Abstract
One of the biggest challenges when teaching chemistry at the elementary and secondary levels arises from the conceptual gap between the ontology of macroscopic objects and agents and an ontology where the relevant objects behind macroscopic phenomena are, in fact, inaccessible to our senses. This work employs tools from the general philosophy of science to uncover the implicit ontology of school chemistry, offering an analysis that aligns with Talanquer’s proposition of a structure–property progression in chemistry learning. This is achieved through examining the distinction between two different theoretical functions in our science: modeling interactions and providing general structural frameworks. We envision that our proposal will help teachers when guiding the conceptual transitions of students along their learning progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the opening lines of his renowned book “The Nature of the Physical World,” Sir Arthur Eddington discusses two tables: a solid one on which he can rest his hands, and a second one composed of minuscule particles, essentially made up of empty space—completely dissimilar to the first table. The progress of science has repeatedly demonstrated that things are not as they appear to us. Navigating this conceptual shift is one of the most crucial objectives of formal education in modern societies.
Within the realm of science education, no other subject is as transformative in shaping our perception of the world as chemistry. One could argue that the primary role of chemistry in formal education is to instill in students a new worldview, completely detached from their sensory experiences. In this paradigm, the behavior of chemical substances is interpreted in terms of invisible, intangible objects (Johnstone, 1993). It is a profound departure from common sense to assert that the substances we encounter are composed of atoms, that all our experiences can be reduced to combinations of a few elements, and that the diversity of observed properties emerges from the interactions of these elemental entities. Such a shift necessitates adopting an ontology entirely different from what our senses report. This alteration of reality raises subtle metaphysical questions that warrant philosophical inquiry.
The roots of this discussion can be traced back to Lavoisier and the origins of the discipline, but it was Mendeleev who explicitly addressed the issue when he proposed a periodic system for the elements. He argued that these elements should not be viewed as simple substances but rather as metaphysically inaccessible entities, of which we can only know one property—their atomic weight (Labarca, 2016; Scerri, 2020).
The transition to understanding sub-microscopic entities presents significant cognitive challenges, creating complex problems for teachers to tackle. Let us consider a concrete example: a student is given the task of distinguishing between two samples of yellow dust, one of sulfur (S) and another of lead oxide (PbO). In such a scenario, the comparison is drawn between substances that appear similar at the macroscopic level and possess comparable structural patterns without further intervention. Herein lies the task for chemistry teachers—to persuade students that these seemingly alike objects are actually composed of distinct entities—microscopic particles—that also serve as the foundational concepts for other essential chemical notions, such as differentiating between elements and compounds. Convincing pupils of this notion is crucial for building a solid understanding of chemistry.
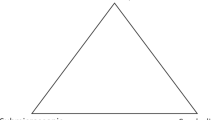
The distinction between the macroscopic and microscopic levels has deeply influenced the discourse on chemistry education, most notably through Johnstone’s well-known triad of thinking levels (Johnstone, 1991, 1993, 2000). In an effort to enhance the teaching and learning of chemistry in schools, Johnstone proposes classifying chemical thinking into three levels: the macroscopic level, which deals with tangible objects; the sub-microscopic level, involving atoms, molecules, ions, and other chemical structures; and the symbolic level, representing concepts expressed through equations, symbols, and graphs. These three levels can be depicted as vertices of a triangle, underscoring their interconnectedness (see Fig. 1). While Johnstone does not assign superiority to any particular level, he acknowledges that the explanatory power of chemistry is grounded in the sub-microscopic level. Interestingly, although Johnstone never explicitly approached the triad from a metaphysical perspective, it becomes evident that the macroscopic and sub-microscopic levels presuppose the existence of two distinct levels of reality. Similar to Eddington’s table analogy, the ontology of water as a macroscopic substance differs significantly from the ontology of hydrogen and oxygen atoms engaging in continual interactions.
The three basic components of chemical descriptions. From Johnstone (1993, p. 703)
Despite its significant influence, Johnstone’s contribution has not fully addressed the learning challenges faced by students. When grappling with explanations related to mixtures or chemical reactions, research has demonstrated that students often conceptualize an (incorrect) intermediate level between the macroscopic and the submicroscopic, known as the semi-particulate level (Galagovsky et al., 2003). Within this framework, students attribute macroscopically observable properties to sub-microscopic particles, for instance, associating red color and malleability with copper atoms. Thus, while Johnstone’s triad implies an ontological distinction between the macroscopic and submicroscopic levels, it alone does not offer complete solutions to the learning difficulties encountered by students during the transition between these levels. These difficulties are often referred to as erroneous (Sanders, 1993; Özalp and Kahveci, 2015) or alternative conceptions (see Chi et al., 1994; 2008). These terms denote non-scientific ideas or explanations that stem from students’ belief systems or prior experiences before formal education, influenced by their culture and general context, ultimately impeding their acquisition of accurate scientific concepts. The process of transitioning from alternative conceptions to conceptual change, understood as learning that modifies pre-existing concepts (Chi et al., 1994), faces a hurdle linked to the ontological assumptions underpinning the explanatory discourse used in the classroom. This work aims to explore this aspect and highlight its significance.
We firmly believe that the ontological distinction between the macroscopic and submicroscopic realms is a pivotal element for unlocking a profound understanding of chemistry. This viewpoint is also shared by Talanquer (2018), who underscores the importance of considering the interplay between the macroscopic and submicroscopic levels when explaining the phenomena studied in school chemistry.
Talanquer draws upon well-established notions of conceptual change and types of human reasoning (Brown, 2014; Chi, 2008) to explore the progression that students undergo as they attempt to explain the properties of macroscopic substances in terms of their composition.
Throughout this learning process, Talanquer identifies intermediate stages of explanation that gradually become more sophisticated in terms of chemical knowledge. These stages represent the changing notions that students grapple with as they develop a deeper understanding of chemistry and its underlying principles. By comprehensively exploring the evolution of student reasoning, Talanquer sheds light on the complexity of conceptual change in chemistry education.
In this study, we build upon the understanding that ontological confusions present a significant obstacle to the effective learning of chemistry, as evidenced in existing literature. We see this as an opportunity to make a meaningful contribution to chemistry education by incorporating perspectives from the philosophy and metaphysics of science.
Specifically, we demonstrate that Talanquer’s proposal for a progression in the teaching process in schools is rooted in the recognition and resolution of these ontological confusions. By employing theoretical tools derived from the philosophy of science, we show how these confusions can be effectively dissolved.
Taking our metaphysical analysis further, we argue that, at the level of school chemistry, it is more appropriate to perceive macroscopic substances not as actual entities but rather as patterns or structural bundles of regularities that emerge from the interactions of more fundamental (submicroscopic) substances.
We firmly believe that such theoretical tools will prove invaluable to chemistry teachers, and it should be an essential topic for discussion and analysis during the training of future teachers. By embracing the insights from philosophy and metaphysics, we aim to enhance chemistry education and contribute to a deeper and more comprehensive understanding of the subject for future educators.
The structure of this work is as follows: Sect. 2 provides a concise overview of the issue of identifying ontological levels within chemistry education. In Sect. 3, we follow Talanquer’s ideas to demonstrate how each stage of learning is based on expanding a structural understanding to encompass more aspects of macroscopic phenomena. Section 4 explores how certain concepts from the general philosophy of science and scientific metaphysics highlight the connection between interactions and material objects. Additionally, it emphasizes that a significant portion of scientific descriptions focuses not on interactions or objects, but on structural frameworks. This has implications at both the ontological and epistemic levels, which we believe are pertinent to science education. Section 5 serves as the bridge between these philosophical concepts and the findings of Talanquer, illustrating how the former provide a solid foundation for the latter. It enables a deeper comprehension of the challenges that students encounter in their learning process. Section 6 briefly demonstrates how our proposal aligns naturally with current didactic strategies, such as modeling, by explicitly linking it to relevant examples from existing literature. Finally, in Sect. 7, we present our conclusions summarizing the key insights and contributions of our work.
