Abstract
A simple and efficient protocol has been developed for the synthesis and characterization of new Cu(II) complex supported into MCM-41 channels modified with adenine (Cu(II)-Adenine-MCM-41) as a reusable and heterogeneous catalyst for the synthesis of 5-substituted 1H-tetrazoles and 1H-indazolo [1,2-b]phthalazine-triones. The key advantages of this method are easy work-up, short reaction times, pure product formation with good to excellent yields and reusability of the catalyst. This catalyst was characterized by TEM, SEM, XRD, TGA, EDS, AAS, BET method and FT-IR spectroscopy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
From last few decades a considerable attention has been focused on synthesis of heterocyclic compounds due to their wide range of biological applications [1]. Among the large variety of heterocyclic compounds, phthalazine derivatives have attracted increasing attention because of the wide range of biological activities [2]. Multicomponent reactions (MCRs) make it possible to synthesize heterocyclic compounds with greater efficiency and atom economy [3, 4]. One-pot multi-component reactions are preferably fast, simple, and efficient for the synthesis of 2H-indazolo[2,1-b]phthalazine-triones. Besides, the growth of tetrazole chemistry over the last two decades has been significant [5, 6]. They are a class of heterocycles that have been utilized in organometallic chemistry as effective stabilizers of metallopeptide structures, peptide chelating agents, in medicinal chemistry as stable surrogates for carboxylic acids and in coordination chemistry as ligands [7, 8]. Furthermore, tetrazoles have a wide range of applications in pharmaceutical science including anti-allergic, anti-asthmatic, anti-neoplastic, anti-inflammatory, and cognition disorder activities [9]. Recently tetrazole moieties were widely used for binding aryl thiotetrazolylacetanilides with HIV-1 reverse transcriptase [10]. One of the major synthetic routes to tetrazole formation is cycloaddition of an organonitrile and an azide salt that requires high temperatures or long reaction times [7, 11]. One of the most successful methods proposed in response to these limitations, is synthesis of tetrazole and its derivatives in the presence of heterogeneous catalysts [12]. Recycling of catalysts is a task of great environmental and economic advantages in pharmaceutical and chemical industry, especially when expensive or toxic heavy metal compounds are used [13]. For this reason, immobilization of homogeneous catalysts on solid supports has been used for synthesis of efficient and reusable heterogeneous catalysts [14]. Different supports have been used to develop the efficient heterogeneous catalyst [15,16,17,18,19,20,21,22]. In recent years, mesoporous molecular sieves (MMS) have gained an increasing interest because of their potential applications; some of which include their uses for catalytic applications [23,24,25], adsorption [26], drug-loading [27], luminescent materials [28], and so on. Mesoporous MCM-41 with two-dimensional channel structure has been widely investigated in various fields such as detection [29], separation [10] and catalysis [30] since its invention by the scientists of Mobil Corporation [31]. Because MCM-41 nanoparticles have some unique properties such as large specific surface area that is more than 1000 m2/g which is led to high capacity of catalyst loading, ease of functionalization due to many silanol groups on its structure, homogeneous hexagonal pore arrays with pore diameter, high thermal stability that is led to use as catalyst for various reactions in high temperatures, relatively hydrophobic nature and versatile separation from reaction media [32,33,34,35]. Adenine is a nucleobase that its structure is shown in Fig. 1.
Previous studies have shown that in adenine complexes, N1 and N3 atoms are electronically favored coordination sites for the metals. Based on these studies, adenine through the nitrogen N3 and N9 have been involved in metal coordination because of tautomerization of the imidazole hydrogen atom between N7 and N9 that can be form a number of different products with suitable conformers [36]. Thus, adenine was selected as a suitable ligand in this work. The aim of this paper is to study synthesis and characterization of Cu(II)-Adenine-MCM-41 and demonstrate the role of this novel complex as an efficient and reusable catalyst for the synthesis of 5-substituted 1H-tetrazole derivatives from sodium azide and 1H-indazolo [1,2-b]phthalazine-triones.
2 Experimental
2.1 Materials
The tetraethylorthosilicate (TEOS, 98%), cetyltrimethylammoniumbromide (CTAB, 98%), solvents and other chemicals used in the current study were purchased from Merck, Aldrich or Fluka companies and used without further purification.
