Abstract
An intriguing trend in the synthesis of heterocyclic compounds is green chemistry, which involves using environmentally friendly reagents and efficient reactions to suit the demands of the pharmaceutical sector. This study is a part of continuous endeavour to advance novel synthetic approaches for the synthesis of heterocyclic molecules. With lanthanum nitrate hexahydrate as a catalyst, a procedure for the synthesis of tetrazoles was described in this work. The ideal 10% mole ratio of La(NO3)3·6H2O catalyst with DMF solvent produced good yields of the intended products, ranging from 85 to 98%, according to the results. High product yields, cost effectiveness, and operational simplicity are just a few benefits of using La(NO3)3·6H2O as a catalyst in this condensation reaction. The chemical structures of the synthesized tetrazoles were analyzed and characterized using 1HNMR, 13C-NMR, and mass spectra (HRMS).
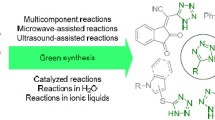
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multicomponent reactions (MCRs) have emerged as a groundbreaking approach in organic synthesis, fundamentally reshaping the landscape of chemical reactions. By combining three or more starting materials simultaneously in a single reaction vessel, MCRs achieve several significant advantages. They streamline the synthesis process by eliminating the need for intermediate purification steps, thereby enhancing efficiency and reducing production costs. This efficiency is particularly appealing in pharmaceutical applications, where rapid and economical synthesis of complex molecules is crucial for drug discovery and development [1,2,3].
Furthermore, MCRs contribute to sustainability by minimizing chemical waste generation compared to traditional sequential reactions. Their ability to produce structurally diverse and intricate molecules in a single operation has revolutionized medicinal chemistry, allowing researchers to explore vast chemical space and optimize the biological activity of potential drug candidates. As the pharmaceutical industry continues to seek innovative solutions to meet therapeutic challenges, MCRs remain at the forefront, offering a powerful toolset that integrates efficiency, sustainability, and creativity to advance pharmaceutical science [4,5,6,7,8].
Tetrazoles represent versatile heterocyclic frameworks highly valued across diverse scientific disciplines, including synthetic organic chemistry and catalysis, as well as applications in the pharmaceutical and organometallic industries [9, 10]. Tetrazoles are characterized by a multifaceted utility stemming from their unique molecular structure, which imparts distinctive chemical properties essential for advanced applications. Beyond their pivotal role in drug discovery, tetrazoles are indispensable in the development of high-performance materials [11], sophisticated coordination polymers [12], and effective corrosion inhibitors [13]. Furthermore, their historical use in photography [14] and contemporary applications in the design of functional materials highlight their enduring significance in modern scientific and technological pursuits [15].
Researchers have been drawn to tetrazole derivatives due to their unique molecular structure and promising pharmacological properties, such as their potential as antihypertensive, antiallergic, antibacterial, anticonvulsant, and anticancer agents [16,17,18,19,20,21,22,23], as illustrated in Fig. 1. Various methodologies have been developed for the synthesis of tetrazoles from substrates ranging from nitriles and amides to thioamides, imidoyl chlorides, heterocumulenes, ketones, amines, alkenes, and isocyanides, demonstrating the robustness and versatility of these synthetic routes [24, 25].
Catalysts are pivotal in the synthesis of diverse heterocycles using different catalysts such as phosphate fertilizers and phosphates modified with metals [26], bimetallic catalytic system [27] nano and Ecofriendly catalysts [28, 29]. Continual advancements in catalyst design and application broaden the horizons of chemical synthesis, fostering sustainable practices and expanding the repertoire of molecules and materials accessible to researchers and industries alike [30, 31].
Tetrazoles have been synthesized using a wide range of catalysts, such as Cu2 (OTf)2 [32], solid support [33], ZnCl2 [34], iPrMgCl [35], DMAP [36], DBU [37], L-Proline [38], Cu(OAc)2 [39]. Furthermore, a variety of heterogeneous catalysts have been used to create 1H-tetrazoles like Pd (0)/FeCl3 [40] and cobalt on modified boehmite nanoparticles [41]. Without a doubt, a large range of tetrazole derivatives can be successfully prepared using the aforementioned techniques. Unfortunately, the majority of these techniques have one or more of the following problems: they require extremely acidic conditions; they take a long time to react; they provide low yields; they require laborious work-up procedures; they demand excess amounts of reagent; or they involve poisonous solvents, catalysts, or reagents. For this reason, it is ideal to prepare substituted tetrazoles using an environmental friendly technique. Particularly, 5-substituted 1H-tetrazoles are the most important and interesting category of tetrazoles due to their extensive applications in the field of medicinal chemistry [42], as shown in Fig. 1. Hence, in this direction, efforts have been undertaken to establish the synthesis of 5-substituted tetrazoles using the diversified catalyst lanthanum (III) nitrate. Recent developments in organic transformations have focused a lot of interest on lanthanum (III) nitrate because of its high acidity, good stability, low toxicity, low cost, and heat stability. Furthermore, it can be seen from recent research that organic synthesis has successfully used lanthanum (III) nitrate as a catalyst [43, 44].
