Abstract
The effluent produced by the dairy industry is characterized by high levels of turbidity and organic load. This issue is commonly addressed by employing coagulation techniques to remove turbidity. However, regarding the proven disadvantages involved in using chemical coagulants, it is necessary to explore natural coagulants. This research compares the efficiency of the chemical coagulant (ferric chloride) with natural coagulant (Plantain leaf) concerning their ability to remove turbidity from industrial wastewater. Results indicate that the turbidity removal is similar between the two coagulants. Moreover, an increase in the concentration of coagulants resulted in a higher percentage of turbidity reduction. The highest and lowest reduction efficiency of the ferric chloride coagulant was 99% and 2%, respectively. Meanwhile, the highest and lowest reduction efficiency of the yellow Plantain coagulant was 98% and 22%, respectively. Ultimately, the coagulant derived from the yellow leaves of the Plantain tree is recommended as an effective and practical alternative to the chemical ferric chloride coagulant. This natural coagulant demonstrated a high ability to remove turbidity, complete coagulation, abundant resources, low cost, environment-friendly, and biodegradable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a major source of environmental pollution, industrial wastewater affects water and soil quality, leading to serious health hazards [1, 2]. One of the most common problems with industrial waste is the presence of high turbidity levels that can harm the environment [3, 4]. This problem can be overcome using coagulation and flocculation techniques to remove turbidity from industrial wastewater. The use of chemical coagulants has been the traditional method for turbidity removal. However, using natural coagulants (e.g., Plantain leaf) has been gaining popularity because of their environmental benefits [5]. Chemical coagulants such as chlorferric are commonly used in industries for turbidity removal. However, the use of these chemicals involves some drawbacks. First, they are expensive and require careful handling and storage. Second, they can lead to the formation of sludge, which can be difficult to dispose of. Third, using chemical coagulants can result in producing harmful byproducts that can be detrimental to the environment [6].
Recently, green approaches in chemical reactions have been the subject of intense research [7, 8]. Natural coagulants have emerged as the most effective method among these approaches because of availability, enhanced selectivity, and green reaction condition [9]. Various materials (e.g., chitosan [10], Robinia [11], Abelmoschus esculentus, Quercus, Salvia hispanica [12], and Vicia faba [13] have been used as highly potent green materials to develop natural coagulants. Plantain’s leaf is a natural coagulant used in many parts of the world for turbidity removal [14, 15]. One of the main advantages of using natural coagulants is that they are eco-friendly and do not produce harmful byproducts [16]. These leaves are readily available and inexpensive [17]. Besides, they can be easily extracted and processed for industrial wastewater treatment [18]. Based on the mentioned points, the present study explores the effectiveness of ferric chloride chemical coagulant and natural coagulant of Plantain leaf. The experiments involved using industrial wastewater collected from the outlet of dairy industry refineries. The effluent turbidity was measured before and after treatment with both coagulants. The results showed that both coagulants effectively removed turbidity from the wastewater. Finally, of the two coagulants examined in this study, Plantain leaf coagulant was found as a good and practical coagulant with a high potential to replace ferric chloride coagulants. Moreover, it has the advantages of low cost, environmentally friendly, versatile, and biodegradable.
Experimental
Measurement of turbidity and other parameters
To remove the turbidity, the wastewater of Pegah Hamedan pasteurized milk company was used. The effluent’s turbidity (TRE) was measured using a turbidimeter model AL200T-IR. For this purpose, first, the device was calibrated using ready standards that are commercially available. The desired effluent was poured into a special tube, placed inside the device, and its turbidity level was read [19]. Equation (1) was used to calculate the turbidity removal efficiency. In this equation, T0 is the initial value of turbidity (before using a coagulant), and T is the secondary value of turbidity (after using a coagulant) in terms of NTU.
Also, other parameters such as pH, total dissolved solids (TDS), total suspended solids (TSS) [20], settleable solids (SS), electrical resistivity and conductivity (EC) [21], calcium hardness (Ca) [22], magnesium hardness (Mg) [23], and total hardness (TH) [24] were measured. The pH was measured using a pH meter (Jenway 3510 model). The amount of SS was measured using a spectrophotometer device (Jenway 6305 model). Also, EC was measured using an EC meter (Jenway 4510 model). Finally, TDS measurement was done using EC value. Equation (2) expresses the TDS calculation method.