2.2 Characterization techniques
Powder X-ray diffraction (PXRD) of catalyst was carried out using a Philips diffractometer with Cu Kα radiation at 40 kV and 30 mA. Fourier transform infrared (FT-IR) spectra were recorded with KBr pellets utilizing a VRTEX 70 model Bruker FT-IR spectrometer. The component analysis was carried out by the energy dispersive spectroscopy (EDS) using a Tacnai TF20 high-resolution transmission electron microscope. Thermogravimetric analyses (TGA) of the samples were obtained between 30 and 900 °C with heating rate of 10 °C/min using Shimadzu DTG-60 automatic thermal analyzer. The content of Cu(II) was measured by Tech flame atomic absorption spectrometer (AAS) in a NovAA 400p Analyticaljena-Germany device. The particle size and morphology were performed using a JEOLJEM-2010 scanning electron microscopy (SEM), using an accelerating voltage of 20 kV. The transmission electron microscopy (TEM) images were taken by a Philips CM10 microscope with operating voltage at 200 kV. Melting point of all compounds were determined using Griffin melting point apparatus. Nitrogen adsorption isotherms were determined using a standard gas manifold at 77 K to surface characteristics of catalyst using BELSORP MINI II device. Also, the catalyst sample was degassed at 120 °C for 2 h using BEL PREP VAC II device before analysis.
2.3 Preparation of catalyst (Cu(II)-Adenine-MCM-41)
Mesoporous Si-MCM-41 was prepared according to our previous works (sol–gel) [37,38,39] by hydrolyzing cetyltrimethylammonium bromide (CTAB) (1 g) as the structure directing agent in solution containing 3.5 mL NaOH (2 M). The mixture was stirred at 80 °C until the mixture became uniform, then 5 mL of the tetraethyl orthosilicate (TEOS) was slowly added and the resulting mixture was refluxed for 2 h at the same temperature. After cooling to room temperature the white solid was obtained, washed with deionized water and heated at 70 °C in an oven for 20 h. The resulting solid was calcined at 823 K for 5 h with rate of 2 °C/min to remove the residual surfactant. Post-synthesis organic modification of pure MCM-41 was performed by refluxing 4.8 g of MCM-41 with 4.8 g (3-chloroopropyl)-triethoxysilane (3-CPTES) in n-hexane for 24 h under nitrogen atmosphere. The product nPrCl-MCM-41 was filtered, washed with n-hexane and dried under vacuum.
The preparation of catalyst was continued by refluxing a mixture of nPrCl-MCM-41 (0.5 g), triethylamine (2 mmol, 0.202 g) and adenine (1 mmol, 0.135 g) in toluene for 24 h. The resulting white solid was filtered, washed with ethanol for several times and dried in a vacuum. Finally Cu(II)-Adenine-MCM-41 was prepared by refluxing a mixture of Cu(NO3)2.6H2O (0.70 mmol, 0.170 g) and prepared functionalized MCM-41 mesoporous (0.25 g) in ethanol (25 mL) for 20 h at 80 °C. The resulting gray solid impregnated with the metal complex was filtered, washed with ethanol and dried at 50 °C in an oven (Scheme 1).
2.4 General procedure for the synthesis of 5-substituted 1H-tetrazoles
A mixture of benzonitrile (1 mmol), sodium azide (0.08 g, 1.2 mmol) and Cu(II)-Adenine-MCM-41 (35 mg) in PEG-400 (2 mL) was stirred at 130 °C for an appropriate time. The progress of reaction was monitored by TLC (eluent; n-hexane: acetone, 8:2). After completion of the reaction, the catalyst was removed by filtration and the residue was treated with ethyl acetate, water and 0.1 N HCl (5 mL). Then the organic layer was separated and the aqueous layer was extracted with ethyl acetate (20 mL). Then, ethyl acetate was evaporated and the products were obtained.
2.5 General procedure for the synthesis of 1H-indazolo [1,2-b]phthalazine-triones
A mixture of aldehyde (1 mmol), dimedon (1.2 mmol) and phthalhydrazide (1 mmol), Cu(II)-Adenine-MCM-41 (25 mg) under free solvent condition was stirred and heated at 100 °C to give the corresponding 2H-indazolo[2,1-b]phthalazine-trione and progress of the reaction monitored by TLC (n-hexane/acetone (8:2)). After completion the reaction, ethanol was added to the reaction mixture and the catalyst was separated by filtration. Finally, the product was recrystallized in ethanol to give pure product.
