Abstract
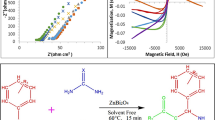
MgFe2O4 and MgFe2ZnxO4+δ composite samples were fabricated using MgCl2.6H2O and FeCl3.6H2O (and Zn(NO3)2.6H2O for the doped sample) in one-step, solid-state reactions at 800 °C for 12 h. The obtained materials were characterized by the powder X-ray diffraction technique. Rietveld analysis data showed that the obtained MgFe2O4 was crystallized in the cubic crystal system with the space group Fd-3m. The morphology of the synthesized materials was studied by a field emission scanning electron microscope. The aim of the present work was the preparation of 5-aryl-1H-tetrazoles from benzonitrile raw material derivatives and sodium azide in the presence of MgFe2O4 and MgFe2ZnxO4+δ heterogeneous and recyclable catalysts under conventional heating conditions. The main superiorities of the method were high output and simplicity. Time, temperature, solvent type, and quantity of the catalyst were considered crucial factors for optimization of the reaction conditions. According to the results, the best conditions for the catalytic reaction were 3 h, 80 °C, 5 mol% catalyst, and dimethyl sulfoxide solvent. At the optimum conditions, a high catalytic yield (97%) was obtained.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heterogeneous catalysts with a nano-scale structure exhibit high efficiency and selectivity in organic transformations [1]. They can be isolated and separated readily from the desired organic compounds. Besides, they are recyclable and reusable while maintaining their catalytic properties [2, 3]. Recently, magnetic nanomaterials have attracted plenty of interest due to their extensive potential applications like catalytic reactions, magnetic resonance imaging, biomedicine, data storage, and batteries [4]. Recently, Fe-based oxides such as MFe2O4 (M = Co, Ni, and Mn) have drawn more attention due to their green chemistry, optical properties, photocatalytic features, and wide ranges of chemical composition [5, 6]. They are magnetically soft and possess n-type semiconductor features. Their properties are favorable to use in heterogeneous catalysts, sensors, and other high-tech magnetic applications [7]. Magnesium ferrite (MgFe2O4) has been used as a catalyst for various applications such as carbon production, water purification, alkylation, dehydrogenation, dechlorination, oxidation [8,9,10]. The physical properties are affected by different factors such as structure, stoichiometry, particle porosity, grain size, and ion distribution in the system [11]. In the last years, the metallic ions such as Al, Cd, Cr, Cu, Ge, Mo, Ni, Ce–Gd, Sm–Gd, Sn, and Y have been doped into MgFe2O4 crystal system [12, 13] by a variety of different techniques such as solid-state [5], combustion [14], melt quenching [15], sonochemical [16], hydrothermal [17], microemulsion, citrate-gel [18], and microwave [19]. Tetrazoles are nitrogen-rich chemical agents that have demonstrated various applications and gained great importance in organic chemistry. For instance, a type of tetrazole compound derived from proline is a ligand that can be used in asymmetric and multicomponent reactions [20]. Hantzsch and Vagt [21] were the first researchers to disclose a common method for the synthesis of 5-aryl-1H-tetrazoles by cycloaddition of azide to match the nitrile (3 + 2) derivative. However, this type of reaction was cost-intensive and dangerous as it contains toxic, explosive, volatile, and water-sensitive compounds [22,23,24]. However, Demko and Sharpless [25] invented a safer aquatic approach using Zn salts as catalysts. Several compounds, including inorganic salts and metal complexes [26], TMSN3, TBAF [27], BF3.OEt2 [23], Pd(PPh3)4 [28], AlCl3 [29], Yb(OTf)3 [30], Zn(OTf)3 [31], Pd(OAc)2/ZnBr2 [32], and others, have been used as catalyst for the cycloaddition processes.
Homogeneous catalysts suffer from major challenges due to their separation and recycling difficulties. So, heterogeneous catalysts compensate for the above drawbacks and are considered a good alternative to them. Nanocrystalline Zn/Al HT, ZnO [33], Zn hydroxyapatite [34], Cu2O [35, 36], CdCl2 [37], FeCl3-SiO2 [38], natural natrolite zeolite [39], ZnS [40], and γ-Fe2O3 [41] are examples of heterogeneous catalysts. In the present study, following our efforts and interest in catalytic reactions [42,43,44,45,46,47] , MgFe2O4 and MgFe2ZnxO4+δ nanoparticles are used as recyclable catalysts in the cycloaddition reaction between aryl nitrile and sodium azide to synthesize 5-aryl-1H-tetrazole compounds. In comparison with monometallic nanoparticle catalysts, the mixed metal oxide nanocatalysts have superior activity due to the cooperation nature of each of the metal oxides.