The current study explores an efficient method for synthesizing 5-substituted 1H tetrazoles, employing Lanthanum (III) nitrate hexahydrate as a catalyst. Scheme 1 shows the multicomponent synthesis of 5-Substituted 1H-Tetrazoles using Lanthanum (III) nitrate hexahydrate as a Catalyst. This marks the first successful application of Lanthanum (III) nitrate hexahydrate in catalysing the synthesis of tetrazoles from nitriles and azides. A pivotal aspect of this investigation involves click chemistry, specifically the [3+2] cycloaddition, which is increasingly recognized as a significant advancement in organic synthesis. Through systematic exploration, it has been observed that Lanthanum (III) nitrate hexahydrate plays a crucial role in facilitating the formation of oximes and nitriles, demonstrating substantial influence on the overall product yield.
Result and discussion
To optimize the reaction conditions, a mixture of anisaldehyde (1), hydroxylamine hydrochloride (2), and sodium azide (3) was used as a model reaction. According to previous literature reports, it was first attempted with FeCl3 catalyst in a DMF solvent system, resulting in a smooth reaction with a yield of 74% (Table 1, entry 1). Subsequently, various non-metal catalysts or reagents such as DDQ, p-TSA, and molecular iodine were also tested.
The reaction did not proceed with DDQ and p-TSA (Table 1, entries 2 and 3) but observed positive results with iodine and with 62% yield (Table 1, entry 4). Next, it was studied with metal-based catalysts like Sc (OTf)3, Cu (NO3)2, and La(NO3)3·6H2O (20 mol%) to optimize the reaction conditions. With the Scandium triflate and copper nitrate catalysts, a reaction was also undergone (Table 1, entry 5,6), but the yield was low and with lanthanum nitrate hexahydrate, a good yield was observed (Table 1, entry 7). Then it was focused on different mol% of catalyst, like 10 mol% and 20 mol%, but there was no change in the yield of product even with, 50 mol%, Thus, the most suitable reaction conditions for the formation of 4 were established at 10 mol% of catalyst.
Various solvents were tested in an effort to optimize the reaction conditions. Dichloromethane (DCM), dimethyl acetamide (DMA), water, dimethyl sulfoxide (DMSO), ethanol (EtOH), and acetonitrile (CH3CN) were all employed. Notably, the reaction failed to proceed when dichloroethane (DCE) was used as the solvent (Table 1, entry 9), whereas DMA proved highly effective (Table 1, entry 10). Water and ethanol yielded the desired product but in relatively low yields (Table 1, entries 11 and 13). Dimethyl sulfoxide (DMSO) and acetonitrile (CH3CN) facilitated the reaction, albeit with moderate yields (Table 1, entries 12 and 14). Among these solvents, N,N-dimethylformamide (DMF) emerged as the optimal choice.
Furthermore, the necessity of a catalyst was evident from the lack of reaction in its absence (Table 1, entry 16), and significantly reduced yields were observed under neat conditions (Table 1, entry 17). Thus, after thorough solvent system evaluation, it was concluded that the preparation of 5-substituted 1H-tetrazoles is best achieved using 10 mmol of aldehyde, 15 mmol of hydroxylamine hydrochloride (2), and 20 mmol of sodium azide (3), in the presence of a 10 mol% catalyst, under a nitrogen atmosphere, and reflux conditions for 3–4 h. The scope of this reaction under optimized conditions and the results were illustrated in Table 2. Similarly, a variety of substituted aldehydes possessing electron-donating and electron-withdrawing functional groups, aliphatic aldehydes, reacted with azide to afford the corresponding 5-substituted tetrazoles 4a–4t in 85–98% yield without any side products. So, based on the above optimized conditions, different analogues of five substituted 1H-tetrazoles was synthesised. The reaction has undergone with different substituents like electron donating and withdrawing groups. Compounds with electron-donating groups at both ortho and para positions exhibited superior yields (Table 2, entries 1, 2, 6, 9, and 11), whereas those with groups at ortho and meta positions resulted in lower yields. (Table 2, entries 13, 20). Electron withdrawing groups at meta positions gave higher yield (Table 2, entry 5 &16) than compounds (Table 2, entries 4, 7,8 &15). In addition to it reactions proceeded with heterocyclic aldehydes offered moderate yield (Table 2, entry 9, 10) and low yield (Table 2, 17) was observed with the aliphatic aldehyde. All the products were purified by silica gel column chromatography using petroleum ether or ethyl acetate as eluent to give the desired products.
All the products were characterized by 1HNMR, 13C NMR, and HRMS. One of the products was formed (5-(4-methylphenyl)-1H-tetrazole) (4b) as a yellow solid and its melting point is 231–232 °C, and it was characterized by different analytical data. In 1H NMR spectral data, a total of 7 protons are present, those chemical shift value of δ7.92 ppm. One doublet of doublet (dd) was observed with a coupling constant of J = 10.0 Hz, which corresponds to two aromatic protons (2H, Ar–H), which are due to the ortho position of the tetrazole ring. Another two aromatic protons (2H, Ar–H) resonate at a chemical shift value of δ 7.40 ppm, which are responsible for the meta position of the tetrazole ring as a doublet. With a chemical shift value of J = 13.0 Hz at methyl group protons appeared as a singlet at δ 2.40 ppm (s, 3H, Ar–CH3). In 13C NMR spectra, the chemical shift value at δ 152.9 ppm indicates the quaternary carbon of tetrazole, and at δ 142.0 ppm indicates the carbon of the benzene ring, which is attached to the methyl group. And the remaining four carbons of the benzene ring, those in ortho and meta positions to the methyl group, resonate at δ 130.5 and 127.4 ppm respectively. The peak appeared at chemical shift value δ 121.4 ppm indicates the carbon of the benzene ring that is attached to the tetrazole ring and peak at δ 21.5 ppm value is because of the methyl group carbon. In ESI-mass spectroscopy, the base peak m/z value of 161 was observed very clearly. High-resolution mass spectroscopy (HRMS) helps to predict the confirmation of molecular formula (mass) and purity of the compound as [M+H]+ that is calculated for C8H9N4 161.0821 and also found to be 161.0825.
