Abstract
New 5-methylisatin including thiocarbohydrazones (1–5) have been synthesized. The chemical structure of synthesized compounds was elucidated with IR, 1H NMR, 13C NMR spectroscopic methods, and elemental analysis. Moreover, the synthesized compounds have been screened for antimicrobial activity. Their antibacterial activities were tested against Gram-positive (Bacillus subtilis ATCC 6623, Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212), Gram-negative (Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 70060, Pseudomonas aeruginosa ATCC 27853), and fungal (Candida albicans ATCC 10231, Aspergillus niger ATCC 16404) microbial strains using the microdilution method. In the isatin series, particularly the compound 2 showed the best antimicrobial activity against E. faecalis strain with MIC values of 64 μg/mL compared to other compounds. This high activity of compound 2 is due to the presence of two electron-donating methoxy groups in its structure. The remaining substituted compounds have shown good and moderate antimicrobial activity compared to standard drugs. The results may provide insights into the target compounds' structure–activity relationships, which may facilitate the development of pharmacological and biological applications for the target compounds.
Graphical abstract
New isatins bearing thiocarbohydrazone were synthesized. Structures of all compounds were elucidated with spectroscopic approaches. The antimicrobial activities of the tested compounds presented promising antimicrobial activity against the eight tested microorganisms.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Isatin and its derivatives (1H-indole-2,3-dione) are an important class of aromatic heterocyclic compounds. Isatin nucleus can be regarded a significant skeleton for the design of biologically active and drug discovery compounds. Numerous biological and medicinal activities can be performed by modifying the isatin scaffold, such as anti-oxidant [1,2,3], anti-fungal [4,5,6], anti-bacterial [4, 7,8,9], anti-cancer [10, 11], thymidine phosphorylase activity [12], enzyme inhibitory [4, 13,14,15], anti-viral [16, 17], anti-tubercular [18], anti-convulsant [19], and analgesic [20].
Thiocarbohydrazones consist of a significant class of dyes and pigments presenting the anti-microbial [21,22,23], anti-bacterial [24], anti-fungal [25], anti-oxidant [26, 27], anti-leishmanial [28], cytotoxic [29], anti-viral [30], and anti-tumoral activity [31]. Furthermore, substituted isatin-thiocarbohydrazones based on Schiff Base are usually called as β-isatin aldehyde-N,N′-thiocarbohydrazones [32]. Thiocarbohydrazones have also reported as corrosion inhibitors in the literature [33,34,35].
In this work, isatins based thiocarbohydrazone and Schiff bases are important not only in possessing medicinal activities, but also in biological properties. We reported that new isatin derivatives were achieved by reaction of isatin-thiocarbohydrazide with various aromatic aldehydes. The chemical structures of the compounds were clarified by using IR, 1H NMR and 13C NMR spectroscopic approaches, and elemental analysis. Moreover, the antimicrobial activities of the compounds were tested against selected strains using the microdilution method.
Experimental section
Measurement and reagents
All reagents and solvents were bought from Aldrich, Sigma, or Merck Chemical Company and were used without further purification. The solvents were spectroscopic grade. Melting points were recorded using Stuart Melting Point 30 apparatus and are uncorrected. The elemental analysis was measured on Eurovector EA3000-Single. IR spectra were recorded on a Bruker Vertex 80v spectrophotometer. 1H NMR and 13C NMR spectra were determined in dimethyl sulfoxide-d6 (DMSO-d6) a Bruker Avance DPX-400 (400 MHz) spectrophotometer using TMS as the internal standard.