2 Ontological Issues in Chemistry Education
Science teachers often encounter a common issue in the classroom: despite providing students with similar conditions and learning opportunities, only some of them successfully make the desired transition to a new conceptual framework. Harrison and Treagust (2001) shed light on this matter, explaining that the problem lies at the ontological, epistemic, and socio-affective levels.
On the ontological level, the authors highlight that students tend to unjustifiably transfer macroscopic properties to microscopic entities. For instance, in a case study, it is shown that when teaching the concept of particles, various analogies are used, inadvertently leading students to overlook the microscopic nature of particles. Furthermore, students may fail to recognize that macroscopic properties are a product of the interactions and behavior of these microscopic constituents. These analog models can unwittingly reinforce the notion that causal explanations function solely at the macroscopic level, resulting in ontological confusions. This becomes apparent in the erroneous transitions students make in their explanations when they describe microscopic entities with macroscopic characteristics or properties.
In a similar vein, Chi et al. (1994) stated that the essence or metaphysical standing of the concept of interest needs to be adjusted along the path of conceptual change. In essence, it is crucial to address the fundamental understanding of the concept, particularly when dealing with the transition from macroscopic to microscopic levels in scientific explanations. By recognizing and addressing these issues at the ontological level, science educators can better support students’ conceptual change and foster a deeper understanding of the subject matter.
Along the same lines, Özalp and Kahveci (2015) conducted an inquiry into students’ understanding of the concept of particles and found that students often attribute macroscopic properties to submicroscopic entities. For instance, they may believe that the shape of a water droplet is directly associated with water molecules, or that molecules behave like melting ice cubes as temperatures rise, thus transferring their common-sense knowledge to the submicroscopic realm. Similarly, students tend to associate characteristics of gold atoms with the hardness and brightness of jewelry they have personally encountered or culturally integrated. They may also mistakenly believe that atoms and molecules can be observed under a microscope, similar to how they observe cells in their biology class. Additionally, students may link color and smell to these submicroscopic particles. When considering fluids, they commonly conceptualize particles as small spheres of fluid, moving in contact with one another, which is a misconception also found among adults.
In summary, students face difficulty in distinguishing between submicroscopic particles and the macroscopic world they constitute. This inability to differentiate between two different realms of phenomena and recognize their causal dependency is what we refer to as a case of ontological confusion. Students struggle to identify the interactions of submicroscopic entities as the underlying cause of the properties and characteristics observed in macroscopic objects and events. Addressing and resolving these ontological confusions is crucial to supporting students’ conceptual change and fostering a more accurate understanding of chemistry and its submicroscopic nature.
Another classic example of these issues arises with acid–base theory, where erroneous conceptions are sometimes reinforced by standard textbooks. These textbooks may loosely traverse from the macroscopic to the submicroscopic level, rather than focusing on the central ideas (Paik, 2015), leading to misleading or inaccurate explanations and applications (Quílez, 2019). As a result, students’ understanding of chemistry is hindered (Quílez, 2021).
When it comes to acid–base phenomena, students often mix their explanations by referring to microscopic entities such as protons or hydroxide ions but attributing to their properties and characteristics observed in the macroscopic reactions, such as the colors they see in the lab. They also struggle to grasp the concept that the same species can act as an acid or a base depending on its interaction with other substances. This highlights the fact that students fail to assign the explanation of the phenomenon to the interaction of entities at the submicroscopic level, and instead, they remain at the macroscopically observable level.
The enumeration of such conceptual mistakes could continue at length, and several recent descriptions of these issues can be found in the literature (Chi & Roscoe, 2002; Gómez et al., 2006; Ayas et al., 2010; Kahveci, 2009; Nyachwaya et al., 2011; Özmen, 2008; Tsitsipis et al., 2011; Bridle and Yeziersky, 2012; Muniz et al., 2018; Lemma & Belachew, 2022). The underlying categorical mistake remains the same: students assign macroscopic properties or characteristics to submicroscopic entities without fully understanding that it is the interaction of objects at an entirely different ontological level that causally explains chemical phenomena. This ultimately hinders students from progressing towards a scientifically accurate conceptualization of matter. Addressing and rectifying these conceptual challenges is crucial for facilitating a deeper and more accurate understanding of chemistry among students.
Indeed, authors such as Posner et al. (1982) (cited in Harrison & Treagust, 2001) have provided analyses of these issues primarily in epistemic terms. However, there is a clear affinity between their ideas and the diagnosis we have presented. The relationship between conceptual change and ontology is explicitly explored in the works of authors like Chi et al. (1994), Thagard (1993), and Vosniadou (1994), who emphasize the significance of ontological analyses in understanding how students explain scientific phenomena.
For instance, Chi et al. (1994) discuss conceptual change based on the premise that scientific entities belong to distinct ontological categories and are an intrinsic part of the world’s makeup. According to this perspective, conceptual change occurs when students are capable of shifting their explanations to the appropriate ontological level (Chi & Roscoe, 2002). In other words, students need to modify the way they perceive the world and place their explanations within the correct ontological framework. Ideally, this process should be guided by a teacher who is aware of the issues we have discussed. It is not sufficient to monitor learning while assuming the base ontology as a given and universally accepted premise. Instead, it is necessary to encourage students to question their implicit ontological commitments to achieve significant learning of the contents in the chemistry curricula.
By promoting a deeper understanding of the ontological underpinnings of scientific explanations, teachers can facilitate meaningful conceptual change and help students develop more accurate and scientifically sound perspectives of the chemical world. Encouraging students to question and critically analyze their implicit ontological assumptions is an essential step in fostering a more profound and enduring comprehension of chemistry concepts.
Indeed, it can be concluded that ontological confusion is a significant factor contributing to the challenges faced by chemistry teachers. When students attempt to provide causal explanations for scientific phenomena, they frequently rely on macroscopic elements rather than understanding the underlying submicroscopic interactions. This reliance on macroscopic explanations demonstrates how misunderstandings about ontology can impede the process of conceptual change in students' understanding of scientific concepts.
This raises important questions about the role of science education in bridging the gap between these two ontological levels in the teaching practice. The ultimate goal is to facilitate the students’ adequate appropriation of scientific phenomena. To achieve this, science educators need to take a deliberate and thoughtful approach to address ontological issues in the classroom.
Teachers should strive to guide students towards a more accurate and scientifically valid understanding of the subject matter by emphasizing the connections between macroscopic observations and the underlying submicroscopic interactions. By making these connections explicit, students can develop a more coherent and consistent conceptual framework that aligns with the principles of chemistry.
Science education must go beyond merely conveying facts and concepts; it should encourage students to think critically about the ontological foundations of scientific explanations. By fostering a deeper understanding of ontology and its role in scientific understanding, educators can help students navigate the conceptual changes necessary to develop a robust and scientifically sound understanding of chemistry.
In summary, addressing ontological confusion and explicitly connecting the macroscopic and submicroscopic levels in the teaching practice are vital steps to facilitate meaningful learning and support students’ appropriation of scientific phenomena in chemistry education.
3 Macro- and Micro-ontologies
The problems we have highlighted in chemistry education share a common feature: confusion at the ontological level. This is not surprising, as chemistry is the primary subject in early formal education where the conventional understanding of the world is challenged. The implicit message of school chemistry is that macroscopic properties, which we naturally associate with attributes of visible objects, are actually the outcomes of interactions among invisible submicroscopic entities. While this message might be evident and uncontroversial for most teachers, we believe that it has not been emphasized to the extent it deserves.
The ontology of school-level chemistry revolves around submicroscopic objects, while the macroscopic materials and phenomena we observe serve the theoretical function of providing guiding structures or constraining the possible behaviors at the atomic and molecular levels that are relevant to the subject. For instance, school chemistry assumes that the physical properties of water, such as density, surface tension, boiling point, and heat capacity, are all explained by interactions between H2O molecules. However, the relationship between these macroscopic properties and the submicroscopic ones is more subtle than it may initially appear.
