med Cell Longev 2017:2056705. https://doi.org/10.1155/2017/2056705
Dimberg LH, Theander O, Lingnert H (1993) Avenanthramides–a group of phenolic antioxidants in oats. Cereal Chem 70:637–641
Sumayya PC, Babu G, Muraleedharan K (2021) Quantum chemical investigation of the antiradical property of avenanthramides, oat phenolics. Heliyon 7:e06125. https://doi.org/10.1016/j.heliyon.2021.e06125
Xue Y, Teng Y, Chen M et al (2021) Antioxidant activity and mechanism of Avenanthramides: Double H+/e– processes and role of the catechol, guaiacyl, and carboxyl groups. J Agric Food Chem 69:7178–7189. https://doi.org/10.1021/acs.jafc.1c01591
Purushothaman A, Jishnu Gopal P, Janardanan D (2022) Mechanistic insights on the radical scavenging activity of oat avenanthramides. J Phys Org Chem 35:e4391. https://doi.org/10.1002/poc.4391
Pizzino G, Irrera N, Cucinotta M et al (2017) Oxidative stress: Harms and benefits for human health. Oxid Med Cell Longev 2017:8416763. https://doi.org/10.1155/2017/8416763
Sharifi-Rad M, Anil Kumar N V, Zucca P, et al. (2020) Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases . Front. Physiol. 11
Mellon I (2004) Transcription-coupled DNA repair, overview. In: Lennarz WJ, Lane MDBT-E of BC (eds). Elsevier, New York, pp 204–208
Islam BU, Habib S, Ahmad P et al (2015) Pathophysiological role of peroxynitrite induced DNA damage in human diseases: A special focus on Poly(ADP-ribose) Polymerase (PARP). Indian J Clin Biochem 30:368–385. https://doi.org/10.1007/s12291-014-0475-8
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. and DJF (2016) Gaussian 16, Revision C.01
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3094. https://doi.org/10.1021/cr9904009
Analyzer AMW, Lu T (2019) Multiwfn. 7:
Humphrey W, Dalke A, Schulten K (1996) VMD: Visual molecular dynamics. J Mol Graph 14:33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Zhang J, Lu T (2021) Efficient evaluation of electrostatic potential with computerized optimized code. Phys Chem Chem Phys 23:20323–20328. https://doi.org/10.1039/D1CP02805G
Khlaifia D, Massuyeau F, Ewels C et al (2017) DFT modeling of novel donor-acceptor (D-A) molecules incorporating 3-hexylthiophene (3HT) for Bulk Heterojunction Solar Cells. ChemistrySelect 2:10082–10090. https://doi.org/10.1002/slct.201701481
Zhan C-G, Nichols JA, Dixon DA (2003) Ionization potential, electron affinity, electronegativity, hardness, and electron excitation energy: molecular properties from density functional theory orbital energies. J Phys Chem A 107:4184–4195. https://doi.org/10.1021/jp0225774
Putz MV (2013) Koopmans’ analysis of chemical hardness with spectral-like resolution. Sci World J 2013:348415. https://doi.org/10.1155/2013/348415
Parr RG, Szentpály LV, Liu S (1999) Electrophilicity Index. J Am Chem Soc 121:1922–1924. https://doi.org/10.1021/ja983494x
Chakraborty D, Chattaraj PK (2021) Conceptual density functional theory based electronic structure principles. Chem Sci 12:6264–6279. https://doi.org/10.1039/d0sc07017c
Dutra FR, de Silva C, S, Custodio R (2021) On the accuracy of the direct method to calculate pKa from electronic structure calculations. J Phys Chem A 125:65–73. https://doi.org/10.1021/acs.jpca.0c08283
Kelly CP, Cramer CJ, Truhlar DG (2006) Aqueous solvation free energies of ions and ion−water clusters based on an accurate value for the absolute aqueous solvation free energy of the proton. J Phys Chem B 110:16066–16081. https://doi.org/10.1021/jp063552y
Mittal A, Vashistha VK, Das DK (2023) Free radical scavenging activity of Gallic acid toward various reactive oxygen, nitrogen, and Sulfur species: a DFT approach. Free Radic Res 1–10. https://doi.org/10.1080/10715762.2023.2197556
Coimbra M, Isacchi B, van Bloois L et al (2011) Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int J Pharm 416:433–442. https://doi.org/10.1016/j.ijpharm.2011.01.056
Abdel-Mottaleb MSA (2016) DFT studies of caffeic acid antioxidant: molecular orbitals and composite reactivity maps correlation with photophysical characteristics and photochemical stability. J Chem 2016:8727130. https://doi.org/10.1155/2016/8727130
Marković Z, Tošović J, Milenković D, Marković S (2016) Revisiting the solvation enthalpies and free energies of the proton and electron in various solvents. Comput Theor Chem 1077:11–17. https://doi.org/10.1016/j.comptc.2015.09.007
Ferreira CA, Ni D, Rosenkrans ZT, Cai W (2018) Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res 11:4955–4984. https://doi.org/10.1007/s12274-018-2092-y
Alfadda AA, Sallam RM (2012) Reactive oxygen species in health and disease. J Biomed Biotechnol 2012:936486. https://doi.org/10.1155/2012/936486
Galano A (2015) Free radicals induced oxidative stress at a molecular level: The current status, challenges and perspectives of computational chemistry based protocols
Bartmess JE (1994) Thermodynamics of the Electron and the Proton. J Phys Chem 98:6420–6424. https://doi.org/10.1021/j100076a029
Najafi H (2013) Theoretical study of the substituent effects on the reaction enthalpies of the antioxidant mechanisms of stobadine derivatives in the gas-phase and water. J Theor Comput Chem 12:
Martínez A, Hernández-Marin E, Galano A (2012) Xanthones as antioxidants: A theoretical study on the thermodynamics and kinetics of the single electron transfer mechanism. Food Funct 3:442–450. https://doi.org/10.1039/C2FO10229C
Mittal A, Kakkar R (2020) The effect of solvent polarity on the antioxidant potential of echinatin, a retrochalcone, towards various ROS: a DFT thermodynamic study. Free Radic Res 54:777–786. https://doi.org/10.1080/10715762.2020.1849670
Acknowledgements
The author Sumayya P.C. expresses sincere gratitude to UGC-MANF for financial support (Research fellowship). The authors are also thankful to the Central Sophisticated Instrumentation Facility (CSIF) of the University of Calicut for the Gaussian 16 software support.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
Author information
Authors and Affiliations
Contributions
S.P.C. involved in idea, methods, software, experiment setup, preparation of data and evaluation, and paper writing and editing. Dr. K.M. involved in monitoring, proof reading, final editing, and guidance.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Human and animal rights
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sumayya, P.C., Muraleedharan, K. Quenching of reactive species by Avenanthramides: theoretical insight to the thermodynamics of electron transfer. Theor Chem Acc 143, 37 (2024). https://doi.org/10.1007/s00214-024-03111-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-024-03111-2