Synthesis of new 5-methylisatin including thiocarbohydrazones
A mixture of 5-methylisatin (8.0 mmol) and thiocarbohydrazide (8.0 mmol) in ethanol (20 mL) and four drops of acetic acid was refluxed for 3 h. The reaction mixture was cooled, and the precipitate formed was filtered and washed with ethanol (96%) to give isatin-β-thiocarbohydrazone. A mixture of isatin-β-thiocarbohydrazone (4.0 mmol), various aromatic aldehydes (4.0 mmol) and two drops of hydrochloric acid in ethanol (20 mL) was refluxed for 5 h. The colour precipitate formed was filtered and washed with ethanol (96%) to give a product. The reaction route is given in Scheme 1. They were obtained according to an earlier procedure [36].
Antimicrobial activity assay
The in vitro antimicrobial activities were observed and the minimal inhibitory concentration (MIC) values were determined [37, 38] by microdilution method using bacterial strains listed in Table 6. The synthesized compounds in this study were dissolved in in dimethyl sulfoxide (DMSO) at the proper concentration. Amoxicillin and Tetracycline were used as the reference standard for antibacterial activity while Ketoconazole was used as the reference standard for antifungal activity.
The cultures were obtained from nutrient broth for all the bacterial strains after 24 h of incubation at 37 °C. Fungal strains were maintained in nutrient broth after incubation for 24 h at 28 °C. Bacterial and fungal microorganisms were suspended in nutrient broth. The turbidity of bacterial and fungi suspensions was set at a concentration of approximately 106 cells/ml. DMSO, pure microorganisms and pure media were used as control wells. 100 μL suspension of each microorganism and 100 μL suspension of compounds were tested in the 96 well microplates. The MIC value of the tested compounds and the standards are presented in Table 6.
Results and discussion
Physical properties
The structure names, melting points, yields and basic analysis data of the synthesized compounds are presented in Tables 1 and 2.
Vibrational frequencies
In the FT-IR spectra of the compounds, the signal of the aldehyde group (–CHO, two bands) of the starting material was not observed at 2830–2680 cm−1. Moreover, the symmetric and asymmetric stretching vibrations of the amino group (–NH2) did not show at 3400–3150 cm−1. Instead, new –C=N stretching vibrations of imine group were observed at 1631–1541 cm−1. These data showed that the intended reaction took place successfully, as expected.
For compounds 1–4, the O–H signals of phenolic region were observed at 3669–3667 cm−1; the –C–O stretching vibrations originating from the methoxy/ethoxy and hydroxy groups were detected in the range 1159–1073 cm−1.
For all compounds (1–5), while the –NH vibrations of isatin moiety were observed at 3524–3310 cm−1, the –NH vibrations of thiocarbohydrazone region were observed in the range 3312–3104 cm−1, the –C=O signals of isatin region were observed at 1699–1688 cm−1, the –C=S signals of thiocarbohydrazone region were observed at 1386–1362 cm−1, the –C–N group vibrations were observed at 1254–1200 cm−1. These frequency values of the compounds are in agreement with those of similar compounds in the literature [1, 39,40,41]. IR vibrations for the compounds are presented in Table 3. IR spectra of all compounds are given in supplementary material (Fig. S1–S4 in Supplementary information).
For compound 1, the O–H signal of phenolic region was observed at 3668 cm−1; the –NH vibrations of thiocarbohydrazide and isatin moiety were observed at 3272, 3144, and 3310 cm−1 as shown in Fig. 1. The C=O signal of isatin ring was observed at 1699 cm−1; the –C=N stretching vibration was appeared at 1592 and 1541 cm−1; the C=S signal of thiocarbohydrazide region was observed at 1376 cm−1; the –C–N stretching vibration was appeared at 1248 and 1204 cm−1; the –C–O signal of aryl ring were observed at 1146 and 1119 cm−1.
1H NMR interpretations
The 1H NMR spectra of all compounds were detected in DMSO-d6 solvent. DMSO-d6 and water in DMSO (HOD, H2O) signals are shown around at 2.00, 2.50 (quintet) and 3.30 ppm (variable, based on the solvent and its concentration), respectively [42]. The chemical shifts of all compounds are given in Table 4.