The macroscopic observations impose constraints on the microscopic states, ensuring that only a small subset of the countless possible submicroscopic behaviors is analyzed in each case. For example, a proton by itself behaves very differently from a proton in an aqueous solution, and it is only the latter that is relevant to acid–base theory. This subtlety is of significant importance and warrants further consideration.
Interestingly, the question of the ontology of chemistry has been a topic of debate within philosophers of science. The literature can be divided into two main currents: microstructuralism and anti-microstructuralism, which differ in their stance on whether the submicroscopic level entirely determines the macroscopic one or not. Similarly, there has been extensive discussion about different kinds of reduction of chemistry to physics.
For readers interested in this topic, we recommend following the academic discussion between Olimpia Lombardi and other renowned authors in the philosophy of chemistry. The debate can be traced through a sequence of articles: Lombardi and Labarca (2005), Needham (2006), Lombardi and Labarca (2006), Labarca and Lombardi (2010), Lombardi and Castagnino (2010), Mulder (2011), Lewowicz and Lombardi (2013), Lombardi (2014), Hettema (2014), and Manafu (2013). While we will not delve into the specifics of these discussions here, it is important to emphasize that the question of whether the submicroscopic world is the entirety of the ontology of chemistry remains an open one. However, at least at the level of school chemistry, we can safely consider the world to be made up of submicroscopic components. What is taught in schools essentially posits that these submicroscopic entities entirely determine the macroscopic world’s phenomenology through their properties and interactions.
The ontological division between the macroscopic and submicroscopic realms lies at the heart of the competences that chemistry aims to develop at the school level. This recognition has been well-established, dating back to the works of Johnstone (1991, 1993, 2000), and it also holds significant importance in Talanquer’s research on the progression of reasoning concerning the structure–property relationship. In particular, Talanquer (2018) identifies a series of intermediate phases of student understanding for each of the two ontological levels. These phases describe the typical progression of chemical knowledge among students, encompassing properties that may appear intrinsic to systems, as well as those related to perceptible changes. A summary of these phases is presented in Table 1.
If we analyze in detail the progression found by Talanquer regarding the understanding of intrinsic properties, it is interesting to note that what distinguishes one stage from the next is the type of component that students invoke to make sense of the properties and phenomena under study, with associative thinking being a common thread. Let us examine some examples from Talanquer’s (2018) classification:
-
(1)
Corpularism: At this stage, students transfer properties from one ontological level to another. For instance, they might think that copper atoms are red and malleable, or that gas particles expand when the temperature rises.
-
(2)
Atomic/functional compositionism: In this stage, students make different connections between ontological levels depending on the type of atoms or molecules known to play a part in the system. For example, they might associate oxygen atoms with flammability or recognize that the OH group in alcohols affects the boiling temperature.
-
(3)
Atomic centralized causalism: When it comes to behaviors that can be directly perceived, such as solubility or reactivity, and are often explained by an agent carrying certain dispositions, students usually establish a linear connection between these dispositions and the observed properties. For instance, at this stage, students might connect the reactivity of some atoms to their size, assuming a direct correlation.
-
(4)
Energetic stabilism: Here, students see chemical stability (or lack of reactivity) as an absolute property of a chemical species, depending on the composition and structure of subcomponents assumed to be static and isolated from each other. For example, students might think that bromide is bigger than chlorine, and therefore more stable.
These stages illustrate the gradual development of students’ understanding of intrinsic properties, and how they progressively link different components at the submicroscopic level to explain macroscopic phenomena.
What is most relevant for this work is the clear ontological distinction between the submicroscopic and macroscopic worlds, which forms the foundation of school-level chemistry. Specifically, the focus is on how students’ understanding of this division evolves towards a state in which the causal mechanisms explaining all chemical phenomena are attributed to the submicroscopic agents, while macroscopic systems provide constraints and boundary conditions for the interplay of these microscopic agents. The main goal of chemistry education at the school level is to guide students to reach this final stage of abstraction, where they can construct causal-mechanistic explanations that connect the scales of electrons, atoms, molecules, and ultimately the macroscopic world.
It is important to note that this progression is not solely about differentiating between the macro and micro scales, as students may incorporate microscopical elements at various stages of learning. Rather, the crucial distinction lies in the functional role that these levels play. The submicroscopic level becomes meaningful only to the extent that it reveals, through interactions, the underlying cause of macroscopic properties and phenomena. In other words, as students progress through their learning journey, they may refer to microscopic elements at different stages, but they gradually approach an understanding of the distinct functional roles of the two ontological levels. It is only in the final stage that they fully grasp the theoretical significance of the submicroscopic world, recognizing its key role in explaining macroscopic phenomena through microscopic interactions.
If we analyze the transition described by Talanquer, the most significant aspect is the gradual shift of causal mechanisms from macroscopic substances to the submicroscopic level, where all causal events ultimately occur in the last stage. This pattern is evident at each stage of Talanquer’s progression, and we can illustrate this using the intrinsic properties of water as an example:
-
(1)
Intuitive eclecticism: At this stage, water’s intrinsic characteristics are attributed to whatever components it is made of, such as being liquid and wet.
-
(2)
Macro-compositionism: In this stage, water’s properties are attributed to macro-components, like being hydrated in the presence of oxygen.
-
(3)
Corpularism: The properties of water are associated with the intrinsic properties of its submicroscopic components. For instance, students might think that water molecules have the shape of droplets or little ice cubes that melt at higher temperatures.
-
(4)
Atomic compositionism: At this stage, water’s properties are directly related to the presence of oxygen and hydrogen, without considering global structural issues. For example, students might attribute the higher boiling point of water to the larger size of oxygen atoms compared to hydrogen atoms.
None of these explanations seems to rely on interactions between agents; instead, they are more focused on structural aspects. This structural perspective descends in scale until it eventually only considers atoms and molecules as the causal agents.
These conceptual transitions are subtle and can lead to various erroneous alternative conceptions among students. Hence, it is crucial to seek conceptual and educational tools that assist teachers in facilitating more fluid transitions. In the next section, we present a classification of theories derived from general arguments in the philosophy of science. These theories may provide the necessary tools to correctly identify ontological and functional roles in theories and models of chemical phenomena, thereby helping to address the challenges in chemistry education.
4 Interactions and Frameworks in Chemistry
Up to this point, our focus has been on the issues in science education related to the incorrect ontological classification of elements in chemistry and how school chemistry aims to provide explanations of phenomena based on the interactions between submicroscopic constituents of matter. These concepts align well with the meta-theoretical distinction proposed within the general philosophy of science between framework and interaction theories, which attributes different ontologies, theoretical functions, and types of explanations to each type of theory or theoretical construct.
The origin of this distinction can be traced back to the writings of Galileo, Newton, and Poincaré, but it gained further prominence in the philosophy of physics after an article by Einstein (1919). More recently, it has been refined by scholars like Flores (1999) and Maltrana et al. (2022). The crux of the distinction is that theoretical products in science, such as theories, laws, and models, can generally be categorized into two groups:
-
(1)
Interaction theories: These theories allow for the elaboration of mechanistic-causal explanations, explicitly modeling the action of agents that are causally responsible for phenomena. In the context of chemistry, these would involve theories that focus on describing and explaining the interactions between submicroscopic entities, revealing the causal mechanisms behind macroscopic properties and events.
-
(2)
Framework theories: On the other hand, framework theories enable the elaboration of unificationist explanations of phenomena through structural constraints that do not depend on any specific agent. These theories provide the broader conceptual frameworks within which interactions between agents can be understood and integrated.
The distinction between these two types of theories offers valuable insights for understanding the nature of scientific explanations and the roles of different theoretical constructs in explaining natural phenomena. In the context of chemistry education, recognizing this distinction can aid in developing effective teaching strategies and conceptual frameworks to facilitate students’ understanding of the causal and structural aspects of chemical phenomena.