Experimental
Materials and procedures
Chemical compounds were bought from Merck and Aldrich suppliers and used at their standard purity. Their melting point was determined by the Philip Harris C4954718 apparatus. The infrared spectra were taken by a Thermo-Nicolet Nexus 670 FT-IR instrument over KBr discs. A Bruker Avance AQS 300 MHz spectrometer recorded 1H NMR spectra. Chemical shifts were demonstrated by DMSO-d6 in comparison with tetramethylsilane as the internal standard. The elemental Vario EL III elemental analyzer took the CHN analyses. The Brunauer-Emmett-Teller (BET) and the Barrett-Joyner-Halenda (BJH) methods were conducted to calculate surface area, pore-volume, and average particle size. The Beckman Coulter SA3100 surface area analyzer was used to save the data.
Synthesis of MgFe2O4 (S 1) and MgFe2ZnxO4+δ (S 2)
The compounds were synthesized by the solid-state method described in the literature [48]. For the purpose, MgCl2.6H2O (1 mmol, 203 mg) and FeCl3.6H2O (2 mmol, 540 mg) were mixed in a mortar and ground until a homogenous powder was formed. The powder was placed into a crucible and treated thermally at 800 °C for 12 h in order to complete the synthesis process in a pre-heated electrical furnace. The crucible was cooled down to room temperature in the furnace normally. For the MgFe2ZnxO4+δ sample, MgCl2.6H2O (1 mmol), FeCl3.6H2O (2 mmol), and Zn(NO3)2.6H2O (0.034 mmol) were poured into a mortar and ground to obtain a homogeneous powder. The crucible was heated at 800 °C for 12 h in the preheated furnace. After the reaction was completed, the product mixture was allowed to cool down to room temperature in the furnace normally.
General process for the transformation of aromatic nitrile (ArCN) into compounds 4a-g
In order to perform a reflux process, all of the raw compounds included in Table 1 were mixed in a condenser and stirred for a certain time. Then, the prepared nanocatalyst (5 mol%) and DMSO (5 mL) were added to the mixture at 80 °C under an air atmosphere.
Reaction completion was monitored by a thin layer chromatography (TLC) test using an ethyl acetate/n-hexane (2:3 volume ratio) solvent mixture. The separation of the catalyst from the reaction mixture solution was done magnetically, and the catalyst recovery was done after the reaction’s completion. Then, the solvent was removed from the product mixture by an evaporation process. The crude solid crystalline 5-aryl-1H-tetrazole was obtained by adding HCl (3 N, 10 mL) to the residue while vigorously stirring. Silica gel column chromatography was used to purify the product.
Results and discussion
Characterization
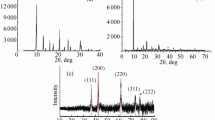
The as-prepared MgFe2O4 and MgFe2ZnxO4+δ nanocomposites were characterized by the PXRD technique. Figure 1a, b shows the PXRD patterns of the obtained materials in the 2θ range of 10–80°. The results of structural analysis performed by the FullProf program employing profile matching with a constant scale factor are also included in the figures. The red lines are the observed intensities, while the black ones are the calculated data. The blue line is the difference: Yobs − Ycalc. The Bragg reflection positions are indicated by blue, red, and green bars, which correspond to the main crystal phase (MgFe2O4) with space group Fd-3m and lattice parameters of about a = b = c = 8.38 Å [49,50,51,52], MgFeO3 as a second crystal phase with space group C2/c and lattice parameters a = 9.72748, b = 8.62991 and c = 5.37184 Å, and α = γ = 90.0°, and β = 90.03° [53], and Fe2O3 as a third and minimum crystal phase with space group R-3c and lattice parameters a = b = 5.02785 and c = 13.73903 Å and α = β = 90.0° and γ = 120.0° [54], respectively. The crystallite sizes of S1 and S2 were calculated by the Scherrer equation using the peaks at 35.37 and 35.23°. The calculated grain sizes were 67 and 59 nm, respectively, for S1 and S2.