TSS measurement was done by weighing the filter paper before and after passing the sample through it and drying in the oven. Then, it was calculated using Eq. (3). In this equation, A is the second weight of the filter paper after passing the samples and drying in the oven, B is the initial weight of the filter paper in grams, and VSample is the volume of the sample in milliliters.
TH and Ca were measured using the titration method, and TH, Ca, and Mg were calculated using Eq. (4). Equations (5) and (6) present the methods of calculating Ca and Mg, respectively. In Eqs. (4) and (5), V EDTA is the consumed volume of EDTA, F EDTA is the EDTA concentration, and VSample is the sample volume in milliliters. In Eq. (6), TH is the total hardness value, and Ca is the calcium hardness value (5). To reduce the error and obtain reliable data, we repeated each experiment step in triplicate for each sample, and the results were the average of these three times.
The Plantain leaves were washed thoroughly with a grease remover and plenty of water. Then, they were dried in a Memert (UNB-400) oven at 100 °C. The dried leaves were then ground into a fine powder using a Moulinex grinder. An extract was prepared by adding the powdered material (1.00 g) to distilled water (1.00 L) and mixing it for 3.00 min using an IKA.RET basic c mixer at 120 rpm. The resulting extract was then passed through a filter paper with a hole diameter of 0.450 μm, yielding a homogeneous solution free of suspended particles, which served as a coagulant. Figure 1 presents the stages involved in producing and preparing the coagulant derived from yellow leaves. Plantain contains iridoids that give it anti-inflammatory activity. In addition, its leaves are also composed of mucilages, flavonoids, including luteolein and apigenin, but also coumarins, tannins, minerals (calcium, iron, magnesium, potassium, selenium) and vitamins (A, B, C, K). Plantain has anti-infectious and antiviral properties against the herpes virus. Studies have shown the antibacterial properties of plantain on Staphylococcus aureus and, among others, on Bacillus cereus, Escherichia coli and Pseudomonas aeruginosa [25].
Methodology
Time, speed of mixing and determination of dosage of coagulants
With excessive mixing, flocculation is not affected, but insufficient mixing causes problems in this step. The proper contact time of the coagulant in the mixing chamber is usually between 1 and 3 min. Proper coagulation and formation of microflocs require proper mixing speed and high energy of coagulants. Jar test is used to determine the amount of coagulant. In this test, various coagulants are added to the wastewater with different doses to determine the best coagulant with the appropriate dose [26].
Sampling
The current study is descriptive-cross-sectional. Natural wastewater was used for the experiments. Raw sewage samples were collected daily from the outlet of the pasteurized milk factory in Hamedan, in 1.5 L plastic bottles and transported to the laboratory.
Preparation of chlorferric coagulant
1 g of chloroferric was added to 1 L of distilled water, and to create a homogeneous solution, the resulting solution was mixed with stirrer for 10 min. It is worth mentioning that the resulting solution is the mother solution of 1000 ppm.
Preparation of coagulant of yellow plantain leaves
Sycamore leaves that have turned yellow were collected from trees in the city of Hamadan province. Then, the leaves were washed well with plenty of water and grease remover and then dried in an oven at a temperature of 100 °C. Then, they were turned into a soft powder with a Moulinex grinder. In order to create the extract, one gram of the created powder was weighed. For this purpose, first the device was set to zero, then the desired powder was weighed, and in the next step, it was added to 1 L of distilled water and mixed for 3 min with the help of the IKA.RET basic c mixer at 120 rpm. Then, the created extract was passed through a filter paper with a hole diameter of 0.45 μm, and finally, a homogeneous solution without suspended particles was obtained, which was used as a coagulant. The above solution was prepared fresh for each purification and to prevent changes in alkalinity and viscosity, the solution was kept in a cool place with a maximum temperature of 20 °C.
Coagulation process
In this study, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5 and 5 mg/L of chlorferric were tested in the purification process. Below are the values of 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130 mg/L of seed powder, green leaf powder, yellow leaf powder, seed powder ash, powder ash. Green leaf and yellow leaf powder ash were tested separately in one liter of wastewater. The selection of the range of coagulant concentration changes was based on the significance of the difference in purification efficiency. In addition, economic foundations were also taken into account.