Einstein used two theories, thermodynamics and the kinetic theory of gasses, as examples to illustrate the distinction between framework and interaction theories. In thermodynamics, the laws are universally applicable and independent of the constitution or specific properties of matter. For instance, the second law of thermodynamics is valid for any system, whether it be an ice cube or a black hole. It provides a structural framework that governs all processes, irrespective of their nature. As thermodynamics applies universally, the explanations derived from its laws do not require specific mechanisms or causal agents to be mentioned.
On the other hand, the kinetic theory of gasses, formulated by Boltzmann, presupposes that gasses consist of microscopic particles that undergo mutual collisions. According to this theory, all the macroscopic properties of gasses, such as pressure and temperature, result from the interactions between these microscopic gas particles.
Flores provides a philosophical analysis of Einstein’s proposal, identifying three dimensions in the distinction: ontological, epistemic, and functional. Each dimension provides elements that differentiate these two types of theories, as depicted in Table 2.
Within interaction theories, agents that interact are essential components, as these theories describe phenomena based on the interactions of actual or modeled pieces of matter. Their functional role at the meta-theoretical level is to provide information about observed interactions in the natural world. Consequently, an ontology of agents and their dispositions to act becomes necessary to fulfill this function. Interactions cannot be conceived without the agents undergoing them, as there are no relations without relata, and these theories focus on studying specific relations in nature.
Ontologically, interactions are what cause a change in the material world. Every effect in the material world has a material cause, which is invariably the result of interactions. Therefore, interaction theories enable bottom-up explanations of phenomena.
On the other hand, framework theories can generate descriptions of the material world without directly appealing to causal mechanisms. Instead, they aim to capture common structural elements that unify a group of phenomena. These regularities explicitly exclude agents and interactions and are grounded in constraints on the modal space in which individual cases are framed. The ontology of framework theories and theoretical elements is in principle empty of objects and purely structural, as they abstract away from individualities to reveal general principles or conditions that apply to any interaction within the realm of interest.
The framework/interaction distinction allows for the recognition of the epistemic, ontological, and functional roles of diverse theoretical elements even before conducting a detailed analysis of the theory. This classification is particularly relevant in chemistry education, where the main objective is to help students transition to a view of the world where macroscopic properties emerge from interactions at the submicroscopic level. This connection between the framework/interaction distinction and chemistry education is further explored in the following section.
5 Interactions as Submicroscopic Causes
In his seminal 1919 article in the Times of London, Einstein explicitly expressed his preference for causal explanations. According to his arguments, only what we now refer to as interaction theories (which he called “constructive” theories) provide genuine explanations of phenomena, as they reveal the causal mechanisms behind them.
However, modern philosophy of science has moved beyond these biases and now considers both causal explanations and “unificationist” explanations as legitimate and complementary approaches. Unificationist explanations, which do not focus on causal processes, are recognized as valuable in their own right. Some authors even argue that this complementarity between causal and unificationist explanations can lead to ideal explanations (see Salmon, 1984; and De Regt, 2006).
Considering this historical bias towards causal explanations, it becomes even more important to take into account the recent works by Talanquer in the context of chemistry education. At each stage of the progression analyzed by Talanquer, we can observe how students construct their understanding of chemical phenomena by introducing spurious agents that they assume to be causally responsible for the observed phenomenon. However, in many cases, there are no actual interactions or causal connections, and these “agents” are merely personifications of essential characteristics.
Talanquer (2018, p. 999) states:
Humans also seem to have implicit causal schemas that guide the identification of plausible causes for given effects (Grotzer, 2003; Keil, 2006). These schemas tend to be general cognitive constructs that are applied to build explanations in different domains (Talanquer, 2006; diSessa, 2014; Coley et al., 2017). We often, for example, explain changes by looking for an agent acting on or within a system in linear and direct ways (linear causality), explain behaviors in terms of the wills or desires of certain agents (teleology), or explain observable properties in terms of hidden components or essences with inherent characteristics (essentialism). These causal schemas allow us to generate tentative hypotheses of why things happen.” (Talanquer, 2018, p 999).
This bias towards causal explanations is evident at every stage of students’ progression in their understanding of chemistry. Whenever students assign a property to “something” (which could be an object, a system of objects, a state, etc.), there is a risk of attributing causal agency to that “something.” Talanquer points out this tendency, as properties are commonly perceived as belonging to agents that possess them, and untrained individuals often struggle to recognize structural properties as modal restrictions without imagining an agent behind them.
This bias contributes to the ontological confusions reported in the literature and summarized in Sect. 1. Students frequently attribute macroscopic properties (such as color, shape, hardness, fluidity) to mesoscopic structures, systems of molecules and atoms, or even submicroscopic particles. This is a result of two interrelated mistakes: firstly, the tendency to see properties as belonging to some identifiable “something” or “someone,” which reinforces the inclination to seek agents, even in cases where the structure is the only relevant factor. Secondly, many of these properties are causally inert and simply emerge as a consequence of interactions at a lower level of reality.
The latter point may seem evident upon detailed analysis. It is clear that the property of “fluidity” relies on the intensity of interactions between molecules or particles. For example, to have a liquid, the interaction among its submicroscopic components should be strong enough to prevent the macroscopic system from transitioning into a gaseous state at room temperature, but also sufficiently weak to avoid forming solid structures. Similarly, we understand that the transparency of certain substances results from photons with energies within the visible spectrum being unable to interact with the electronic levels of the submicroscopic elements that constitute the substance. However, the idea that material entities can be singularized by their causal profile is so counterintuitive that it is probably the source of a profound and well-reported misconception, namely, that some students think of particles as embedded in matter, failing to recognize that matter is no more than the resultant of those particles’ interactions (Beerenwinkel et al., 2011; Griffiths & Preston, 1992; Renström et al., 1990; Wiser & Smith, 2009).Footnote 1
Grasping the abstract notion that the properties we typically associate with material substances are, in most cases except for the most fundamental dispositions (as discussed in Benitez, 2019, where a dispositionalism related to fundamental charges in physics is defended), the outcome of interactions among elements belonging to lower levels or scales than the one observed, constitutes a conceptual leap that is particularly challenging to make. This understanding usually arises implicitly within the contents taught in chemistry class, making it a difficult concept to fully comprehend.
Indeed, students often do not initially learn that the properties we observe are emergent from interactions between submicroscopic elements. Instead, this understanding tends to develop gradually over time, as depicted in Talanquer’s research findings. However, it is important to recognize that this need not be the case, as these concepts directly stem from the distinction between theories proposed by Einstein and later refined by Flores and other scholars. The underlying message is simple yet transformative: not all theoretical products resulting from scientific endeavors require descriptions in terms of agents, but some offer structural elements that constrain interactions. Agents only come into play when interactions occur.
We firmly believe that conveying this message can significantly impact the teaching and learning of chemistry. By highlighting the role of interactions in shaping macroscopic properties, students can better comprehend the emergence of properties from underlying interactions at the submicroscopic level, leading to a more profound understanding of the subject.
In the context of school chemistry, interactions are primarily observed at the submicroscopic level. Content that explicitly focuses on interaction theories and mechanistic-causal explanations naturally aligns with this ontological level. However, this does not imply that at higher scales, there are no interacting agents or theories describing such interactions. In biology, for instance, interactions between bacteria and host cells or between higher animals are discussed, and engaging with these interaction theories commits us ontologically to the agents involved (such as viruses, cells, and animals).
What we aim to emphasize is that, in the case of school chemistry, the focus remains implicitly on interactions among submicroscopic elements. The description of interactions between macroscopic substances is generally left for physics or biology classes. This pedagogical decision has significant ontological implications, as it implies that the dispositions responsible for macroscopic interactions ultimately result from more fundamental interactions at the submicroscopic level. Consequently, these macroscopic properties become structural regularities that can be explained by referencing the submicroscopic level.