Scheme 2 shows the plausible mechanism for the synthesis of 5-substituted 1H-tetrazoles. Lanthanum nitrate hexahydrate acts as a Lewis acid catalyst. Initially, Ln(III) attaches to the lone pair of oxygen in the aldehyde, increasing its electrophilicity. Subsequently, hydroxylamine attacks the carbonyl carbon, forming a nitrile (I) and then an oxime (II) through the expulsion of water molecules [45,46,47]. Lanthanum nitrate hexahydrate stabilizes the transition state, lowering the activation energy for the reaction. In the process of forming aldoximes from aromatic aldehydes using hydroxylamine, lanthanum nitrate hexahydrate enhances the formation of nitriles (III) from aldoximes by acting as a catalyst in the Beckmann rearrangement reaction [38, 41, 48].
Its role is to facilitate the rearrangement process, thereby promoting the conversion of aldoximes to nitriles under suitable reaction conditions. Lanthanum ions play a critical role in activating sodium azide (NaN3) (IV) through coordination with the azide anion, thereby increasing its nucleophilicity. This activation allows nitriles (RC≡N) to transform into nitrile imines (RC=N2+). Subsequently, these activated nitrile imine intermediates undergo [3+2] cyclization with another molecule of NaN3, resulting in the formation of tetrazole rings (V) (RN4−) [49].These steps are essential for the efficient synthesis of tetrazoles from nitriles and azides. Finally, a 1,3-H-shift produces the 5-substituted 1H-tetrazole product upon acidic work-up (VI) (Table 3).
Finally, the synthesis of tetrazoles through the reaction involving aldehydes, hydroxylamine, sodium azide, and lanthanum nitrate hexahydrate represents a paradigm of high atom economy in organic chemistry. This method affords tetrazoles directly from easily accessible starting materials, circumventing the generation of substantial waste products. The choice of lanthanum nitrate hexahydrate as a catalyst offers significant environmental advantages, as lanthanum salts are generally recognized for their lower toxicity and reduced environmental impact compared to conventional heavy metal catalysts employed in tetrazole synthesis. Furthermore, the reaction conditions enabled by lanthanum nitrate hexahydrate catalysts often permit mild reaction conditions, facilitating the use of safer solvents and thereby contributing to a diminished overall environmental footprint. This approach underscores a pivotal advancement in sustainable synthetic methodologies, aligning efficiency with environmental stewardship in chemical synthesis.
Experimental section
General procedure for the synthesis of 5 phenyl 1H tetrazole from aldehyde
Aldehyde (10 mmol) is added with hydroxylamine hydrochloride (15 mmol) and sodium azide (20 mmol) in 5 mL DMF, then catalyst La(NO3)3·6H2O (10 mol%) was added. The mixture was refluxed for an appropriate time, and the progress of the reaction was monitored by TLC. After completion of the reaction, the solution was treated with HCl (4 N,10 mL), and then the solution was poured into 50 mL of water and extracted with ethyl acetate, which was washed several times with water. The combined organic mixture was dried over anhydrous Na2SO4, concentrated, and the residue was purified by silica gel column chromatography at 60–120 mesh using petroleum ether/ethyl acetate (the ratio depends on the polarity of the tetrazole formed in the reaction mixture from the corresponding aldehyde) as eluent to afford the pure solid tetrazole.
5-(4-Methoxyphenyl)-1H-tetrazole (4a)
Yield: 84%, yellow solid, 1H NMR (400 MHz, DMSO-d6), δ 8.06–7.90 (m, 2H, Ar–H), 7.21–7.09 (m, 2H, Ar–H), 3.85 (s, 3H, Ar–OCH3). 13C NMR (100 MHz, DMSO-d6), δ 161.91, 155.32, 129.10, 116.87, 115.21, 55.94. IR (ʋ, neat) 2922, 2852, 1662, 1606, 1499, 1254, 1219, 1177, 772 cm−1, MS (ESI) m/z 177, [M+H]+ HRMS (ESI) [M+H]+Calcd: For C8H9ON4 177.0770 Found 177.0776.2.
5-(4-Methylphenyl)-1H-tetrazole (4b)
Yield: 87%, Yellow solid, 1H NMR (400 MHz, DMSO-d6), δ7.92 (dd, J = 10.0 Hz, 2H, Ar–H), 7.40 (dd, J = 13.0 Hz, 6.7, 2H, Ar–H), 2.40 (s, 3H, Ar–CH3). 13C NMR (100 MHz, DMSO-d6), δ 152.9, 142.0, 130.5, 127.4, 121.4, 21.5. IR (ʋ, neat), 3400, 2254, 2128, 1657, 1220, 1048, 1023, 997, 823, 761 cm−1, MS (ESI) m/z 161 [M+H]+, HRMS (ESI), [M+H]+ calcd: For C8H9N4 161.0821 Found 161.0825.