Perform mixing operations
The mixing operation was done mechanically with Jartest SK 2008 model and with the ability to select variable speeds. After preparing the samples in the jar test machine, they were subjected to direct mixing with 120 revolutions in 2 min and slow mixing with speeds of 60, 40, 20, respectively, for 8, 8, 5 min and at the end for 10. They were kept in a state of rest for 30 min in order to settle down. At the end, total hardness, calcium hardness, magnesium hardness, turbidity, pH, TSS, TDS, SS, and EC were measured in the samples.
Turbidity measurement
Effluent turbidity was measured using AL200T-IR turbidity meter. For this purpose, first the device was calibrated using ready standards that are commercially available, then the desired effluent was poured into a special tube and then it was placed inside the device and the turbidity level was read.
pH measurement
The pH was measured using a Jenway 3510 pH meter. For this purpose, the device was first calibrated using buffer solutions with known pHs that are suitable for this purpose. Then, the electrode was washed with distilled water and dried, and finally the desired effluent was poured into the beaker and the electrode was placed inside the beaker and the pH was read.
Measurement of settleable solids (SS)
The amount of SS was measured using a Jenway 6305 spectrophotometer. For this purpose, the device was first turned on and calibrated. For calibration, some distilled water was poured into a small glass container that is special for the device and placed inside the device. Then, it was set to the desired wavelength for SS reading which is 810 nm. After calibration, the desired effluent was poured into special glass containers and placed in the machine, and the amount of SS was read.
Electrical conductivity (EC) measurement
The amount of EC was measured using a Jenway 4510 EC meter. For this purpose, the device was first calibrated using the EC meter solution that is suitable for this purpose. Then, the sensor of the device was washed with distilled water and dried, and finally the desired effluent was poured into the beaker and the sensor was placed inside the beaker and the EC level was read.
Jar test
This test is done using a number of glass containers that have a capacity of at least 1 L and are the same in terms of size and shape. The speed of the machine was set to rapid mixing (80–120 rpm). Because the test must be repeatable. A 5-mL graduated pipette was filled with the original coagulant and gradually added to the mixing sample; As soon as the first clots were observed, the injection of coagulant was stopped and the amount consumed was noted, this amount of coagulant is considered as the base amount.
Then, we filled all six containers of the jar machine with one liter of mixed sample. The round of the machine was set to the round of the previous step. Adjust the injection amount of coagulant in each container so that the number obtained from the previous step is placed in one of the two middle containers. We added the coagulant to all six containers at the same time and kept the time. (60 s) Then, we set the speed of the machine to slow mixing (20 rpm) and continued slow mixing for 20 min. Turn off the device and let the samples settle for 30 min (it is better). Now we compared the results using the turbidity meter and reported the best result [27].
Examining the characteristics of the surface morphology of the samples (FESEM)
Jeol.JSM-840A Scanning Electron Microscopy SEM (Scanning Electron Microscopy) model was used to investigate the surface morphology characteristics of dry samples that include produced sludge and various coagulants. To prepare the samples, first, some of the created sludge and some of the examined coagulants were placed separately on the slide and dried in the Memert. UNB-400 oven at 60 °C for 24 h. Then, the samples were placed in Jeol.JFC-1100E gold coating machine for 10 min, and finally, they were placed in the scanning electron microscope and photographed in different magnifications. At the end, in order to identify the elements, EDAX (Energy-dispersive X-ray spectroscopy) qualitative analysis was taken using X-ray energy diffraction spectrometer.
FT-IR analysis
Fourier-transform infrared spectroscopy (FTIR) was performed using Spectrum 65 model FT-IR spectrophotometer in order to determine the presence of functional groups in different coagulants. For this purpose, first some KBr (potassium bromide) was ground with a mortar. Then, 100 to 1 ratio of leaves and coagulant powder was mixed and re-ground and the material was placed inside the ring and placed in the Petro Tech model press machine to turn into tablets. Finally, in order to determine and read the presence of functional groups in the coagulants, the obtained tablets were placed in the FT-IR spectrophotometer.
Statistic analysis
The statistical processing of the data in this research was done using SPSS software version 20 and the corresponding graphs were drawn with Excel software. The efficiency of each coagulant in removing the above parameters was calculated by percentage. At first, the normality of the obtained data was investigated by the Kolmogorov–Smirnov test. After determining the normality of the data, parametric tests were used. In order to compare the average of each of the investigated parameters with the standard limit before and after the use of coagulant, a sample t test was used. Also, in order to compare the average concentration of each of the investigated parameters, analysis of variance (ANOVA) test was used. Duncan's test was used for statistical grouping and it was drawn in the form of a graph, and correlation test was used to measure the relationship between variables.