3 Results and discussion
In this work, we report synthesis and characterization of Cu(II)-Ademine-MCM-41 and its application as an efficient, facile, and environmentally friendly mesoporous catalyst for the synthesis of 5-substituted 1H-tetrazole derivatives and 1H-indazolo [1,2-b]phthalazine-triones in good to excellent yields. The synthesized catalyst was characterized by various techniques such as TEM, SEM, XRD, TGA, EDS, AAS, BET method and FT-IR spectroscopy.
3.1 Catalyst characterization
3.1.1 TEM, SEM and AAS
The TEM (transmission electronic microscopy) micrograph and SEM (scanning electron microscopy) images of Cu(II)-Adenine-MCM-41 are shown in Fig. 2 that the hybrid nanomaterial has spherical shapes and 30–50 nm of diameter of particles [32, 37]. This result clearly indicates that the catalyst was hexagonal regularly shaped of mesoporous channels. The amount of Cu(II) immobilized on MCM-41 nanostructure was found to be 0.74 mmol g−1 that was determined by atomic absorption spectroscopy (AAS).
3.1.2 Thermogravimetric analysis
The thermogravimetric analysis (TGA) curves of MCM-41 (red line) and Cu(II)-Adenine-MCM-41 (green line) show several mass loss steps of the organic materials as they decompose upon heating (Fig. 3). The TGA curve of MCM-41 sample exhibited a stage of weight loss with approximate amount of 4% below 100 °C that is due to desorption of physically absorbed water and solvent [40]. Also according to the curve of Cu(II)-Adenine-MCM-41, three weight loss steps were observed. The first mass loss occurs at a temperature range of 100–200 °C that is related to the loss of physical adsorbed water and other solvents. The second mass loss occurs at a temperature range of 200–450 °C that can explain by decomposition of organic moieties. The third mass loss at a temperature range of 500 to ~ 750 °C that is related to the decomposition of silanol groups.
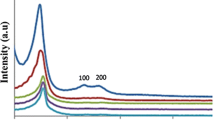
3.1.3 X-ray diffraction
The XRD patterns of MCM-41 and Cu(II)-Adenine-MCM-41 are shown in Fig. 4. The diffractogram of the MCM-41, indicated the three reflections in the 2° < 2θ < 6° range, indexed to a hexagonal cell as (1 0 0), (1 1 0) and (2 0 0) plans. In the XRD pattern of Cu(II)-Adenine-MCM-41 these observed peaks intensity clearly reduced. This observation is due to an X-ray scattering contrast reduction between the silica walls and pore-filling material that indicate functionalization occurred mainly inside the MCM-41 channels.
3.1.4 FT-IR spectroscopy
Figure 5 compares the FTIR spectra of the starting materials with MCM-41 modified using (3-chloropropyl)-triethoxysilane (nPrCl-MCM-41), adenine-MCM-41 and Cu(II)-Adenine-MCM-41. FT-IR spectrum of MCM-41 shows three characteristic bands: the asymmetric and symmetric stretching and bending vibrations of Si–O–Si are present about 1059, 962 and 454 cm−1, respectively. Also, the O–H stretching vibration bands appeared at 3443 cm−1 (Fig. 5a). In Fig. 5b, the presence of the anchored 3-chloropropyl-triethoxysilane (nPrCl-MCM-41) is confirmed by C–H stretching vibrations around 2900 cm−1. The spectrum of adenine shows strong bands at 1671 and 1369 cm−1 that may be attributed to the C=N and C–N stretching frequencies respectively Fig. 5c. Reaction of nPrCl-MCM-41 with adenine was confirmed by the presence of these bands in FT-IR spectrum of adenine-MCM-41 as shown in Fig. 5d. Moreover, in FT-IR spectrum of Cu(II)-Adenine-MCM-41 these bands are shifted to the lower frequencies, which indicate the formation of metal–ligand bonds (Fig. 5e). The FT-IR spectrum of adenine (Spectrum c) shows several bands at 3553, 1601, 1671, 1452, 1414, 1368 and 649 cm−1 that are attributed to the N–H, C=C, C=N and C–N stretching vibrations respectively, that are specific to the structure of adenine, whereas these bands are not observed in FT-IR spectrum of nPrCl-MCM-41 [39, 40]. These evidence confirmed that adenine has been immobilized on the MCM-41 instead of chlorine. In addition, in the spectrum of Cu(II)-Adenine-MCM-41 the broading in the range of 1200–1650 cm−1 are attributed to the formation of copper complex on MCM-41 [41].