FESEM images of the fabricated samples are shown in Fig. 2. The images show that the morphology of the obtained samples is a prism with a homogeneous shape. However, it is found that S2 has a more ordered shape than S1. The particle sizes on the prisms are about 80–100 nm.
FT-IR spectra
The FTIR spectra of the prepared magnesium ferrite nanoparticles are illustrated in Fig. 3. The OH in the bending and stretching modes of water is revealed by the expanded bands at 1630 and 3426 cm−1, respectively. The band at 558 cm−1 displays intrinsic metal atom stretching vibrations at the tetrahedral site, Mtetra ↔ O (ν1), whereas the band at 449 cm−1 (ν2) band discloses metal-atom streching type vibration which is an octahedral bond, Mocta ↔ O. The longer and shorter bond lengths, related to oxygen–metal bands for octahedral Fe–O and tetrahedral Mg–O sites, can be associated with the discrepancy between two vibrations (ν1 and ν2) [55].
BET and BJH texture analysis
The produced powders surface area, average pore volume, and average pore size were investigated. The samples were degassed for 120 min at 150 °C in N2 environment before measurement. Then, using N2 adsorption–desorption isotherms at 77 K, the specific surface area (SBET) of the collected samples was calculated. Table 2 presents the findings of the measurements. The surface area and the pore volumes of the targets are 5.90 to 4.23 m2 g−1 and 0.07 to 0.06 cm3 g−1, respectively (Table 3). Besides, the pore diameter sizes of the obtained materials are 45 and 58 nm for S1 to S2, respectively. BTH data are demonstrative of pore diameter value and in Table 3.
Magnetic property
Figure 4, shows the vibrating-sample magnetometer (VSM) data for the samples prepared by the solid-state method at room temperature. According to the figure, the values of saturation magnetization (Ms) change in the range of 24.4 (S1) to 28.1 (S2) emu/g. The data are reported associated with standard deviation (SD) values. The saturation values for the samples were higher than the values of 21.9 emu/g for sol–gel/combustion-synthesized MgFe2O4 [56] and 12.9 emu/g for co-precipitation-synthesized MgFe2O4 [5]. The outcomes revealed that the magnetic properties of the magnesium ferrite compounds have a direct correlation with the preparation technique. The magnetization strength of materials is defined by remnant magnetization Mr. This equals the magnetization that is retained when the external magnetic field reaches zero level (H = 0). The magnitudes of squareness are the ratio of the remnant per saturation magnetization (Mrs = Mr/Ms). Magnetic parameters are defined in Table 4 in which remanent magnetization, saturation magnetization, and coercive field strength corresponding to Mr, Ms, and Hc, respectively. The squareness ratio will be less than 0.5 when a particle has an isotropic distribution and uniform magnetization without intergrain interactions. The formation of a multi-domain structure over the exchange coupling between adjacent grains explains this value. Because the value of Mrs is 0.11, it can be seen that the samples have no preferred magnetization direction and show a typical (S-shaped) narrow hysteresis loop. A low coercivity rating is shown by the narrow loop. As a result, the demagnetization of the prepared samples is simple.
During the preparation of 5-aryl-1H-tetrazoles using benzonitrile derivatives, the performance of the synthesized nanoparticles was analyzed for their catalytic activity (Scheme 1). The optimization was carried out to improve reaction efficacy. ArCN reaction using NaN3 and different solvents was carried out by helping the prepared catalyst (5 mol%, Table 5). Organic compounds such as Tetrahydrofuran (THF), Dimethyl sulfoxide (DMSO), Dimethylformamide (DMF), dioxane, toluene, and water were also used as solvent in the catalytic reactions. According to Table 5, DMSO is the most suitable solvent to obtain the maximum yield (Table 5, entry 9).
Table 5, entries 6–10, clarifies the temperature role in the reaction yield. To do the reactions, the catalytic process was started at room temperature and then, the reactions were performed at 40, 60, 80, and 100 °C, distinctly, to find the optimum temperature condition. The data revealed that the reaction yield maximized at 80 °C and remained nearly constant at higher temperatures. Finding the best catalyst quantity for the reaction is the subsequent stage. The procedure was carried out under the following optimum temperature at the absence and presence of nanocatalyst (2, 5 and 10 mol%): The best results are shown at 5 mol% (Table 5, entries 9–13).