For compounds 1–5, the proton signals of imine (–CH=N) were detected as a singlet in the ranges 8.59–8.06 ppm. The amino signals (N2H and –N1H) of thiocarbohydrazone moiety were observed as a singlet in the ranges 14.62–14.50 and 12.56–12.36 ppm, respectively. The –NH signals of the isatin region were observed as a singlet at 11.17–11.11 ppm. The aromatic proton signals of phenolic region (H4–H8) were observed between 7.71 and 6.74 ppm. The aromatic proton (H1–H3) signals of the isatin ring were detected in the ranges 7.41 and 6.83 ppm. The methyl (–CH3) proton signals were resonated as a singlet at 3.35 and 3.31 ppm (Fig. S5–S8 in Supplementary information).
For compounds 1–4, the hydroxyl (–OH) proton signals were detected as a singlet at in the ranges 9.68–9.01 ppm. For compounds 1–3, the methoxy (–OCH3) proton signals were resonated as a singlet in the range of 3.93, 3.88, and 3.84 ppm, respectively. In Fig. 2, the methyl (CH3) and methoxy (OCH3) proton peaks of compound 1 were observed as a singlet at 3.55 and 3.93 ppm.
For compound 4, the proton signal of the methylene group (–CH2) was observed as a quartet at 4.22–4.17 ppm (q, J = 6.9 Hz, 2H); the –CH3 proton signal was detected as a triplet at 1.44–1.41 ppm (t, J = 6.9 Hz, 3H). For compound 5, the proton signal of the amino group (N(CH3)2) was detected as a singlet at 3.03 ppm. These data consistent with the values of earlier reported for similar compounds [1, 16, 43, 44].
13C NMR interpretations
The 13C NMR spectra of all compounds were obtained in DMSO-d6 a solvent. The carbon chemical shift of all compounds are presented in Table 5. For compounds 1–5, the characteristic –C=S peaks of the thiocarbohydrazone moiety were detected at 175.5–174.8 ppm. The characteristic –C=O and –C=N of isatin region peaks were observed at 163.3–163.1 and 152.3–148.6 ppm, respectively. The other characteristic –CH=N (imin) peaks were detected in the ranges 145.8–140.3 ppm. The carbon signals of the –CH3 group were observed at 21.0 and 20.9 ppm (Fig. S9–S12 in supplementary information).
The aromatic carbon signals (C1–C6) of isatin region were observed at between 138.1 and 111.3 ppm. The aromatic carbon atoms (C7–C12) of phenolic region were detected in the ranges 148.8–105.6 ppm. For compounds 1, 2, and 4, the resonance of the C9 and C10 carbon atoms shifted down-field due to the presence of electron-withdrawing group of –OCH3/–OC2H5 and –OH as shown in Fig. 3. For compound 3, the resonance of the C8 (147.3) and C9 (140.4) carbon atoms shifted down-field due to the presence of electron-withdrawing group of –OCH3 and –OH.
For compounds 1–3, the methoxy (–OCH3) carbon signals were resonated at 56.2, 56.6, and 56.4 ppm, respectively. For compound 4, the carbon signal of the methylene (–CH2) and methyl (–CH3) groups were observed at 64.4 and 15.2 ppm, respectively. For compound 5, the carbon signal of the (N(CH3)2) was detected as a singlet at 41.3 ppm. These spectroscopic data are consistent with the values of previously reported similar compounds and the literature [2, 26, 36, 45].