3.1.5 Surface characteristics
Nitrogen is commonly gas used for surface probing by BET methods. In order to determine the surface phenomena of catalyst, the nitrogen adsorption/desorption isotherms were evaluated using a BEL sorp mini II volumetric adsorption analyzer. The catalyst sample was degassed at 100 °C under argon gas flow for 3 h before analysis.
The N2 adsorption and desorption isotherms of Cu(II)-Adenine-MCM-41 are shown in Fig. 6. Based on the IUPAC classification, these materials display a typical type IV isotherm, which is the characteristics of mesoporous material [42]. The Brunauer–Emmett–Teller (BET) surface area, pore volume, pore diameter and wall diameter were determined for Cu(II)-Adenine-MCM-41. In our previous work, we have reported N2 adsorption/desorption analysis of MCM-41 [43]. The BET surface area of MCM-41 was decreased from 1372 to 457 m2 g−1, average pore volume and pore diameter were decreased from 1.51 cm3 g−1 and 2.45 nm to 0.34 cm3 g−1 and 1.29 nm for Cu(II)-Adenine-MCM-41 respectively [42]. Meanwhile, the wall diameter was increased from 1.02 to 2.97 nm for Cu(II)-Adenine-MCM-41. These results could be attributed to the immobilization of Cu- complex within channels of MCM-41.
3.1.6 Evaluation of the catalytic activity of Cu(II)-Adenine-MCM-41
3.1.6.1 Synthesis of 5-substituted 1H-tetrazoles
For evaluation of the catalytic properties of Cu(II)-Adenine-MCM-41 in the synthesis of 5-substituted 1H-tetrazoles, we evaluated the reaction of benzonitrile (1 mmol) with sodium azide (1.2 mmol) as a model reaction to synthesis of corresponding tetrazoles (Scheme 2).
Initially, the catalyst was applied to the [3 + 2] cycloaddition reaction of benzonitrile (1 mmol) and sodium azide (1.2 mmol) at 120 °C. When we tested the reaction in the absence of catalyst, no desirable product was obtained (Table 1, entry 1). It can be seen from Table 1, increasing the amount of catalyst led to relatively higher yield. Therefore, the best amount of catalyst is 35 mg (Table 1, entry 5). Use of low temperature gave significantly lower levels of the yield and the yield increased slightly with increasing of temperature (Table 1, entries 5 and 7–10). However, the reaction at 130 °C showed highest level of conversion (92%) (Table 1, entry 7). Also it was found that solvent influenced on outcome of reaction and PEG-400 was the best solvent for this reaction (Table 1, entry 7). The results obtained from the different approaches are summarized in Table 1.
Such this catalytic system has been successfully applied to the synthesis of 5-substituted 1H-tetrazoles (Table 2). This investigation indicated that presence of electron donating or withdrawing groups has no significant role in reaction. All the desired products were obtained in good to excellent of yields. Interestingly, dicyano derivatives (Table 2, entries 4, 8, 12, 13) afford the monoaddition products.
3.1.6.2 Synthesis of 1H-indazolo [1,2-b]phthalazine-triones
In continuation of this work we evaluated the catalytic properties of Cu(II)-Adenine-MCM 41 in the synthesis of 1H-indazolo [1,2-b]phthalazine-triones. For this aim, the reaction of 4-chlorobenzaldehyde (1 mmol), phthalhydrazide (1 mmol) and dimedon (1 mmol) was chosen as model reaction to optimize the reaction conditions and establish the feasibility of the strategy (Scheme 3).