The concept of the catalytic mode on the respective tetrazoles ring preparation from benzonitrile derivatives was tested using optimum reaction conditions. The activity of nitrile compounds toward azide ions in cycloaddition reaction is crucial. Empirically, we found that aryl nitriles with electron-withdrawing groups raise the polarity of the cyanide group inductively leads to higher yields within a shorter time of reaction than other nitrile compounds (Table 6, entry 6). According to the data included in Table 6, when a reactant that possesses an electron-donating agent (methyl/methoxy/hydroxy) is utilized in the reaction mixture, the tetrazole derivative compound is produced in a high yield (entries 4, 5 and 7) at a longer reaction time duration. Benzonitriles with halogen substituents (4-Cl and 4-Br) interacted smoothly and produced the desired results (Table 6, entries 2 and 3). The data included in Table reveal that the efficiency of S2 is more than S1 at the optimum reaction conditions.
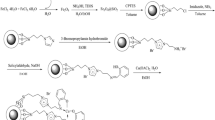
As shown in Scheme 2, an appropriate route for the synthesis of 5-aryl-1H-tetrazoles using the prepared nanocatalysts is proposed. Intermediate I is formed by the coordination of nitrogen atom in nitrile group of Ar-CN 1a–g with Mg(II), which speeds up the cyclization process. This claim was supported by the observation when the reaction was performed without the presence of MgFe2O4. Even after a long period of time, the cycloaddition reaction without a catalyst did not complete (Table 5, entry 11). The intermediate II was generated via a concerted [3 + 2] cycloaddition reaction of the nitrile's group with azide ion. The desired tetrazoles 4a–g as final product and MgFe2O4 catalyst were obtained by protonolysis of intermediate II with NH4OAc H+ or extraction using hydrochloric acid. Ammonium acetate can form ammonium azide in situ by reacting it with sodium azide, which increases the accessibility of the N3− for cycloaddition with C≡N, and it can also be utilized as a proton source into the corresponding tetrazoles [59,60,61].
During the tetrazole ring formation under the optimum conditions, the catalyst (MgFe2O4) recovery and reusability were studied. When the benzonitrile-sodium azide reaction was completed, the catalyst was isolated from the reaction mixture by applying an external magnet, washed with acetone and then water, and kept in an oven at 120 °C for 4 h. The reusability of the catalyst was tested by repeating the reaction until the yield decreased considerably. It is worth noting that the recovered catalyst maintains its peak up to a 4 s run (Fig. 5).
In Fig. 6, the PXRD pattern of S2 after 4 runs of catalytic reaction is presented. It is found that the catalyst suffers from crystal phase decreases. Besides, it is clear that the impurity phases, including MgFeO3 and Fe2O3, increase.
The efficiency of the catalyst was assessed by comparing it to that of other heterogeneous catalytic systems which demonstrates its priority over other systems in the literature (Table 7).
Conclusion
In this study, magnesium ferrite and Zn-doped samples were synthesized by the solid-state method and used as effective heterogeneous catalysts for [3 + 2] cycloaddition reactions of benzonitriles with azide as the starting material to give 5-aryl-1H-tetrazoles with high yields. FESEM images showed that the morphology of the prepared samples was prism. The VSM data revealed that the samples had ferromagnetic properties with a considerable saturation magnetization of 28 emu/g. The data showed that the optimum reaction temperature was 80 °C when DMSO was used as the reaction mixture solvent. A high reaction yield (97%) was obtained when 5 mol% of the prepared nanocatalyst was used. Separation and recovery of the catalyst from the reaction solution were done easily by an external magnet. The reaction was feasible for four successive runs without a noticeable fall in catalytic activity.