5-Phenyl-1H-tetrazole (4c)
Yield: 79%, White solid, 1H NMR (400 MHz, DMSO-d6), δ 8.04 (dd, J = 6.5 & 3.0 Hz, 2H, Ar–H), 7.72–7.55 (m, 3H, Ar–H).13C NMR (100 MHz, DMSO-d6), δ 155.6, 131.5, 129.7, 128.5, 127.6, 127.1, 124.3. IR (ʋ, neat), 3387, 3191, 2922, 2851, 2703, 1644, 1574, 1564, 1402, 1219, 1161, 772 cm−1, MS (ESI) m/z 147 [M+H]+, HRMS (ESI) [M+H]+ calcd: For C7H7N4 147.0665 Found 147.0669.
5-(4-Bromophenyl)-1H-tetrazole (4d)
Yield: 90%, White solid,1H NMR (400 MHz, DMSO-d6), δ 7.99 (d, J = 8.5 Hz, 2H, Ar–H), 7.84 (d, J = 8.5 Hz, 2H, Ar–H), 13C NMR (100 MHz, DMSO-d6), δ 154.9, 132.9, 129.3, 125.1, 124.0. IR (ʋ, neat), 2984, 2930, 2862, 2726, 2618, 2316, 1610, 1499, 1392, 1220, 1019, 772 cm−1, MS (ESI) m/z224 [M+H]+, HRMS (ESI), [M+H]+ calcd: For C7H6N4Br 224.9770 Found 224.9781.
5-(2-Bromo 6-Fluorophenyl)-1H-tetrazole (4e)
Yield: 83% Semi solid, 1H NMR (400 MHz, DMSO-d6), δ 7.92 (dd, J = 8.9, 5.2 Hz, 1H, Ar–H), 7.69 (dd, J = 9.0, 3.1 Hz, 1H, Ar–H), 7.47 (td, J = 8.6, 3.1 Hz, 1H, Ar–H).13C NMR (100 MHz, DMSO-d6), δ 162.1, 159.6, 135.4, 128.3, 119.4, 118.8, 116.5. IR (ʋ, neat), 3393, 2924, 2853, 1655, 1468, 1219, 993, 772 cm−1 MS (ESI) m/z 242 [M+H]+, HRMS (ESI), [M+H]+ calcd: For C7H5N4BrF 242.9676 Found 242.9687.
4-(1H-Tetrazol-5-yl) phenol (4f)
Yield80%, Yellow solid, 1H NMR (400 MHz, DMSO-d6), δ 7.88 (d, J = 8.6 Hz, 2H, Ar–H), 6.94 (t, J = 13.5 Hz, 2H, Ar–H), 13C NMR (100 MHz, DMSO),δ 160.0, 154.7 128.6, 116.0, 114.5. IR (ʋ, neat), 3393, 2924, 2853, 1655, 1468, 1219, 993, 772 cm−1.MS (ESI) m/z 163 [M+H]+ HRMS (ESI) [M+H]+ calcd: For C7H7ON4 163.0614 Found 163.0619.
5-(2-Chlorophenyl)-1H-tetrazole (4g)
Yield: 81%, Yellow solid, 1H NMR (400 MHz, DMSO-d6), δ 7.82 (dd, J = 7.6, 1.7 Hz, 1H, Ar–H), 7.72 (dd, J = 8.0, 1.2 Hz, 1H, Ar–H), 7.64 (tt, J = 5.3, 2.6 Hz, 1H, Ar–H), 7.57 (ddd, J = 8.8, 5.5, 1.3 Hz, 1H, Ar–H), 13C NMR (100 MHz, DMSO-d6), δ 153.3, 132.5, 131.8, 131.7, 130.3, 127.7, 124.1.IR (ʋ, neat) 3122, 3063, 2959, 2852, 2606, 1658, 1601, 1552, 1467, 1441, 1056, 772 cm−1.MS (ESI) m/z 181 [M+H]+ HRMS (ESI) [M+H]+ calcd: For C7H6N4Cl 181.0275 Found 181.0283.
5-(2,4-Dichlorophenyl)-1H-tetrazole (4h)
Yield: 91%, Yellow solid, 1H NMR (400 MHz, DMSO-d6) δ 7.93 (d, J = 2.1 Hz, 1H, Ar–H), 7.87 (d, J = 8.4 Hz, 1H, Ar–H), 7.70 – 7.65 (m, 1H, Ar–H). 13C NMR (100 MHz, DMSO-d6), δ 153.1, 136.96, 133.54, 133.41, 130.58, 128.58, 123.82, IR (ʋ, neat) 3073, 2989, 2921, 2698, 1730, 1603, 1238, 1104, 772 cm−1 MS (ESI) m/z 214 [M+H]+ HRMS (ESI) [M+H]+ calcd: For C7H5N4Cl 214.9885 Found 214.9895.