Initially, we tried to optimize the amount of catalyst under solvent free conditions at 100 °C. As shown in Table 3, 25 mg of catalyst resulted in higher yield than other amount of catalyst (Table 3, entries 1–3). Therefore, 25 mg of catalyst was selected for this reaction. For increasing the yield, the amount of dimedon was increased. Interestingly we show that the yield of product was increased when 1.2 mmol of dimedon was used (Table 3, entry 4). Next, role of different solvent systems such as PEG-400, ethanol, ethyl acetate and solvent free were investigated (Table 3, entries 5–7). We observed that the best manner occurs under solvent free conditions (Table 3, entry 4). Finally, to find optimize temperature on this model reaction, various temperature were applied and the best results were obtained at 100 °C. Also higher temperature was evaluated that we observed only 2% rising in yield at 110 °C. Therefore, 1.2 mmol dimedon, 25 mg of catalyst at 100 °C under solvent free conditions were selected as optimized reaction conditions.
The scope and generality of this method was investigated using several derivatives of aldehydes under the optimized reaction conditions. The results showed that reactions proceeded very well (Table 4).
In the recyclability experiment for synthesis of tetrazoles, a mixture of benzonitrile (1 mmol) with sodium azide (1.2 mmol) was selected under the optimized conditions. After completion of the reaction, the catalyst was recovered by centrifugation, washed with ethyl acetate, dried at 50 °C and reused in the next run. It is found that the catalyst can be reused up to six times without any significant loss in yield and catalytic activity (Fig. 7).
4 Conclusions
In summary, Cu(II)-Schiff-base complex supported on MCM-41 has been successfully prepared by means of Post-Grafting method and characterized by SEM, TEM, XRD, TGA, EDS, AAS and FT-IR techniques. Synthesis of 5-substituted 1H-tetrazoles and 1H-indazolo [1,2-b]phthalazine-triones have been efficiently catalyzed by Cu(II)-Adenine-MCM-41. The catalyst can be easily separated from the reaction mixture and reused several times without significant loss of its catalytic activity.
References
M. Nasrollahzadeh, Y. Bayat, D. Habibi, S. Moshaee, Tetrahedron Lett. 50, 4435 (2009)
V.V. Sureshbabu, R. Venkataramanarao, S.A. Naik. G. Chennakrishnareddy, Tetrahedron Lett. 48, 7038 (2007)
S. Kumar, S. Dubey, N. Saxena, S.K. Awasthi, Tetrahedron Lett. 55, 6034 (2014)
Z. Noroozi Tisseh, M. Dabiri, M. Nobahar, A. Abolhasani Soorki, A. Bazgir, Tetrahedron. 68, 3351 (2012)
D. Habibi, M. Nasrollahzadeh, A.R. Faraji, Y. Bayat, Tetrahedron. 66, 3866 (2010)
P. Sharma, J.K. Seong, Y.H. Jung, S.H. Choi, S.D. Park, Y.I.I. Yoon, I.-H. Baek, Powder Technol. 219, 86 (2012)
P. Mani, A.K. Singh, S.K. Awasthi, Tetrahedron Lett. 55, 1879 (2014)
B. Afzalian, J.T. Mague, M. Mohamadi, S.Y. Ebrahimipour, B. Pour amiri, E. Tavakolinejad Kermani, Chin. J. Catal. 36, 1101 (2015)
M. Kidwai, A. Jahan, R. Chauhan, N.K. Mishra, Tetrahedron Lett. 53, 1728 (2012)
A. Hasaninejed, M. Rasekhi Kazerooni, A. Zare, Catal. Today. 196, 148 (2012)
F. Dehghani, A.R. Sardarian, M. Esmaeilpour, J. Organometal. Chem. 743, 87 (2013)