Awareness of the distinction between framework and interaction theories can prove invaluable for teachers in identifying the root of alternative erroneous conceptions and designing effective teaching tools to facilitate the desired conceptual change. The philosophical insight that there is no agent without interactions is crucial, as it reminds us that our inherent bias often leads us to perceive agents even in cases where interactions might be the key driving factor. This is a common finding in the literature on chemistry teaching.
Equipped with these philosophical tools, teachers can more readily discern the causally responsible interactions related to the specific phenomenon they are teaching. They can assess whether their students are capable of recognizing these interactions and create appropriate pedagogical approaches to facilitate their understanding. By anticipating possible ontological challenges in the learning progression, teachers can guide their students more effectively, leading to a deeper and more accurate understanding of the subject matter.
6 Teacher Input for the Classroom
Our main goal of this manuscript is to highlight and justify the relevance of the meta-theoretical distinction between framework and interaction theories for chemistry teachers in abstract terms, by arguing that the school’s chemistry curricula implicitly rest on it. There is much work to be done to bridge the gap between a conceptual clarification in ontological, epistemic, and functional terms and different strategies for the implementation of these ideas by teachers. Nevertheless, it is worth showing, however briefly, how our proposal materializes.
It is interesting to note that Lavoisier, when proposing the law of conservation of mass, never refers to agents, but rather systematizes certain regularities—in this case, the amount of mass present in reactants and products. The same is true with other ponderal laws, such as Proust’s law (definite proportions) and Dalton’s law (multiple proportions), which were established in terms of the regularities that occur when measuring the mass of elements that combine to form compounds. These laws are all framework laws, general constraints that do not depend on specific materials or the action of any agent, whether submicroscopic or otherwise. Already at this stage, the framework/interaction distinction could work as a tool to better understand conservation laws and their constraining character. But we can try to do better and explore how things are done with specific content in the classroom.
Certainly, there are differences in the curricula in different countries, and the approach to each topic is far from unique, but here, we consider that the case of combustion is such a fundamental issue for chemistry that its treatment is more or less standardized around the world.
In the case of teaching combustion, this phenomenon is described as an everyday chemical reaction that is important for living beings. Descriptions usually begin by observing situations in which “things burn,” particularly with experiments like the classic candle experiment. The guiding questions for the discussion are typically: what kind of change does it correspond to (physical or chemical)? What makes the candle stay lit? What is released during combustion?
Then, considering that part of what the candle needs to stay lit is in the air, ways of scientifically validating this hypothesis are agreed upon (by placing a jar that covers the candle, then jars of different sizes, recording the time it remains lit). Finally, to explain the phenomenon of combustion, microscopic agents are necessarily used: what particles are consumed in the combustion? What happens to them? In this way, the chemical reaction is explained as a process of atomic reorganization, with breaking and formation of bonds, which generates products and can be represented in symbolic language such as chemical equations, subject to the law of conservation of mass. Overall, the case of combustion is used to encourage students to understand the composition of matter as particles at the submicroscopic level (atoms), which interact (breaking and forming bonds, for example) and produce changes at the macroscopic level.
However, this microscopic explanation of combustion is “framed” or constrained by a macroscopic fact: for the reaction to start, it needs the input of energy, for example, by raising the temperature of the substance. So, the microscopic explanation gives the causal chain of the goings-on at the submicroscopic level, but these chains of causes and effects only occur under circumstances that are structurally determined by macroscopic situations.
The basic building blocks of our proposal are well recognized in the extant literature. Mensah et al. (2016) link conceptual change theories with epistemic and ontological issues, highlighting that conceptual change should transition from preconceived common-sensical notions to scientific theories. Already Nakhleh (1994) recommended different methodological approaches and activities that allow such a transition, while Cheng and Gilbert, Davidowitz and Chittleborough in Gilbert & Treagust (2009), and Vosniadou (2003) remark on the centrality of this transition in achieving educational success. Conceptual change is a slow, gradual, and constructive process of concept revision and formation (Vosniadou et al., 2008), rather than a sudden substitution of a naïve idea for a scientific theory. As discussed in Sect. 2, alternative conceptions are not isolated elements but complex structures that often coexist with scientific theories (Vosniadou & Brewer, 1993). The implications of our approach to the teaching–learning process involve considering these alternative conceptions when planning instruction, thereby encouraging students to modify or create a cognitive structure for new knowledge (Amin et al., 2014). Our proposal, with its roots in the philosophy of science, can also be followed in terms of a pedagogical strategy that promotes conceptual change. Its intervention is most effective at the level of the metaconceptual discourse mediated by the chemistry teacher, which enhances the sophistication of school science explanations. Let us exemplify how this can be achieved.
Iturra et al. (2021), using New Year’s “sky lanterns” and the potential risk of fire as a significant situated context, investigated the scientific explanations elaborated by ninth-grade students regarding the concept of the limiting reagent (LR). Sky lanterns, also known as Chinese lanterns or Kongming lanterns, are small hot air balloons made of lightweight paper, with an opening at the bottom where a small fire is suspended. They have a lightweight frame, typically made of bamboo, that holds the paper in shape, and come in various colors and sizes. When the fuel is ignited, the lantern ascends into the sky, drifting with the wind, and creates a glowing spectacle as it ascends until the fuel is exhausted, after which it gently descends to the ground. This uncontrolled landing involves a potential risk of fire.
The activity promotes the integration of the three levels of representation: macroscopic, microscopic, and symbolic. It consists of an introduction to the use of the “sky lantern,” its components, and its functioning, as a macroscopic model with which students have some degree of familiarity that allows bridging their past experiences to the abstract concept of combustion and limiting reactant. The students were not exposed to experiment with actual sky lanterns, and although they are fairly simple objects, the model used is an idealized over-simplification of these lanterns, used to motivate the study of closed systems without reactant flow, even though actual lanterns are not closed systems.
Before asking the students to provide explanations, they are shown the balanced chemical equation \(C{H}_{4}+2{O}_{2}-> C{O}_{2}+2{H}_{2}O\) and two figures with molecular models of methane (as a representation of paraffin, to simplify the representation) and oxygen in different proportions: the first figure shows four molecules of methane, whereas the second figure shows only two methane molecules, and in both cases, seven molecules of \({O}_{2}\) are shown.
The students must select and justify which of three alternative representations of the products best symbolizes the final situation of the chemical reaction, considering the LR. The first figure includes as products two molecules of \({H}_{2}O\) and one of \(C{O}_{2}\), and two molecules of \({O}_{2}\) and one of \(C{H}_{4}\) as a non-reacting reactant. The second figure shows the products of two molecules of \({H}_{2}O\) and two of \(C{O}_{2}\) and four molecules of \({O}_{2}\) as a non-reacting reactant. Finally, the third figure shows four molecules of \({H}_{2}O\) and two of \(C{O}_{2}\) and three molecules of \({O}_{2}\) as a non-reacting reactant. The study reports that 24% of the students selected the correct answer, and from those, 67% used the concept of LR in their justifications. Notoriously, 27% of the students who selected wrong answers did use the concept of LR in their justifications.
Finally, the students are asked: “From your answers above, how would you explain the relation between the initial proportion of the reactants in the two situations above and the course of the chemical reaction (end products)? To do this, think about the following situation: ‘Why does the sky lantern go down after it has been rising for a while?'” (p. 95).
From the reported findings, 60% of the answers are classified as descriptive, even when they include microscopic entities in the explanation:
It descends because the fuel is running out, because the fundamentals of the sky lanterns need fuel to rise.
The balloon starts to descend once the oxygen atoms are completely gone, as well as when the paraffin and the flame are finished (our translation).
In the two previous answers explaining why the sky lantern descends after rising for a while, students do not link the notion of a limiting reactant in terms of the interaction of agents necessary for the chemical reaction to take place and form products. Neither do they consider stoichiometry according to the balanced chemical equation, which represents the proportion in which the reactants react. Instead, they view the reactant as the entity/substance that is consumed first, independent of the proportions in which they are initially found (such as methane in activity 1, with oxygen being the LR in that case).