References
Z. Zhuang, D. Liu, Nano-Micro Lett. 12, 1 (2020)
S. Kumar, S. Jain, M. Nehra, N. Dilbaghi, G. Marrazza, K.H. Kim, Coord. Chem. Rev. 420, 213407 (2020)
M.A. Ashraf, Z. Liu, C. Li, D. Zhang, Appl. Organomet. Chem. 35, e6133 (2021)
K. Kirchberg, A. Becker, A. Bloesser, T. Weller, J. Timm, C. Suchomski, R. Marschall, J. Phys. Chem. C. 121, 27126 (2017)
Z. Wang, P. Lazor, S.K. Saxena, H.S.C. O’Neill, Mater Res. Bull. 37, 1589 (2002)
Z.J. Zhang, Z.L. Wang, B.C. Chakoumakos, J.S. Yin, J. Am. Chem. Soc. 120, 1800 (1998)
S.A. Oliver, R.J. Willey, H.H. Hamdeh, G. Oliveri, G. Busca, Scr. Mater. 33, 1695 (1995)
D.H.K. Reddy, Y.S. Yun, Coord. Chem. Rev. 315, 90 (2016)
N. Kaur, M.M. Kaur, Process Appl. Ceram. 8, 137 (2014)
S.M. Yakout, M.R. Hassan, M.I. Aly, Water Sci. Technol. 77, 2714 (2018)
A. Sankaramahalingam, J.B. Lawrence, Synth. React. Inorg. Met. 42, 121 (2012)
N.M. Deraz, O.H. Abd-Elkader, Int. J. Electrochem. Sci. 8, 8632 (2013)
S.M. Hoque, M.A. Hakim, A. Mamun, S. Akhter, M.T. Hasan, D.P. Paul, K. Chattopadhayay, Mater Sci. Appl. 2, 1564 (2011)
Y.S.Z. Rúbia, G.K. Claudir Jr., K.A. Annelise, P.B. Carlos, FME Trans. 46, 157 (2018)
S.E. Shabrawy, C. Bocker, C. Rüssel, Solid State Sci. 60, 85 (2016)
M.I.M. Omer, A.A. Elbadawi, O.A. Yassin, J. Appl. Ind. Sci. 1, 20 (2013)
S. Ilhan, S.G. Izotova, A.A. Komlev, Ceram Int. 41, 577 (2014)
M.E. Rabanal, A. Varez, B. Levenfeld, J.M. Torralba, Bol. Soc. Esp. Ceram Vidrio. 39, 277 (2000)
T. Iwamoto, Y. Komorida, M. Mito, A. Takahara, J. Coll. Interface. Sci. 345, 143 (2010)
R.N. Butler, A.R. Katritzky, C.W. Rees, E.F.V. Scriven (eds.), Comprehensive Heterocyclic Chemistry (Pergamon, Oxford, UK, 1996)
A. Hantzsch, A. Vagt, Justus Liebigs Ann. Chem. 314, 339 (1901)
D.P. Curran, S. Hadida, S.-Y. Kim, Tetrahedron. 55, 8997 (1999)
A. Kumar, R. Narayanan, H. Shechter, J. Org. Chem. 61, 4462 (1996)
K. Koguro, T. Oga, S. Mitsui, R. Orita, Synthesis. 6, 910 (1998)
Z.P. Demko, K.B. Sharpless, J. Org. Chem. 66, 7945 (2001)
J. Bonnamour, C. Bolm, Chem.Eur. J. 15, 4543 (2009)
D. Amantini, R. Beleggia, F. Fringuelli, F. Pizzo, L. Vaccoro, J. Org. Chem. 69, 2896 (2004)
Y.S. Gyoung, J.-G. Shim, Y. Yamamoto, Tetrahedron Lett. 41, 4193 (2000)
D.P. Matthews, J.E. Green, A.J. Shuker, J. Comb. Chem. 2, 19 (2000)
W.K. Su, Z. Hong, W.G. Shan, X.X. Zhang, Eur. J. Org. Chem. 2006, 2723 (2006)
S. Hajra, D. Sinha, M. Bhowmick, J. Org. Chem. 72, 1852 (2007)
Z. Yizhong, R. Yiming, C. Chun, Helv. Chim. Acta. 92, 171 (2009)
M.L. Kantam, K.B.S. Kumar, K.P. Raja, J. Mol. Catal. A. 247, 186 (2006)
M.L. Kantam, V. Balasubrahmanyam, K.B.S. Kumar, Synth. Commun. 36, 1809 (2006)
T. Jin, F. Kitahara, S. Kamijo, Y. Yamamoto, Tetrahedron Lett. 49, 2824 (2008)
T. Jin, F. Kitahara, S. Kamijo, Y. Yamamoto, Chem. Asian J. 3, 1575 (2008)