Evaluation of antimicrobial activities
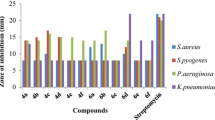
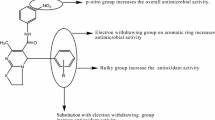
The compound 2 of among all compounds showed the highest antimicrobial activity against all strains tested while the compound 5 exhibited the lowest that. The 3,5-diOCH3-4-OH substituted possessed compound 2 had the highest activity against E. faecalis strain (64 µg/mL) of Gram-positive and Gram-negative bacteria species compared with other derivatives. For this compound, introduction of an extra methoxy group to the aromatic ring dramatically increased the antimicrobial activity. The 3-OCH3-4-OH substituted compound 1 and the 2-OH-3-OCH3 substituted compound 3, this two compound bearing the same groups on the ring showed high activity against B. subtilis (128 µg/mL) and the compound 3 was also showed the same value activity against E. faecalis (128 µg/mL) microorganism. The 3-OCH2CH3-4-OH substituted compound 4, which carrying an ethoxy and hydroxy group on the ring, demonstrated the same activity value of compound 3 against E. faecalis (128 µg/mL) microorganism. This result showed that the binding of the ethoxy group in compound 4 at the same position instead of the methoxy group in compound 3 did not change the activity against E. faecalis microorganism. The N,N′-diCH3 substituted compound 5 of the isatins based thiocarbohydrazone series showed a significant reduction in activity compared to the other compounds. Among all microorganisms, this compound showed the highest activity only against K. pneumoniae against with MIC values of 256 μg/mL. All of the compounds exhibited lower activity against fungi than the Ketoconazole standard. Considering all tested compounds, compound 2 had the highest antifungal activity against A. niger (128 µg/mL). The 3,5-diOCH3-4-OH substituted compound 2 showed the best activity against C.albicans with MIC values of 256 μg/mL, while the 3-OCH2CH3-4-OH substituted compound 4, exhibited the same value activity against A. niger (256 µg/mL). These results revealed that the excellent potency of these compounds is probably due to the presence of electron donating groups such as –OH, –OCH3, –OC2H5 on the phenyl rings attached to the azomethine group (–CH=N), eliciting enhanced antimicrobial activity against certain strains [29, 45,46,47,48] (Table 6).
Conclusions
New isatin derivatives based on thiocarbohydrazone have been synthesized and isolated in acceptable yields with 55–78%. IR, 1H NMR and 13C NMR spectroscopic approaches, and elemental analysis were used to elucidate the chemical structures of all compounds. Moreover, the antimicrobial activities of the newly synthesized compounds were tested. Among the tested compounds, the compound 2 exhibited exceptionally good antimicrobial activities compared to other derivatives against E. faecalis with MIC values of 64 μg/mL. The good microbial activity of this compound due to the presence of two methoxy group at C3 and C5 and an additional of a hydroxyl group at C4 in the phenyl ring. Compounds 1 and 3, which have a methoxy group and a hydroxyl group in the ortho position on the phenyl ring, showed the same activity against B. subtilis with MIC values of 128 μg/mL. The compound 2 showed good antimicrobial activity against E. faecalis compared to other strains, with MIC values of 64 μg/mL. Compound 5 of the isatin-based thiocarbohydrazone series showed a significant reduction in activity compared to other compounds. All of the compounds showed lower activity against fungi than the Ketoconazole standard. The obtained results revealed that the presence of electron donating groups such as a hydroxyl group or methoxy group in the structures of the compounds can provide better activity.