5-(Thiophen-2-yl)-1H-tetrazole (4i)
Yield: 76%, Yellow solid,1H NMR (400 MHz, DMSO-d6), δ 7.73 (dd, J = 3.0, 2.0 Hz, 2H, Ar–H), 7.13 (dd, J = 4.8, 3.9 Hz, 1H, Ar–H). 13C NMR (100 MHz, DMSO-d6), δ 162.7, 140.2, 130.9, 128.5, 127.8, IR (ʋ, neat) 3437, 2252, 2126, 1662, 1220, 1050, 1023, 1001, 821, 759 cm−1.
3-(1H-Tetrazol-5-yl)-1H-indole (4j)
Yield:84%, Yellow solid,1H NMR (400 MHz, DMSO-d6) δ 11.87 (s, 1H, Indole Ar–H), 8.31–8.19 (m, 1H, Ar–H), 8.10 (d, J = 2.9 Hz, 1H, Ar–H), 7.61 – 7.52 (m, 1H, Ar–H), 7.34–7.20 (m, 2H, Ar–H). 13C NMR (100 MHz, DMSO-d6) δ 150.5, 136.3, 126.9, 124.3, 122.5, 120.7, 120.2, 112.2, 99.2. IR (ʋ, neat), 3293, 2934, 2843, 1615, 1448, 1229, 994, 771 cm−1.MS (ESI) m/z 186 [M+H]+ HRMS (ESI) [M+H]+ calcd: For C9H8N5 186.07742 Found 186.07834.
5-(4-Ethoxyphenyl)-1H-tetrazole (4k)
Yield: 89% Yellow solid, 1H NMR (400 MHz, DMSO-d6), δ 8.01–7.91 (m, 2H, Ar–H), 7.19–7.10 (m, 2H, Ar–H), 4.12 (q, J = 7.0 Hz, 3H, -OCH2), 1.39–1.33 (m, 3H, –OCH3).13C NMR (100 MHz, DMSO-d6)δ 160.6, 154.6, 128.5, 116.0, 115.1, 63.3, 14.4. IR (ʋ, neat) 2980, 2926, 2865, 2740, 2316, 1653, 1608, 1499, 1391, 1244, 1221, 919, 772 cm−1. MS (ESI) m/z 191 [M+H]+ HRMS [M+H]+ calcd: For C9H11ON4 191.0927 Found 191.09294.
5-(4-Nitrophenyl)-1H-tetrazole (4l)
Yield: 94%, Yellow solid,1H NMR (400 MHz, DMSO-d6) δ 8.48–8.44 (m, 2H, Ar–H), 8.35– 8.30 (m, 2H, Ar–H). 13CNMR (100 MHz, DMSO-d6) δ 148.5, 136.6, 130.9, 128.0, 124.5. IR (ʋ, neat) 3395, 2923, 2853, 1645, 1525, 1219, 993, 772 cm−1. MS (ESI), m/z 192 [M+H]+ HRMS [M+H]+ calcd: For C9H11ON4 192.0927 Found 192.09294.
2-Methoxy-4-(1H-tetrazol-5-yl) phenol (4m)
Yield 88%, Yellow solid, 1H NMR (400 MHz, DMSO-d6), δ 7.58 (d, J = 2.0 Hz, 1H, Ar–H), 7.53 – 7.46 (m, 1H, Ar–H), 7.00 – 6.94 (m, 1H, Ar–H), 3.82(s, 3H, -OCH3), 13C NMR (100 MHz, DMSO-d6), δ 149.3, 148.0, 120.3, 115.9, 110.6, 55.6.IR (ʋ, neat) 3423, 2991, 2924, 2852, 1636,, 1455, 1217, 1051, 1025, 1006, 742 cm−1MS (ESI) m/z 193 [M+H]+ HRMS (ESI) [M+H]+ calcd: For C8H9O2N4 193.07200 Found 193.0726.
5-(4-Fluorophenyl)-1H-tetrazole (4n)
Yield: 82%, Yellow solid, 1H NMR (400 MHz, DMSO-d6) δ 8.15 – 8.06 (m, 2H, Ar–H), 7.47 (ddd, J = 7.6, 4.7, 2.5 Hz, 2H, Ar–H). 13C NMR (100 MHz, DMSO-d6) 166.7, 130.1, 129.4, 116.6, 115.1. IR (ʋ, neat) 3394, 2923, 2853, 2256, 1663, 1502, 1390, 1220, 1023, 995, 771 cm−1. MS (ESI) m/z 165 [M+H]+,HRMS (ESI) [M+H]+ calcd: For C7H6N4F 165.0571 Found 165.0578.
5-(4-Chlorophenyl)-1H-tetrazole (4o)
Yield: 86%, White solid, 1H NMR (400 MHz, DMSO-d6) δ 8.03 – 7.94 (m, 2H, Ar–H), 7.19 – 7.13 (m, 2H, Ar–H).13C NMR (100 MHz, DMSO-d6) 160.0, 154.7, 128.6, 116.0, 114.5. IR (ʋ, neat), 3385, 2925, 2853, 2258, 1646, 1023, 990, 825, 764 cm−1.MS (ESI) m/z 181 [M+H]+, HRMS (ESI) [M+H]+ calcd: For C7H6N4Cl 181.0275 Found 181.0283.