Alternative conceptions reported in this research, also previously documented in the literature (Órdenes et al., 2013; Raviolo & Lerzo, 2016), include the idea that the LR is only the entity/substance found in the smallest amount, or that the reaction stops independently of the amounts of reactants. In the latter conception, students perceive the concept of LR as a property of a particular substance. Under this perspective, the LR would not depend on either the amount or the proportion in which it is reacting; instead, there would be “limiting” substances. This notion could arise from the difficulty in moving between different modes of representation addressed in the activity (Iturra et al., 2021, p. 92). In this sense, there was a “substantialisation of properties,” linked to the difficulty of moving through different ontological levels, as reported by Chi (2008).
From here, teacher-mediated dialogue can be key to promoting an adequate understanding of the limiting reagent, moving from descriptive explanations to causal explanations. Materials that make up physical reality and the phenomena observed at the macroscopic level have the theoretical function of imposing structural restrictions that must be explained by the interactions of the atoms and molecules of which they are composed.
In this example, students respond to what the pedagogical situation allows them to see. This is because the choice of the model, the symbolic levels, and the questionnaire design do not invite students to place themselves at the ontological level to which they should have access in their explanations.
Our proposal could be implemented by appropriately situating the ontological level through the formulation of the questions posed by the teachers, explicitly designing them to encourage causal explanations. Given that the model used (“the sky lantern”) is macroscopic, the transposition to a microscopic level of the problem is not facilitated.
The teachers ask, “Why does the sky lantern go down after having risen for a while?” and the students use statements such as “less quantity” and “the reaction stops” in their descriptions, both also situated at a macroscopic level. This does not facilitate or guide the students to situate the problem as one of causality at the level of entities. Students respond to the material supplied to them in the description, the design, and the questions of the activity, not based on what is happening at the level of interactions of the causal agents, which is to be expected. Even though the images in activities 1 and 2 move on another symbolic plane, where students should reorient the explanation from the macroscopic to the microscopic, if they are not guided in their questioning about the phenomenon, the students’ answers fail to reach the expected ontological level.
Instead, if teachers are not explicit about ontological levels, they end up with observational descriptions from the macroscopic domain. The different models and symbolic representations seem to contribute to confusing students. This approach to the ontology of questions used by teachers to develop scientific explanations of causality is developed in the literature in the context of mediating questions (Roca et al., 2013; Márquez et al., 2005; Márquez et al., 2004), as one of the elements of their design.Footnote 2
The ontological level in the design of the questions is an aspect that is useful for this analysis, and in which our proposal can play a role. It has been reported that an incorrect ontological level is a learning obstacle, and teachers do not usually use them adequately in the questions they develop in their classes, accentuating alternative conceptions or modifying them for others that are far from the scientific explanation of the phenomenon under study (Joglar & y Rojas, 2019).
In this particular case and taking into account some stages of Talanquer’s progression mentioned above, we can see how it is possible to merge our proposal with the formation of guiding questions that mediate the transition of students’ explanations from the macroscopic to the microscopic level. The questions we propose are the following:
(1) For the macro level: With the image of the “sky lantern” in mind, one could state that the lit candle inside the lantern is used to heat up the air inside it. One might ask what relationship exists between the hot air and the movement of the lantern, if any. This invites students to explore two different macroscopic relations:
-
(a)
Charles and Gay-Lussac’s Law, which connects changes in temperature with changes in the volume of gasses
-
(b)
Archimedes’ principle, which relates differences in density to buoyancy forces
Charles and Gay-Lussac’s Law and Archimedes’ principle are structural relationships that describe phenomena macroscopically but do not reveal the causal mechanisms behind them.
Depending on the students’ prior knowledge of buoyancy or Charles and Gay-Lussac’s laws, a previous step of questions can be used to guide those who are less familiar with the aforementioned laws by providing the necessary information. A possible question allowing us to introduce Charles and Gay-Lussac’s Law could be the following: Lit lanterns rise into the air, while unlit lanterns remain on the ground. Compare a lit lantern with an unlit lantern. In which of them is the air inside warmer? In which of them is there more light? In your opinion, is it the amount of light or the higher temperature that causes the balloon to rise? Do you think that it is possible to lift an object by pointing a flashlight at it? Once more, these questions are meant to explore the macroscopic relations between hot air and buoyancy without any causal descriptions in terms of agents.
The movement to the microscopic causal mechanism behind Charles and Gay-Lussac’s or Archimedes laws rests on models of gasses (the kinetic theory of gasses, for instance) in which agents (molecules) exchange momentum with other molecules and with the walls through collisions (the interaction). Further questions might be implemented to reach this level. However, as the activity is not meant to teach the physics behind the rising of hot balloons but just to motivate the study of LR, these questions can be left aside.
(2) Ask why the lit candle inside the lantern goes out, inviting the students to question themselves based on what has been observed.
Note that following our proposal, the question at this level suggests agents (candle, gas in the lantern) and a type of interaction. Although this is not the ontological level that the teacher wishes to reach with the activity, this first step serves as a bridge to bring the student to the appropriate type of inquiry.
(3) For the micro level: Two consecutive scenarios can be proposed for the occurrence of the phenomenon.
-
(a) When the candle is still burning, what chemical species are involved in the process that allows the candle to burn?
-
(b) When the candle has gone out, in relation to your answer to the previous question, how might the chemical species you identified be related or involved with each other in causing the candle to go out?
With questions (a) and (b), the student is progressively placed at the corpuscular and atomic levels.
To conclude, we suggest the following question:
-
(c) When the combustion of the candle stops the lantern finally starts to descend. How can you relate the phenomenon observed at the macroscopic level of the descent to the interaction of the chemical species involved in the combustion reaction of the candle at the microscopic level? Here, the students might arrive at the conclusion that the chemical reaction is exothermic, moving beyond the study of LR.
These suggestions address how the use of question design focuses on ontological elements explicitly, guiding the students’ learning process in a progressive transition from observations at the macroscopic level to invite answers on how the causal agents of the combustion process are determining the occurrence of what the students observe. This process of “progression in their learning” is mediated by a series of intermediate steps that are generally ordered in terms of increasing complexity and causal importance, as suggested by Corcoran et al. (2009).
In summary, our proposal can help teachers when designing questions or activities with a correct ontological level at each step of the learning progression. This is just one example of how our proposal can be implemented in the meta-analysis of chemist teachers facilitating the design of activities promoting learning progression.
We take these few examples as evidence of the connection between our proposal and current trends in science education. Certainly, a much more elaborate study exploring the points of convergence, opportunities for complementation, and joint areas for further development between the theoretical distinction and didactic approaches should be conducted with care in future works. However, we believe these examples are clear bridges connecting our proposal and recent developments in the field. They demonstrate the power of the mechanistic/structural distinction for making the ontological separations assumed by school chemistry clearer.
If teachers of the subject are introduced to the relevant parts of the distinction—the notion that there are causal and unificationist explanations, that the latter do not need agents to explain a phenomenon, that only interactions require agents and can be behind causal mechanisms, that frameworks are general constraints valid for any reaction, etc.—then we strongly believe that some confusions described in the science education literature could be alleviated.
7 Conclusions
Science education confronts multiple challenges: social, economic, political, and institutional. Without diminishing the importance of all these aspects, in this work, we try to make a contribution to chemistry education from a perspective that is usually less discussed within the existing literature: that of philosophy of science.
From the specific point of view of a chemistry teacher, one of the biggest hurdles to overcome—but also one of the greatest contributions of the subject at the school level—is to introduce the students to a scientific vision of the world that is radically different from the everyday or intuitive image of matter with which students arrive initially, based on the submicroscopic description in terms of atoms, molecules, and chemical reactions between them at scales that are very difficult to imagine.
The difficulty of this conceptual leap has given rise to works in chemistry pedagogy, such as Johnston’s classic triad, and the recent studies by Talanquer, which we have used as starting points for our discussion. These works, among others, make it clear the need for a deeper reflection on ontological aspects of chemistry, justifying the use of philosophical analysis of the subject as a tool for improving teaching.