References
H. Yakan, T.K. Bakır, M.S. Çavuş, H. Muğlu, Res. Chem. Intermed. 46, 5417 (2020)
H. Yakan, M.S. Çavuş, B.Z. Kurt, H. Muğlu, F. Sönmez, E. Güzel, J. Mol. Struct. 1239, 130495 (2021)
S.H. Sumrra, F. Mushtaq, F. Ahmad, R. Hussain, W. Zafar, M. Imran, M.N. Zafar, Chem. Pap. 76, 3705 (2022)
S. Pandeya, D. Sriram, G. Nath, E. DeClercq, Eur. J. Pharm. Sci. 9, 25 (1999)
A.Y. Alzahrani, Y.A. Ammar, M.A. Salem, M. Abu-Elghait, A. Ragab, Arch. Pharm. 355, 2100266 (2022)
G. Singh, A. Arora, A. Singh, P. Kalra, S. Rani, K. Singh, I.K. Maurya, R.S. Mandal, ChemistrySelect 3, 1942 (2018)
H. Guo, Eur. J. Med. Chem. 164, 678 (2019)
A.Y. Alzahrani, Y.A. Ammar, M. Abu-Elghait, M.A. Salem, M.A. Assiri, T.E. Ali, A. Ragab, Biorgan. Chem. 119, 105571 (2022)
A. Jarrahpour, Z. Jowkar, Z. Haghighijoo, R. Heiran, J.A. Rad, V. Sinou, F. Rouvier, C. Latour, J.M. Brunel, N. Özdemir, Med. Chem. Res. 31, 1026 (2022)
R. Meleddu, V. Petrikaite, S. Distinto, A. Arridu, R. Angius, L. Serusi, L. Škarnulytė, U. Endriulaitytė, M. Paškevičiu̅tė, F. Cottiglia, ACS Med. Chem. Lett. 10, 571 (2018)
Y.A. Ammar, A.M.S. El-Sharief, A. Belal, S.Y. Abbas, Y.A. Mohamed, A.B. Mehany, A. Ragab, Eur. J. Med. Chem. 156, 918 (2018)
H. Ullah, A. Liaqat, Q.U. Khan, M. Taha, F. Khan, F. Rahim, I. Uddin, Z.U. Rehman, Chem. Pap. 76, 213 (2022)
Ö. Güzel-Akdemir, A. Akdemir, N. Karalı, C.T. Supuran, Org. Biomol. Chem. 13, 6493 (2015)
Y. Kaya, A. Erçağ, Y. Zorlu, Y. Demir, İ Gülçin, J. Biol. Inorg. Chem. 27, 271 (2022)
V.S. Pawar, D.K. Lokwani, S.V. Bhandari, K.G. Bothara, T.S. Chitre, T.L. Devale, N.S. Modhave, J.K. Parikh, Med. Chem. Res. 20, 370 (2011)
S.Y. Abbas, A.A. Farag, Y.A. Ammar, A.A. Atrees, A.F. Mohamed, A.A. El-Henawy, Monatsh. Chem. 144, 1725 (2013)
A. Jarrahpour, J. Sheikh, I. El Mounsi, H. Juneja, T.B. Hadda, Med. Chem. Res. 22, 1203 (2013)
T. Aboul-Fadl, F.A. Bin-Jubair, O. Aboul-Wafa, Eur. J. Med. Chem. 45, 4578 (2010)
E.A. Fayed, A. Ragab, R.R.E. Eldin, A.H. Bayoumi, Y.A. Ammar, Biorgan. Chem. 116, 105300 (2021)
R.P. Chinnasamy, R. Sundararajan, S. Govindaraj, J. Adv. Pharm. Technol. Res. 1, 342 (2010)
K. El-Mahdy, A. El-Kazak, M. Abdel-Megid, M. Seada, O. Farouk, Acta Chim. Slov. 63, 18 (2016)
A.R. Božić, S.K. Bjelogrlić, I.T. Novaković, N.R. Filipović, P.M. Petrović, A.D. Marinković, T.R. Todorović, I.N. Cvijetić, ChemistrySelect 3, 2215 (2018)
G. Mrđan, A. Tot, M. Vraneš, M. Rašeta, P. Knežević, T. Verbić, B. Matijević, Bull. Chem. Soc. Jpn. 95, 185 (2022)
Z.H. Chohan, H. Pervez, K.M. Khan, C.T. Supuran, J. Enzym. Inhib. Med. Chem. 20, 81 (2005)
G.B. Bagihalli, P.G. Avaji, P.S. Badami, S.A. Patil, J. Coord. Chem. 61, 2793 (2008)
M.S. Çavuş, H. Yakan, C. Özorak, H. Muğlu, T.K. Bakır, Res. Chem. Intermed. 48, 1593 (2022)