Investigating the appearance characteristics of sludge volume produced by natural coagulant yellow leaf and chemical coagulant chlorophrenic through direct observation
The color of the sludge produced by the natural coagulant of yellow leaves is dark brown, and the color of the sludge produced by the chemical chlorferric coagulant is orange to light brown. The settling speed of the clot obtained from the yellow leaf coagulant is lower than the clot obtained from the chlorferric coagulant. The cohesion of the clot obtained from the yellow leaf coagulant is higher than the clot obtained from the chlorferric coagulant. The volume of sludge obtained from yellow leaf coagulant is less than that obtained from chlorferric coagulant.
Examining the dry powder obtained from the yellow leaves of the plantain tree after gelatinization (mixing with distilled water)
After gelatinization, the suspended particles absorb water and swell, and with the increase in viscosity, the grains break, and the materials inside dissolve in water, and after mixing with wastewater, they react with colloidal particles and precipitate. The charge of the colloidal particles is negative and inside the coagulant of the yellow leaves of the plantain tree there are minerals that have a negative charge. As a result, both the names attract each other and increase the size and weight of the particles, and they settle and form clots.
Results and discussion
In continuation of our previous efforts on applying adsorbent in wastewater treatment, herein, we investigate for the first time the synthesis, efficiency, and characterization of an environmentally friendly novel natural coagulant (i.e., Plantain leaf) with a chemical coagulant (i.e., ferric chloride) concerning their ability to remove turbidity from industrial wastewater.
Figure 2 displays the effluent turbidity before and after applying both chemical and natural coagulants.
Figure 3 illustrates the structure of clots formed by the chemical coagulant. This study presents the ferric chloride and natural coagulants derived from the yellow leaves of the Plantain tree.
Characterization
FTIR spectrum of yellow leaf coagulant is presented in Fig. 4a. The broad absorption band at 3420–2900 cm−1 is related to O–H groups. In addition, the absorption bands at 1725 cm−1, 1685 cm−1 and 1646 cm−1 belong to the stretching vibrations of the C=O bond of ester, acid, and amide groups, respectively. Furthermore, it shows bands at 3000–3600 cm−1, corresponding to the asymmetric stretching and the OH groups [28]. Figure 4b shows the FTIR spectra of the ferric chloride coagulant. The broad absorption band at 3420–2900 cm−1 is related to N–H and O–H groups. Also, the absorption bands at 1719 cm−1 and 1643 cm−1 are indexed to the stretching vibrations of C=O in acid and amide groups, respectively.
The field emission scanning electron microscope (FESEM) images in Fig. 5 show the clots generated from the jar test using two coagulants: chlorferric chemical coagulant and natural yellow Plantain coagulant. As illustrated in Fig. 5a, the sludge resulting from wastewater treatment with chlorferric coagulant exhibits a solid and rigid nature. The clots formed possess an irregular shape and are primarily comprised of coarse and pointed polygonal particles. These particles display an uneven surface and are interconnected, forming larger particles resembling rock crystals. Similarly, the sludge obtained from wastewater treatment with yellow Plantain leaf coagulant also exhibits a robust and firm consistency (Fig. 5b). The formed flocs indicate irregular shapes and contain small, wide, cylindrical, and pointed particles with smooth surfaces. These particles join together to form larger particles, resembling rod-like junctions that exhibit a flower-like appearance.
Figure 6 illustrates the removal percentage of wastewater turbidity after using a ferric chloride coagulant. The results demonstrate that the turbidity reduction percentage increases with increased coagulant concentration. The turbidity without coagulants was equal to 35 NTU. The highest turbidity reduction efficiency, observed at a concentration of 2.00 mg L−1, was 99%. It is of note that the turbidity reduction percentage decreased at concentrations of 2.50, 3.00, 3.50, 4.00, 4.50, and 5.00 mg L−1. The concentration of 5.00 mg L−1 yielded the lowest turbidity reduction percentage, i.e., 2% (Table 1).