S. Bhunia, S. Koner, Polyhedron. 30, 1857 (2011)
P. Sivaguru, K. Bhuvaneswari, R. Ramkumar, A. Lalitha, Tetrahedron Lett. 55, 5683 (2014)
C. Bowers, P.K. Dutta, J. Catal. 122, 271 (1990)
M. Salavati-Niasari, Inorg. Chem. Commun. 8, 174 (2005)
M.R. Maurya, S.J.J. Titinchi, S. Chand, J. Mol. Catal. A Chem. 214, 257 (2004)
F. Minutolo, D. Pini, A. Petri, P. Salvadori, Tetrahedron Asymmetry. 7, 2293 (1996)
S.M. Drechsel, R.C.K. Kaminski, S. Nakagaki, F. Wypych, J. Colloid Interface Sci. 277, 138 (2004)
M. Salavati-Niasari, J. Mol. Catal. A. 229, 159 (2005)
B. Movassagh, F.S. Parvis, M. Navidi, Appl. Organometal. Chem. 29, 40 (2015)
B.M. Bhanage, S. Fujita, M. Arai, J. Organometal. Chem. 687, 211 (2003)
O. Aksın, H. Turkmen, L. Artok, B. Cetinkaya, C. Ni, O. Buyukgungo r, E. Ozkal, J. Organometal. Chem. 691, 3027 (2006)
P.A. Vigoto, V. Peruzzo, S. Tamburini, Coord. Chem. Rev. 256, 953 (2012)
A. Garoufis, S.K. Hadjikakou, N. Hadjiliadis, Coord. Chem. Rev. 253, 1384 (2009)
B. Lei, H. Li, S. Zhang, Z. Lu, W. Zheng, Y. Li, Wang, Adv. Funct. Mater. 16, 1883 (2006)
A. Corma, Chem. Rev. 97, 2373 (1997)
J.S. Beck, J.C. Vartuli, W.J. Roth, M.E. Leonowicz, C.T. Kresge, K.D. Schmitt, C.T.W. Chu, D.H. Olson, E.W. Shepard, S.B. McCullen, J.B. Higgins, J.L. Schlenker, J. Am. Chem. Soc. 114, 10834 (1992)
X.G. Zhou, X.Q. Yu, J.S. Huang, S.G. Li, L.S. Li, C.M. Che, Chem. Commun. 1789 (1999)
M. Nandi, P. Roy, H. Uyama, A. Bhaumik, Dalton Trans. 40, 12510 (2011)
S.A. Shaker, Y. Farina, S. Mahmmod, M. Eskender, Sains Malays. 39, 957 (2010)
M. Nikoorazm, A. Ghorbani-Choghamarani, M. Khanmoradi, Appl. Organometal. Chem. 30, 236 (2016)
M. Nikoorazm, A. Ghorbani-Choghamarani, M. Khanmoradi, J. Porous. Matter. 23, 261 (2016)
M. Nikoorazm, A. Ghorbani-Choghamarani, M. Khanmoradi, RSC Adv. 6, 56549 (2016)
A. Tahmasbi, Ghorbani-Choghamarani, Appl. Organometal. Chem. 31, e3644 (2017)
A. Ghorbani-Choghamarani, P. Moradi, B. Tahmasbi, RSC Adv. 6, 56638 (2016)
R. Ghorbani-Vaghei, R. Karimi-Nami, Z. Toghraei-Semiromi, M. Amiri, M. Ghavidel, Tetrahedron. 67, 1930 (2011)
A. Rostami, B. Tahmasbi, A. Yari, Bull. Korean Chem. Soc. 34, 1521 (2013)
H. Shaterian, M. Ghashang, M. Feyzi, Appl. Catal. A Gen. 345, 128 (2008)
A. Ghorbani-Choghamarani, B. Tahmasbi, P. Moradi, RSC Adv. 6, 43205 (2016)
A. Ghorbani-Choghamarani, B. Tahmasbi, P. Moradi, N. Havasi, Appl. Organometal. Chem. 30, 619 (2016)
A. Ghorbani‑Choghamarani, B. Tahmasbi, N. Noori, R. Ghafouri‑nejad, J. Iran. Chem. Soc. 14, 681 (2017)
F. Havasi, A. Ghorbani-Choghamarani, F. Nikpour, Microporous Mesoporous Mater. 224, 26 (2016)
M. Nikoorazm, N. Noori, B. Tahmasbi, S. Faryadi, Transit. Metal Chem. 42, 469 (2017)
Acknowledgements
This work was supported by the research facilities of Ilam University, Ilam, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nikoorazm, M., Ghorbani-Choghamaranai, A., Khanmoradi, M. et al. Synthesis and characterization of Cu(II)-Adenine-MCM-41 as stable and efficient mesoporous catalyst for the synthesis of 5-substituted 1H-tetrazoles and 1H-indazolo [1,2-b]phthalazine-triones. J Porous Mater 25, 1831–1842 (2018). https://doi.org/10.1007/s10934-018-0597-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-018-0597-0