5-(3-Nitrophenyl)-1H-tetrazole (4p)
Yield: 83%, Yellow solid, 1H NMR (400 MHz, DMSO-d6) δ 8.89 – 8.81 (m, 1H, Ar–H), 8.45 (m, 2H, Ar–H), 7.92 (t, J = 8.1 Hz, 1H, Ar–H). 13CNMR (100 MHz, DMSO-d6) 155.0, 148.2, 132.9, 131.1, 126.2, 125.4, 121.4. IR (ʋ, neat) 3398, 2923, 2853, 2254, 1660, 1531, 1352, 1023, 1000, 771 cm−1 MS (ESI) m/z 192 [M+H]+HRMS (ESI) [M+H]+ calcd: For C7H6N5O2 192.1548 Found 192.1748.
5-Isopropyl-1H-tetrazole (4q)
Yield: 34%, Yellow solid, 1H NMR (400 MHz, DMSO-d6) 3.26 (m, 1H, -CH (CH3)2), 1.33 – 1.29 (d, 6H, 2CH3).13C NMR (100 MHz, DMSO-d6) 160.8, 23.9, 20.9. IR (ʋ, neat) 3417, 2922, 2852, 1740, 1463, 1219, 1024, 1002, 771.cm−1MS (ESI) m/z 113 [M+H]+HRMS (ESI) [M+H]+ calcd: For C4H9N4 113.0821 Found 113.0828.
5-(2-Bromo-6-methoxyphenyl)-1H-tetrazole (4r)
Yield: 84%, Yellow solid,1H NMR (400 MHz, DMSO-d6) 8.19 (d, J = 2.6 Hz, 1H, Ar–H), 7.75 (m, 1H, Ar–H), 7.28 (dd, J = 11.1, 5.8 Hz, 1H, Ar–H), 3.98 (s, 3H, -OCH3).13C NMR (100 MHz, DMSO-d6) 155.6, 135.1, 131.1, 114.5, 114.2, 112.0, 56.1. IR (ʋ, neat), 3417, 2922, 2852, 1740, 1463, 1219, 1024, 1002, 771 cm−1MS (ESI) m/z 254 [M+H]+, HRMS (ESI) [M+H]+ calcd: For C8H8ON4Br 254.9876 Found 254.9888.
5-(2, 4-Dimethoxyphenyl)-1H-tetrazole (4s)
Yield: 78%, Yellow solid, 1H NMR (400 MHz, DMSO-d6) 7.85 (d, J = 8.4 Hz, 1H, Ar–H), 6.67 – 6.61 (m, 2H, Ar–H), 3.90 (s, 3H, -OCH3), 3.82 (s, 3H, -OCH3). 13C NMR (100 MHz, DMSO-d6) 171.9, 165.5, 162.5, 132.6, 106.5, 105.4, 98.2, 55.8, 55.3. IR (ʋ, neat) 3437, 2985, 2252, 2126, 1731, 1245, 1048, 1023,821, 760 cm−1. MS (ESI) m/z 207 [M+H]+, HRMS (ESI) [M+H]+ calcd: For C9H11O2N4 207.0876 Found 207.0881.
5-(2, 5-Dimethoxyphenyl)-1H-tetrazole (4t)
Yield: 77%, Yellow solid, 1H NMR (400 MHz, DMSO-d6) 7.62 (d, J = 2.7 Hz, 1H, Ar–H), 7.27–7.13 (m, 2H, Ar–H), 3.92 (s, 3H,-OCH3), 3.80 (s, 3H, -OCH3).13C NMR (100 MHz, DMSO-d6) 154.2, 153.0, 150.5, 118.5, 113.4, 113.3, 55.9, 55.5. IR (ʋ, neat) 3421, 2985, 2253, 1731, 1660, 1375, 1245, 1048, 1023, 822, 760 cm−1. MS (ESI) m/z 207[M+H]+, HRMS (ESI) [M+H]+ calcd: For C9H11O2N4 207.0876 Found 207.0878.
Conclusion
In conclusion, we have developed a novel method for the synthesis of 5-substituted 1H-tetrazole using Lanthanum nitrate hexahydrate as a Lewis acid catalyst. This method involves a one-pot reaction utilizing readily available aldehydes, hydroxylamine hydrochloride, and sodium azide under reflux conditions. The methodology offers several distinct advantages: it boasts an uncomplicated work-up process, employs less hazardous organic solvents, and utilizes water-soluble, low-toxicity catalysts, thereby minimizing environmental impact. Additionally, the catalyst promotes high yields of the desired products, contributing significantly to atom economy by minimizing by-product formation. This efficient approach not only maximizes the conversion of starting materials into valuable products but also signifies a promising pathway in the realm of organic synthesis for the development of novel heterocyclic compounds.
Data availability
No datasets were generated or analysed during the current study.