H. Muğlu, B.Z. Kurt, F. Sönmez, E. Güzel, M.S. Çavuş, H. Yakan, J. Phys. Chem. Solids 164, 110618 (2022)
M.T. Muhammad, N. Ghouri, K.M. Khan, M.I. Choudhary, S. Perveen, Med. Chem. 14, 725 (2018)
M.T. Gabr, N.S. El-Gohary, E.R. El-Bendary, N. Ni, M.I. Shaaban, M.M. El-Kerdawy, Synth. Commun. 48, 2899 (2018)
K. Gangarapu, S. Manda, A. Jallapally, S. Thota, S.S. Karki, J. Balzarini, E. De Clercq, H. Tokuda, Med. Chem. Res. 23, 1046 (2014)
M. Sathisha, V. Revankar, K. Pai, Met.-Based Drugs (2008)
G. Kiran, T. Maneshwar, Y. Rajeshwar, M. Sarangapani, J. Chem. 2013 (2012)
A.R. Sayed, H.M.A. El-Lateef, Coatings 10, 1068 (2020)
N. Esmaeili, J. Neshati, I. Yavari, J. Ind. Eng. Chem. 22, 159 (2015)
D.S. Chauhan, K. Ansari, A. Sorour, M. Quraishi, H. Lgaz, R. Salghi, Int. J. Biol. Macromol. 107, 1747 (2018)
H. Muğlu, H. Yakan, A.G.A. Misbah, M.S. Çavuş, T.K. Bakır, Res. Chem. Intermed. 47, 4985 (2021)
R. Schwalbe, L. Steele-Moore, A.C. Goodwin, Antimicrobial susceptibility testing protocols (Crc Press, Florida, 2007)
K. Canli, M.E. Bozyel, E.M. Altuner, Int. J. Pharm. Sci. Invent. 6, 19 (2017)
O. Bekircan, H. Bektas, Molecules 13, 2126 (2008)
H. Muğlu, H. Yakan, T.K. Bakir, Turk. J. Chem. 44, 237 (2020)
H. Muğlu, H. Yakan, J. Inst. Sci. Technol. 10, 439 (2020)
D. Williams, I. Fleming, (McGraw-Hill, New York, 1996)
A. Jarrahpour, D. Khalili, E. De Clercq, C. Salmi, J. Brunel, Molecules 12, 1720 (2007)
H. Muğlu, M.S. Çavuş, T. Bakır, H. Yakan, J. Mol. Struct. 1196, 819 (2019)
G. Kiran, M. Sarangapani, T. Gouthami, A.R. Narsimha Reddy, Toxicol. Environ. Chem. 95, 367 (2013)
S. Cakmak, J. Mol. Struct. 132932 (2022)
P.M. Chauhan, S.N. Thummar, K.H. Chikhalia, J. Iran. Chem. Soc. 15, 1261 (2018)
M. Hassan, R. Ghaffari, S. Sardari, Y.F. Farahani, S. Mohebbi, Res. Pharm. Sci. 15, 281 (2020)
Acknowledgements
This study was not supported by any organization. We are also grateful to Dr. İkbal Agah İnce at Acıbadem Mehmet Ali Aydınlar University for checking and proofreading the article.
Author information
Authors and Affiliations
Contributions
HY contributed to spectroscopic characterization, writing—review, visualization and editing, supervision. ŞÇ contributed to spectroscopic characterization, biological studies, writing—review. OB contributed to synthesis, spectroscopic characterization. AV contributed to biological studies, writing—review. HM contributed to synthesis methodology, writing—review. NTK contributed to synthesis methodology, review, editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yakan, H., Çakmak, Ş., Buruk, O. et al. New 5-methylisatin including thiocarbohydrazones: preparation, structure elucidation and antimicrobial activity. Res Chem Intermed 48, 4331–4345 (2022). https://doi.org/10.1007/s11164-022-04799-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04799-2