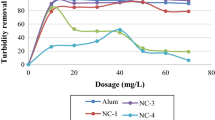
Figure 7 depicts the removal percentage of wastewater turbidity after using yellow Plantain leaf coagulant. An increase in coagulant concentration increased the turbidity reduction percentage. The highest turbidity reduction efficiency, observed at a concentration of 70.0 mg L−1, was 98%. In contrast, the turbidity reduction percentage decreased at concentrations of 80.0, 90.0, 100, 110, 120, and 130 mg L−1. The lowest turbidity reduction percentage, i.e., 22%, was observed at a concentration of 10.0 mg L−1 (Table 2). Plantain leaves contain a significant amount of water-soluble proteins with low molecular weight that have a positive charge of one electron. When added to water, proteins increase the positive charge and attract charged particles that are mainly negative. Coagulation happens when proteins bind to negatively charged molecules and, while neutralizing the charge, they cause the accumulation of particles. When a polymer is used as a coagulant, the long chain and many side branches, as well as sometimes being charged, cause the creation of bridges between the particles and lead to the creation of larger particles. Also, according to the characteristics of natural coagulant, it can be said that the dominant mechanism in removing turbidity is absorption, neutralization, and bridging between particles, while the chemical coagulant only works by bridging between particles.
Normality of data and checking the data variance homogeneity in ferric chloride coagulant and yellow plantain leaves
Table 3 presents the results of checking the normality of the concentration of physical and chemical parameters in the wastewater (Kolmogorov–Smirnov and Shapiro–Wilk tests) and checking the homogeneity of variance of the data (Lon test) in the ferric chloride coagulant and the yellow Plantain coagulant. Based on these results, at p > 0.05, the data have a normal distribution, and data variance is homogeneous with a probability of 95%. Therefore, the parametric analysis of variance test was used to compare the average data.
Statistical grouping of turbidity in different concentrations in ferric chloride coagulant
The results of the statistical grouping of the turbidity parameter at different concentrations in ferric chloride coagulant are presented in Fig. 8. The statistical grouping of the turbidity parameter classifies that concentrations of 0.500 and 3.00 mg L−1, concentrations of 1.00, 1.50, and 2.00 mg L−1, concentration of 2.50 mg L−1, concentration of 3.50 mg L−1, concentration of 4.00 mg L−1, concentration of 4.50 mg L−1, and concentration of 5.00 mg L−1 in the corresponding statistical groups. Also, in terms of turbidity removal content, there was a statistically significant difference between the concentrations of 2.50, 3.50, 4.00, 4.50, and 5.00 mg L−1 (p < 0.050). This result contradicts the fact that there was no statistically significant difference between other concentrations in this respect (p > 0.050).
Statistical grouping of investigated parameters in different concentrations in yellow leaf coagulant
Figure 9 presents the results of the statistical grouping of the turbidity parameter at different concentrations in yellow leaf coagulant. The statistical grouping of the turbidity parameter classifies the concentration of 10.0 mg L−1, the concentration of 20.0 mg L−1, the concentration of 30.0 mg L−1, the concentrations of 40.0 and 130 mg L−1, concentrations of 50.0 and 120 mg L−1, concentrations of 60.0, 70.0, and 80.0 mg L−1, and concentrations of 90.0, 100, and 110 mg L−1 in the corresponding statistical groups. Also, in terms of turbidity removal percentage, there was a statistically significant difference between concentrations of 10.0, 20.0, and 30.0 mg L−1 (p < 0.050). This result contradicts the fact that there was no statistically significant difference between other concentrations in this regard (p > 0.050).
Correlation between investigated parameters in ferric chloride coagulant and plantain yellow leaf coagulant
Table 4 presents the results of the correlation between the investigated parameters in the ferric chloride coagulant and the yellow leaves of the Plantain tree. Based on Table 6, in the ferric chloride coagulant, there is a direct correlation between chlorferric concentration and turbidity with a correlation coefficient (r) of 0.841 and a significance level of 0.010 (p < 0.010). Also, there is a negative (inverse) correlation between the yellow leaf concentration and turbidity with r = 0.678 at the significance level of 0.050 (p < 0.050) in the coagulant of yellow leaves of Plantain tree. The surface charge of the natural coagulant was calculated by DRIFT method [29]. The zero load of this material was found to be 7.2. At pHs lower than this value, the surface charge of the substance is positive, and at pHs higher than this value, the charge of the substance is negative [30]. Considering that the pH of water without coagulant is equal to 8 and negative. At pH between 7 and 8, the anti-coagulant charge is positive and the pollutant charge of water is negative, and this coagulant causes the formation of clots in water at this pH.