References
(a) V.R.K. Velpula, T. Ketike, A. Rajajagdeesan, M.A. Bora, S. Ganji, D.R. Burri, K.M. Surapaneni, Mater. Adv. 3, 7960 (2022); (b) G. Neochoritis, T. Zhao, A. Dömling, Chem. Rev. 119, 1970 (2019)
K. Gullapelli, N. Ramesh, R. Konakanchi, Res. Chem. Intermed. 49, 4713 (2023)
Y. Merroun, S. Chehab, A. El Hallaoui, S. Boukhris, R. Ghailane, A. Souizi, J. Mol. Struct. 1306, 137838 (2024)
K. Gullapelli, G. Sadanadam, K. Ledwaba, R. Maroju, J. Photochem. Photobio. A Chem. 429, 113888 (2022)
H.T. Ji, J. Jiang, W.B. He, Y.H. Lu, Y.Y. Liu, X. Li, W.M. He, J. Org. Chem. 89, 4113 (2024)
R. Taghavi, S. Rostamnia, Chem. Methodol. 6, 639 (2022)
Y. Merroun, S. Chehab, A. El Hallaoui, T. Guedira, S. Boukhris, R. Ghailane, A. Souizi, J. Mol. Struct. 1294(2), 136383 (2023)
K. Gullapelli, G. Brahmeshwari, M. Ravichander, Bull. Chem. Soc. Ethiop. 33, 143 (2019)
R. Mittal, S.K. Awasthi, Synth 51, 3765 (2019)
N. Taherzad, L. Kafi-Ahmadi, A. Poursattar Marjani, Appl. Organomet. Chem. 37(6), e7089 (2023)
B. Chen, H. Lu, J. Chen, Top Curr. Chem. (Z) 381, 25 (2023)
X. Min Kang, M. Hua Tang, G. Li Yang, B. Zhao, Coord. Chem. Rev. 422(1), 213424 (2020)
M. Esmaeilzadeh Khabazi, A. Najafi Chermahini, ACS Omega 8, 9978 (2023)
O.I. Shmatova, V.G. Nenajdenko, J. Org. Chem. 78, 9214 (2013)
C.G. Neochoritis, T. Zhao, A. Dömling, Chem. Rev. 119, 1970 (2019)
W.C. Xi, B. Ming, G.G. Hua, Molecules 20, 5528 (2015)
J.J. Li, H. Wang, J. Li, F. Qu, S.G. Swartz, A.S. Hernández, S.A. Biller, J.A. Robl, J.A. Tino, D. Slusarchyk, R. Seethala, P. Sleph, M. Yan, G. Grover, N. Flynn, B.J. Murphy, D. Gordon, Bioorg. Med. Chem. Lett. 18, 2536 (2008)
R.S. Upadhayaya, S. Jain, N. Sinha, N. Kishore, R. Chandra, S.K. Arora, Eur. J. Med. Chem. 39, 579 (2004)
A.H. Kategaonkar, R.U. Pokalwar, S.S. Sonar, V.U. Gawali, B.B. Shingate, M.S. Shingare, Eur. J. Med. Chem. 45, 1128 (2010)
A.A. Bekhit, O.A.E. Sayed, E. Aboulmagd, J.Y. Park, Eur. J. Med. Chem. 39, 249 (2004)
B.J.A. Hourani, S.K. Sharma, J.Y. Mane, J. Tuszynski, V. Baracos, T. Kniess, M. Suresh, J. Pietzsch, F. Wuest, Bioorg. Med. Chem. Lett. 21, 823 (2011)
C.N.S.S.P. Kumar, D.K. Parida, A. Santhoshi, A.K. Kota, B. Sridhar, V.J. Rao, Med. Chem. Commun. 2, 486 (2011)
G.S. Jedhe, D. Paul, R.G. Gonnade, M.K. Santra, E. Hamel, T.L. Nguyen, G.J. Sanjayan, Bioorg. Med. Chem. Lett. 23, 4680 (2013)
M.C. Leech, A. Petti, N. Tanbouza, A. Mastrodonato, I.C.A. Goodall, T. Ollevier, A.P. Dobbs, K. Lam, Org. Lett. 23, 9371 (2021)
K. Ishihara, T. Shioiri, M. Matsugi, Org. Lett. 22(16), 6244 (2020)
(a) S. Chehab, Y. Merroun, T. Ghailane, Russ. J. Org. Chem. 55, 1380 (2019); (b) Y. Merroun, S. Chehab, A. El Hallaoui, T. Guedira, S. Boukhris, R. Ghailane, N. Habbadı, A. Hassıkou, B. Lakhrıssı, A. Souizi, JOTCSA. 5(1), 303 (2018); (c) Y. Merroun, S. Chehab, A. El Hallaoui, T. Guedira, S. Boukhris, R. Ghailane, N. Habbadı, A. Hassıkou, B. Lakhrıssı, A. Souizi, Mediterr. J. Chem. 10(6), 553 (2020); (d) Y. Merroun, S. Chehab, T. Ghailane, Reac. Kinet. Mech. Cat. 126, 249, (2019)
(a) A. El Hallaoui, T. Ghailane, Y. Merroun, S. Chehab, T. Guedira, S. Boukhris, R. Ghailane, A. Souizi, Mediterr. J. Chem, 11(3), 215 (2021); (b) A. El Hallaoui, Y. Merroun, S. Chehab, Monatsh. Chem. 154, 231, (2023)
(a) S. Bibak, A. Poursattar Marjani, Sci. Rep. 13, 17894 (2023); (b) A. Farajollahi, A. Poursattar Marjani, J. Alloys Compd. 988, 174240 (2024)
(a) Y. Merroun, S. Chehab, A. El Hallaoui, T. Guedira, S. Boukhris, R. Ghailane, A. Souizi, J. Mol. Chem. 1294, 136383 (2023); (b) S. Chehab, Y. Merroun, S. Boukhris, R. Ghailane, A. Souizi, Polycycl. Aromat. Comp. 43(6), 4906 (2023)