Indeed, such a conceptual leap between what we can call different levels of reality has been repeatedly analyzed within the philosophy of science. For the philosopher, chemistry is at the crossroads between two images of the world: the common-sense macroscopic image and the microscopic image that fundamental physics provides. This has generated deep disagreements about the place that chemistry occupies or deserves to occupy at the time of choosing a scientific image of the world—disagreements that are ongoing in many ways.
In this work, we assumed a pluralistic metaphysics of the material world, justifying it by the diverse scientific theories we use to describe it. As discussed in the main text, recent developments within the general philosophy of science allow us to classify any theory or theoretical element into two possible groups, known as framework and interaction theories. Crucially, these two classes of theories stand out for offering different types of scientific explanations: causal-mechanistic for interaction theories, and structural-unificationist for framework theories.
Science teachers, in general, and specifically chemistry teachers would greatly benefit from considering this meta-theoretical tool. It provides a clear understanding of the ontological, functional, and epistemic aspects of the theory under examination, enabling deeper insights for making informed didactic decisions.
The classification allows us to achieve a higher level of conceptual clarity when analyzing our science, not only in terms of explanations (which are obviously central in the educational context) but also in ontological terms, as Talanquer argues, playing a much more subtle role in developing students’ understanding of chemistry. Let us emphasize that ontological issues pose challenges not only for students but also for well-trained teachers. The meta-theoretical tool we are proposing here, while potentially beneficial for anyone analyzing scientific content, would likely be most effectively utilized by science teachers.
The structural/mechanistic classification has the additional advantage of being easy to parse, breaking down scientific explanations into interacting agents on the one hand and structural constraints on the other. This is why we consider that this input, which comes from the philosophy of science, can be highly useful in designing the school curriculum in chemistry. Teachers can guide students to grasp new intuitions, such as the concepts of causal agent and structural constraint, to navigate the difficulties described by Talanquer in the transition from an everyday vision of the world to a scientific vision—a transition that is unquestionably one of the most urgent tasks of formal education today.
In this work, we have emphasized how these ideas connect with those of Johnston and Talanquer (among others) in chemistry education. Additionally, we have shown how our conceptual meta-theoretical framework allows for a structural explanation of the conceptual errors with which chemistry teachers are confronted daily. Finally, we have demonstrated, through explicit examples such as the properties of water and combustion, how our proposal connects and potentially enhances the conceptual clarity in the analysis of significant chemical phenomena.
Awareness about the distinction between framework and interaction theories can help teachers identify the source of erroneous intuitions and design educational tools to better promote the desired conceptual transition. We hope that our proposal will become a valuable tool for the entire teaching community and, perhaps more importantly, for future students who will indirectly receive clearer guidance along their paths towards a modern and scientific conception of the world.
Notes
We would like to thank the anonymous referee for bringing this example to our attention.
Other elements of question design include contextualisation, being concrete, open-ended and inviting further questions.
References
Amin, T., Smith, C., & Wiser, M. (2014). Student conceptions and conceptual change: Three overlapping phases of research. In N. Lederman & S. Abell (Eds.) Handbook of research on science education, vol. II (pp. 57–81). New York: Routledge. https://doi.org/10.4324/9780203097267.ch4
Ayas, A., Özmen, H., & Çali, M. (2010). Students’ conceptions of the PNM at secondary and tertiary level. International Journal of Science and Mathematics Education, 8, 165–184.
Beerenwinkel, A., Parchmann, I., & Gräsel, C. (2011). Conceptual change texts in chemistry teaching: A study on the particle model of matter. International Journal of science and mathematics Education, 9, 1235–1259.
Benitez, F. (2019). Selective realism and the framework/interaction distinction: A taxonomy of fundamental physical theories. Foundations of Physics, 49(7), 700–716.
Bridle, C. A., & Yezierski, E. J. (2012). Evidence for the effectiveness of inquiry-based, particulate-level instruction on conceptions of the PNM. Journal of Chemical Education, 89, 192–198.
Brown, D. E. (2014). Students’ conceptions as dynamically emergent structures. Sci. & Educ., 23, 1463–1483.
Chi, M. T. H. (2008). Three kinds of conceptual change: Belief revision, mental model transformation, and ontological shift. In S. Vosniadou (Ed.), International handbook of research on conceptual change (pp. 61–82). Routledge.
Chi, M. T. H., & Roscoe, R. D. (2002). The processes and challenges of conceptual change. In M. Limon & L. Mason (Eds.), Reconsidering Conceptual Change: Issues in Theory and Practice (pp. 3–27). Kluwer Academic Publishers.
Chi, M. T. H., Slotta, J. D., & De Leeuw, N. (1994). From things to processes: A theory of conceptual change for learning science concepts. Learning and Instruction, 4(1), 27–43.
Coley J. D., Arenson M., Xu Y. and Tanner K. D. (2017). Intuitive biological thought: Developmental changes and effects of biology education in late adolescence. Cognitive Psychology, 92, 1–21.
Corcoran, T., Mosher, F. A., & Rogat, A. (2009). Learning progressions in science: An evidence-based approach to reform. CPRE Research Report# RR-63. Consortium for Policy Research in Education.
De Regt, H. W. (2006). Wesley Salmon’s complementarity thesis: Causalism and unificationism reconciled? International Studies in the Philosophy of Science, 20(2), 129–147.
diSessa A. A. (2014). The construction of causal schemes: Learning mechanisms at the knowledge level. Cognitive Science, 38, 795–850.
Einstein, A. (1919). What is the theory of relativity? In Ideas and Opinions, 1982, 227–232.
Flores, F. (1999). Einstein’s theory of theories and types of theoretical explanation. International Studies in the Philosophy of Science, 13(2), 123–134.
Galagovsky, L., Rodríguez, M., Morales, L., & Stamati, N. (2003). Representaciones mentales, lenguajes y códigos en la enseñanza de ciencias naturales: Un ejemplo para el aprendizaje del concepto de reacción química a partir del concepto de mezcla. Enseñanza De Las Ciencias, 21(1), 107–122.
Gilbert, J. K., & Treagust, D. F. (2009). Models and modeling in science education: Multiple representations in chemical education (Vol 4). Scotland: Springer.
Gómez, E. J., Benarroch, A., & Marín, N. (2006). Evaluation of the degree of coherence found in students’ conceptions concerning the PNM. Journal of Research in Scıence Teachıng, 43(6), 577–598.
Griffiths, A. K., & Preston, K. R. (1992). Grade-12 students’ misconceptions relating to fundamental characteristics of atoms and molecules. Journal of Research in Science Teaching, 29(6), 611–628.
Grotzer T. A. (2003). Learning to understand the forms of causality implicit in scientifically accepted explanations. Studies of Science Education, 39, 1–74.
Harrison, A., & Treagust, D. (2001). Conceptual change using multiple interpretive perspectives: Two case studies in secondary school chemistry. Instructional Science, 29, 45–85.
Hettema, H. (2014). Linking chemistry with physics: A reply to Lombardi. Foundations of Chemistry, 16(3), 193–200.
Iturra, M., Mallea, J, Quintanilla, M., Yin, Y., Herrera-Melin, A. (2021). Explicaciones escolares respecto al concepto reactivo limitante. Educación Química, 32(4). https://doi.org/10.22201/fq.18708404e.2021.5.78128
Joglar, C., & y Rojas, S. P. (2019). Superación de obstáculos para la formulación y uso de preguntas en el aula de ciencias: Análisis desde un taller de reflexión docente. Investigación en Educación Científica, 49, 1125–1139.
Johnstone, A. H. (1991). Why is science difficult to learn? Things are seldom what they seem. Journal of Computer Assisted Learning, 7, 75–83.
Johnstone, A. H. (1993). The development of chemistry teaching: A changing response to a changing demand. Journal of Chemical Education, 70(9), 701–705. https://doi.org/10.1021/ed070p701
Johnstone, A. H. (2000). Chemical education research: Where from here? University Chemistry Education, 4(1), 34–38.