Plantain leaves contain a significant amount of water-soluble proteins with low molecular weight that have a positive charge of one electron. When added to water, proteins increase the positive charge and attract charged particles that are mainly negative. Coagulation happens when proteins bind to negatively charged molecules and, while neutralizing the charge, they cause the accumulation of particles.
The effect of ferric chloride chemical coagulant and natural yellow plantain coagulant on reducing other parameters of the used wastewater
In this study, in addition to investigating the effect of concentration and type of coagulants on wastewater turbidity, parameters such as pH, total dissolved solids (TDS), total suspended solids (TSS), settleable solids (SS), electrical conductivity (EC), calcium hardness (Ca), magnesium hardness (Mg) and total hardness (TH) were investigated. Figure 10 compares some wastewater parameters before and after using chemical and natural coagulants. Figure 10 shows the comparison of the effect of coagulant dose on various parameters. For example, with the increase of the coagulant dose, the total hardness has increased after adding the coagulant, which has increased with the increase of the coagulant cycle. But the dose of coagulant does not affect the pH of the solution and the coagulation and flocculation process [31].
Table 5 lists the values of wastewater parameters before and after using coagulants and the World Health Organization standard for reusing wastewater. The examined coagulants have caused a significant decrease in pH value, total dissolved solids, total suspended solids, settleable solids, electrical conductivity, calcium hardness, magnesium hardness, and total hardness. Notably, this comparison was obtained in the best case, and the highest removal efficiency by the two investigated coagulants. The results listed in Table 5 indicate that the removal of the mentioned parameters by the two investigated coagulants is lower than the World Health Organization (WHO) standards for use in agriculture and discharge to surface waters. Therefore, there is no problem with using these coagulants.
Comparison of the average effluent turbidity parameter with the standard threshold of the WHO
Based on Table 6, the significance level (sig) for the average concentration of turbidity is less than 0.050, indicating a significant difference with the WHO guideline for use in agriculture and discharge to surface water. As can be seen, the average concentration of turbidity in wastewater samples is higher than the WHO guidelines.
Comparison of the average effluent turbidity parameter with the standard limit
Table 7 indicates that the average turbidity parameter in FeCl3 at concentrations of 0.50, 1.00, 1.50, 2.00, 2.50, and 3.00 mg L−1 is lower than the permitted limit for use in agriculture and discharge to surface water and has a statistically significant difference with the permitted limit (p < 0.050). Meanwhile, the average turbidity in FeCl3 coagulant at concentrations of 3.50, 4.00, 4.50, and 5.00 mg L−1 is higher than the permissible limit for use in agriculture and discharge to surface water and has a statistically significant difference with the permissible limit (p < 0.050).
Based on Table 8, the average turbidity parameter in yellow leaf coagulant at a concentration of 10.0 mg/liter is higher than the permissible limit for use in agriculture and discharge to surface water. Also, it has a statistically significant difference with the permissible limit (p < 0.050). In addition, the average turbidity parameter in yellow leaf coagulant at concentrations of 30.0, 40.0, 50.0, 60.0, 70.0, 80.0, 90.0, 100, 110, 120, and 130 mg L−1 is lower than the permissible limit for use in agriculture and discharge to surface water, while having a statistically significant difference with the permissible limit (p < 0.050). Meanwhile, the average turbidity parameter in yellow leaf coagulant at a concentration of 20.0 mg L−1 is equal to the permissible limit for use in agriculture and discharge to surface water while. Moreover, it does not show a statistically significant difference with the permissible limit (p > 0.050).
Conclusion
In conclusion, using natural coagulants such as Plantain leaf can be an effective and eco-friendly alternative to chemical coagulants for removing turbidity from industrial wastewater. Also, using natural coagulants can help reduce the environmental impact of industrial wastewater treatment and provide a cost-effective solution to the effluent turbidity problem. However, further research is needed to investigate the long-term effects of natural coagulants on the environment.
Availability of data and materials
The authors declare that they haven't need research data support with this submission. Also, the authors are sure that all data and materials as well as software application or custom code support their published claims and comply with field standards.