Y. Merroun, S. Chehab, A. El Hallaoui, S. Boukhris, R. Ghailane, A. Souizi, Res. Chem. Intermed. 50, 3411 (2024)
A. Farajollahi, A. Poursattar Marjani, Sci. Rep. 14, 11475 (2024)
L. Bosch, J. Vilarrasa, Angew. Chem. 46, 3926 (2007)
S. Swami, S.N. Sahu, R. Shrivastava, RSC Adv. 11, 39058 (2021)
S. Rostamizadeh, H. Ghaieni, R. Aryan, A. Amani, Chin. Chem. Lett. 20, 1311 (2009)
S. Vorona, T. Artamonova, Y. Zevatskii, L. Myznikov, Synth 46, 781 (2014)
S.A. Ghumro, S. Saleem, M. Rashida, N. Iqbal, R.D. Alharthy, S. Ahmed, S.T. Moin, A. Hameed, RSC Adv. 7, 34197 (2017)
M.A. Siddiqui, M.H. Shaikh, A.A. Nagargoje, T.T. Shaikh, V.M. Khedkar, P.P. Deshpande, B.B. Shingate, Res. Chem. Intermed. 48, 5187 (2022)
S.B. Bhagat, V.N. Telvekar, Synlett 29, 874 (2018)
X. Xingquan, Y. Chao, L. Xu, L. Shilin, Tetrahedron Let. 60, 402 (2019)
M. Nasrollahzadeh, Y. Bayat, D. Habibi, S. Moshaee, Tetrahedron Lett. 50, 4435 (2009)
A. Jabbari, P. Moradi, B. Tahmasbi, RSC Adv. 13, 8890 (2023)
T. Jin, F. Kitahara, S. Kamijo, Y. Yamamoto, Tetrahedron Lett. 49, 2824 (2008)
M. Hatanoaand, K. Ishihara, Chem. Commun. 49, 1983 (2013)
M. Narasimhulu, T. Srikanth Reddy, K. Chinni Mahesh, S. MallaReddy, A. Vijender Reddy, Y. Venkateswarlu, J. Mol. Catal. A Chem. 264, 288 (2007)
K. Ishihara, M. Kawashima, T. Matsumoto, T. Shiori, M. Matsugi, Synth 50, 1141 (2018)
D.S. Bolotin, N.A. Bokach, M.Y. Demakova, VYu. Kukushkin, Chem. Rev. 117, 13039 (2017)
(a) U.B. Patil, K.R. Kumthekar, J.M. Nagarkar, Tetrahedron Lett. 53, 3706 (2012); (b) G. Sravanthi Devi, P. Santosh Kumar, A. Nagarsenkar, K.K. Gupta, B. NagendraBabu, Synlett 27, 1241 (2016); (c) B. Mitra, S. Mukherjee, G.C. Pariyar, P. Ghosh, Tetrahedron Lett. 59, 1385 (2018)
R.S. Pathare, A.J. Ansari, S. Verma, A. Maurya, A.K. Maurya, V.K. Agnihotri, A. Sharon, R.T. Pardasani, D.M. Sawant, J. Org. Chem. 83, 9530 (2018)
W. Zhao, Y. Zhou, L. Jiang, Org. Lett. 15(21), 5540 (2013)
G. Qi, W. Liu, Z. Bei, Chin. J. Chem. 9, 131 (2011)
B. Sreedhar, A. Suresh Kumar, D. Yada, Tetrahedron Lett. 52, 3565 (2011)
S.M. Agawane, J.M. Nagarkar, Catal. Sci. Technol. 2, 1324 (2012)
V. Rama, K. Kanagaraj, K. Pitchumani, J. Org. Chem. 76, 9090 (2011)
L. Lang, H. Zhou, M. Xue, X. Wang, Z. Xu, Mater. Lett. 106, 443 (2013)
M. Nikoorazm, A. Ghorbani-Choghamaranai, M. Khanmoradi, P. Moradi, J. Porous Mater. 25, 1831 (2018)
F. Dehghani, A.R. Sardarian, M. Esmaeilpour, J. Organomet. Chem. 743, 87 (2013)
G. Aridoss, K.K. Laali, Eur. J. Org. Chem. 2011, 6343 (2011)
A. Jabbari, B. Tahmasbi, M. Nikoorazm, A. Ghorbani-Choghamarani, Appl. Organometal. Chem. 32, e4295 (2018)
Acknowledgements
The authors gratefully acknowledge Management, Principal of Mahatma Gandhi Institute of Technology, Hyderabad and IICT Hyderabad for providing facilities to carry out this work.
Author information
Authors and Affiliations
Contributions
Gullapelli Kumaraswamy: Conceptualization, Investigation, Writing –original draft, Supervision, Writing review. Ramesh Nukala: Validation, Visualization, Methodology. Saidulu Ganji: Interpretation of spectral data and Writing review Ramesh Kola: Supervision and editing. Ravichandar Maroju: Data curation,formal analysis.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gullapelli, K., Nukala, R., Ganji, S. et al. One-pot multicomponent approach towards the synthesis of 5-substituted 1H-tetrazoles using lanthanum (III) nitrate hexahydrate as a catalyst. Res Chem Intermed 50, 4407–4423 (2024). https://doi.org/10.1007/s11164-024-05365-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-024-05365-8