Kahveci, A. (2009). Exploring chemistry teacher candidates’ profile characteristics, teaching attitudes and beliefs, and chemistry conceptions. Chemistry Education Research and Practice, 10, 109–120.
Keil F. (2006). Explanation and understanding. The Annual Review of Psychology, 57, 227–54.
Labarca, M., & Lombardi, O. (2010). Why orbitals do not exist? Foundations of Chemistry, 12(2), 149–157.
Labarca, M. (2016). “Filosofía de la química”. En Diccionario Interdisciplinar Austral, editado por Claudia E. Vanney, Ignacio Silva y Juan F. Franck. http://dia.austral.edu.ar/Filosofía_de_la_química. Accessed April 2023.
Lemma, A., & Belachew, W. (2022). Ontological orientations of educators’ sense of the atom and underlying source domains: A case study of Kotebe Metropolitan University. Ethiopia. Chemistry Education Research and Practice, 23(4), 885–897.
Lewowicz, L., & Lombardi, O. (2013). Stuff versus individuals. Foundations of Chemistry, 15(1), 65–77.
Lombardi, O. (2014). Linking chemistry with physics: Arguments and counterarguments. Foundations of Chemistry, 16(3), 181–192.
Lombardi, O., & Castagnino, M. (2010). Matters are not so clear on the physical side. Foundations of Chemistry, 12(2), 159–166.
Lombardi, O., & Labarca, M. (2005). The ontological autonomy of the chemical world. Foundations of Chemistry, 7(2), 125–148.
Lombardi, O., & Labarca, M. (2006). The ontological autonomy of the chemical world: A response to Needham. Foundations of Chemistry, 8(1), 81–92.
Maltrana, D., Herrera, M. and Benitez, F. (2022). Einstein’s theory of theories and mechanicism. International Studies in the Philosophy of Science, 1–18.
Manafu, A. (2013). Internal realism and the problem of ontological autonomy: A critical note on Lombardi and Labarca. Foundations of Chemistry, 15(2), 225–228.
Márquez, C., Roca, M., Gómez, A. A., Sardà, A., & Pujol, R. M. (2004). La construcción de modelos explicativos complejos mediante preguntas mediadoras. Investigación en la escuela, 53, 71–81. https://ddd.uab.cat/record/202456. Accessed July 2024.
Márquez, C., Bonil, J., & Pujol, R. M. (2005). Las preguntas mediadoras como recursos para favorecer la construcción de modelos científicos complejos. Enseñanza de las Ciencias, (Extra), 1–5. https://ddd.uab.cat/record/70646. Accessed July 2024.
Mensah, A., Morabe, O. & Golightly, A. (2016). Teaching chemical equilibrium through conceptual change approach: A synthesis and analysis of the literature. Science and Technology.
Mulder, P. (2011). Are orbitals observable? International Journal for Philosophy of Chemistry, 17(1), 24–35.
Muniz, M. N., Crickmore, C., Kirsch, J., & Beck, J. P. (2018). Upper-division chemistry students’ navigation and use of quantum chemical models. Chemistry Education Research and Practice, 19(3), 767–782. https://doi.org/10.1039/c8rp00023a
Nakhleh, M. B. (1994). Students’ models of matter in the context of acid-base chemistry. J. Chemical Education., 71(6), 495–499.
Needham, P. (2006). Ontological reduction: A comment on Lombardi and Labarca. Foundations of Chemistry, 8(1), 73–80.
Nyachwaya, J. M., Mohamed, A. R., Roehrig, G. H., Wood, N. B., Kern, A. L., & Schneider, J. L. (2011). The development of an open-ended drawing tool: An alternative diagnostic tool for assessing students’ understanding of the PNM. Chemistry Education. Res, 12, 121–132.
Órdenes, R., Arellano, M., Jara, R., & Merino, C. (2013). Representaciones macroscópicas, submicroscópicas y simbólicas sobre la materia. Educación Química, 25(1), 46–55.
Özalp, D. and Kahveci, A. (2015). Diagnostic assessment of student misconceptions about the particulate nature of matter from ontological perspective. Chemistry Education Research and Practice, 1–58.
Özmen, H. (2008). Turkish primary students’ conceptions about the PNM. International Journal of Environmental & Science Education, 6(1), 99–121.
Paik, S. (2015). Understanding the relationship among Arrhenius, Brønsted−Lowry, and Lewis theories. Journal of Chemical Education, 92, 1484–1489.
Posner, G. J., Strike, K. A., Hewson, P. W., & Gertzog, W. A. (1982). Accommodation of a scientific conception: Toward a theory of conceptual change. Science education, 66(2), 211–227.
Quílez, J. (2019). A categorisation of the terminological sources of student difficulties when learning chemistry. Studies in Science Education, 55(2), 121–167.
Quílez, J. (2021). Le Châtelier’s principle a language, methodological and ontological obstacle: An analysis of general chemistry textbooks. Science & Education, 30, 1253–1288.
Raviolo, A., & Lerzo, R. (2016). Enseñanza de la estequiometría: Uso de analogías y comprensión conceptual. Educación Química, 27(3), 195–204.
Renström, L., Andersson, B., & Marton, F. (1990). Students’ conceptions of matter. Journal of Educational Psychology, 82(3), 555.
Roca, M., Márquez, C., & Sanmartí, N. (2013). Las preguntas de los alumnos: Una propuesta de análisis. Enseñanza De Las Ciencias, 31(1), 95–114.
Salmon, W. (1984). Scientific explanation and the causal structure of the world. Princeton University Press.
Sanders, M. (1993). Ideas erróneas sobre la respiración: El factor maestro. Diario De Investigación En Enseñanza De Las Ciencias, 35(3), 265–296.
Scerri, E. (2020). On chemical natural kinds. Journal for General Philosophy of Science, 51(3), 427–445. https://doi.org/10.1007/s10838-020-09511-9
Talanquer V. (2006). Common sense chemistry: a model for understanding students’ alternative conceptions. Journal of Chemistry Education, 83(5), 811–816.
Talanquer, V. (2018). Progressions in reasoning about structure-property relationships. Chemistry Education Research and Practice, 19, 998–1009. https://doi.org/10.1039/C7RP00187H
Thagard, P. (1993). Conceptual revolutions. Princeton University Press.
Tsitsipis, G., Stamovlasis, D., & Papageorgiou, G. (2011). A probabilistic model for students’ errors and misconceptions on the structure of matter in relation to three cognitive variables. International Journal of Science and Mathematics Education, 10, 777–802.
Vosniadou, S. (1994). Capturing and modeling the process of conceptual change. Learning and Instruction, 4(1), 45–69.
Vosniadou, S. (2003). Exploring the relationships between conceptual change and intentional learning. In Intentional Conceptual Change (pp. 373–402). Taylor and Francis. https://doi.org/10.4324/9781410606716-20
Vosniadou, S., & Brewer, W. F. (1993). Mental models of the earth: A study of conceptual change in childhood. Cognitive Psychology, 24, 535–585.
Vosniadou, S., Vamvakoussi, X. and Skopeliti, I. (2008). The framework theory approach to the problem of conceptual change. International Handbook of Research on Conceptual Change. 3–34.
Wiser, M., & Smith, C. L. (2009). Learning and teaching about matter in grades k–8: When should the atomic-molecular theory be introduced?. International handbook of research on conceptual change (pp. 233–267). Routledge.
Funding
Open access funding provided by University of Bern This work was supported by Pontificia Universidad Católica de Valparaíso under Grant 039-460/2024, and ANID-Subdirección de Capital Humano, Doctorado Nacional, under Grant 2024- 21242479.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maltrana, D., Guíñez, R., Herrera, A. et al. A Map for the Ontological Crossroads. Sci & Educ (2024). https://doi.org/10.1007/s11191-024-00555-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s11191-024-00555-7