References
J.-R. Jeon et al., Chemosphere 77(8), 1090 (2009)
F. Talebzadeh, R. Zandipak, S. Sobhanardakani, Desalin. Water Treat. 57(58), 28363 (2016)
A. Kharazmi, R. Ghorbani-Vaghei, S. Alavinia, ChemistrySelect 5(4), 1424 (2020)
S. Alavinia et al., Mater. Chem. Phys. 293, 126915 (2023)
A.G. Georgiadis, N.D. Charisiou, M.A. Goula, Catalysts 10(5), 521 (2020)
R. Aghyani et al., Environ. Energy Econ. Res. 7(3), 1 (2023)
S. Alavinia, R. Ghorbani-Vaghei, J. Mol. Struct. 1270, 133860 (2022)
A. Gharehkhani, R. Ghorbani-Vaghei, S. Alavinia, RSC Adv. 11(59), 37514 (2021)
S. Katayon et al., Bioresour. Technol. 97(13), 1455–1460 (2006)
F. Hesami, B. Bina, A. Ebrahimi, Int. J. Environ. Health Eng. 3(April), 46 (2014)
M.B. Šćiban, M.T. Klašnja, J.L. Stojimirović, Acta Period. Technol. 36, 81 (2005)
M.E. Oliva et al., Food Funct. 12(24), 12407 (2021)
D.V. Kukić et al., Ecol. Eng. 84, 229 (2015)
J. Obiefuna, Sci. Hortic. 11(1), 31 (1979)
Y. Tamura, S. Nishibe, J. Agric. Food Chem. 50(9), 2514 (2002)
S.Y. Choy et al., J. Environ. Sci. 26(11), 2178 (2014)
W.L. Ang, A.W. Mohammad, J. Clean. Prod. 262, 121267 (2020)
V. Kumar, N. Othman, S. Asharuddin. EDP Sciences (2017)
A. Basu et al., Res. Chem. Intermed. 40, 447 (2014)
X. Cao et al., Res. Chem. Intermed. 44, 5123 (2018)
K. Suhailath, M. Thomas, M. Ramesan, Res. Chem. Intermed. 46, 2579 (2020)
M. Jabri et al., Synthesis and optimization of a new calcium phosphate ceramic using a design of experiments. Res. Chem. Intermed. 39, 659 (2013)
W. Chen, J. Huang, J. Peng, Res. Chem. Intermed. 41, 1257 (2015)
R.A. Reza, M. Ahmaruzzaman, Removal of naproxen from aqueous environment using porous sugarcane bagasse: impact of ionic strength, hardness and surfactant. Res. Chem. Intermed. 42, 1463 (2016)
O. Sojinu et al., The implications of ripening agents on chemical compositions of plantain (Musa paradisiaca). Heliyon. 7(6), 1–5 (2021)
N. Zaki et al., Sep. Sci. Technol. 1 (2023)
K. Fujisaki, J. Civ. Eng. Environ. Sci. 4, 38 (2018)
J. Babamoradi, R. Ghorbani-Vaghei, S. Alavinia, CuI nanoparticles supported on a novel polymer-layered double hydroxide nanocomposite: an efficient heterogeneous nanocatalyst for the synthesis of bis-N-arylsulfonamides. RSC Adv. 11(31), 19147 (2021)
J.S. Noh, J.A. Schwarz, J. Colloid Interface Sci. 130(1), 157 (1989)
M.P. Gatabi, H.M. Moghaddam, M. Ghorbani, J. Mol. Liq. 216, 117 (2016)
B. Bina et al., J. Water Wastewater 61, 24 (2007). ((In Persian))
Acknowledgements
The authors are thankful to the Hamedan Branch, Islamic Azad University for providing instruments to conduct and complete this study.
Funding
Not Applicable.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection and analysis were performed by NG, BL, SS, MC, and NK. The first draft of the manuscript was written by NG and SS, and all authors commented on previous versions of the manuscript. The corresponding author ensuring that all the listed authors have approved the manuscript before submission, including the metadata.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals and human subjects. The authors confirm that all the research meets ethical guidelines and adheres to the legal requirements of the study country.
Consent for publication
The authors declare that this manuscript does not contain any individual person’s data and material in any form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Golzadeh, N., Lorestani, B., Sobhanardakani, S. et al. Comparing the effectiveness of ferric chloride chemical coagulant and natural coagulant of plane tree leaves in turbidity removal from industrial wastewater. Res Chem Intermed 49, 5613–5633 (2023). https://doi.org/10.1007/s11164-023-05151-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-023-05151-y