G. Venkateshwarlu, A. Premalatha, K.C. Rajanna, P.K. Saiprakash, Synth. Commun. 39, 4479 (2009)
M. Nasrollahzadeh, Y. Bayat, D. Habibi, S. Moshaee, Tetrahedron Lett. 50, 4435 (2009)
M. Nasrollahzadeh, D. Habibi, Z. Shahkarami, Y. Bayat, Tetrahedron. 65, 10715 (2009)
L. Lang, B. Li, W. Liu, L. Jiang, Z. Xu, G. Yin, Chem. Commun. 46, 448 (2010)
G. Qi, Y. Dai, Chin. Chem. Lett. 21, 1029 (2010)
M. Aslanpanjeh, A.P. Marjani, J. Khalafy, N. Etivand, Res. Chem. Intermed. 46, 165 (2020)
A. Nouri, A.P. Marjani, J. Khalafy, N. Etivand, Res. Chem. Intermed. 46, 3025 (2020)
L. Kafi-Ahmadi, A.P. Marjani, E. Nozad, Appl. Organomet. Chem. 35, e6271 (2021)
F. Majidi Arlan, A.P. Marjani, R. Javahershenas, J. Khalafy, New J. Chem. 45, 12328 (2021)
F. Azimi, A.P. Marjani, S. Keshipour, Sci. Rep. 11, 23769 (2021)
B.P. Habashi, A.P. Marjani, Res. Chem. Intermed. 48, 2325 (2022)
F. Yousefzadeh, L. Kafi-Ahmadi, Sh. Khademinia, Catal. Lett. 149, 1660 (2019)
H. Das, T. Arai, N. Debnath, N. Sakamoto, K. Shinozaki, H. Suzuki, N. Wakiya, Adv. Powder Technol. 27, 541 (2016)
J. Nonkumwong, P. Pakawanit, A. Wipatanawin, P. Jantaratana, S. Ananta, L. Srisombat, Mater. Sci. Eng. C. 61, 123 (2016)
C. Fragassa, L. Berardi, G. Balsamini, FME Trans. 44, 333 (2016)
A. Ivanets, M. Roshchina, V. Srivastava, V. Prozorovich, T. Dontsova, S. Nahirniak, V. Pankov, A. Hosseini-Bandegharaei, H.N. Tran, M. Sillanpää, Colloids Surf. A Physicochem. Eng. Asp. 571, 17 (2019)
N.T. Hung, L.H. Bac, N.N. Trung, N.T. Hoang, P.V. Vinh, D.D. Dung, J. Magn. Magn. Mater. 451, 183 (2018)
S. Zhang, X.J. Chen, C.R. Gu, Y. Zhang, J.D. Xu, Z.P. Bian, D. Yang, N. Gu, Nanoscale Res. Lett. 4, 70 (2009)
H. Kavas, N. Kasapoğlu, A. Baykal, Y. Köseoğlu, Chem. Pap. 63, 450 (2009)
A. Pradeep, P. Priyadharsini, G. Chandrasekaran, J. Magn. Magn. Mater. 320, 2774 (2008)
V. Rama, K. Kanagaraj, K. Pitchumani, J. Org. Chem. 76, 9090 (2011)
L.V. Myznikov, A. Hrabalek, G.I. Koldobskii, Chem. Heterocycl. Chem. 43, 1 (2007)
M. Nasrollahzadeh, B. Jaleh, A. Jabbari, RSC Adv. 4, 36713 (2014)
D.R. Patil, M.B. Deshmukh, D.S. Dalal, J. Iran. Chem. Soc. 9, 799 (2012)
H. Sharghi, S. Ebrahimpour Moghaddam, M.M. Doroodmand, J. Organomet. Chem. 738, 41 (2013)
B. Sreedhar, A.S. Kumar, D. Yada, Tetrahedron Lett. 52, 3365 (2011)
M. Lakshmi Kantam, K.B. Shiva Kumar, C. Sridhar, Adv. Synth. Catal. 347, 1212 (2005)
B. Sreedhar, A.S. Kumar, D. Yada, Tetrahedron Lett. 52, 3565 (2011)
F. Abrishami, M. Ebrahimikia, F. Rafiee, Appl. Organometal. Chem. 29, 730 (2015)
S.M. Joshi, R.B. Mane, K.R. Pulagam, V. Gomez-Vallejo, J. Llop, C. Rode, New J. Chem. 41, 8084 (2017)
L. Zamani, B.B.F. Mirjalili, K. Zomorodian, S. Zomorodian, S. Afr, J. Chem 68, 133 (2015)
C. Tao, B. Wang, L. Sun, J. Yi, D. Shi, J. Wang, W. Liu, J. Chem. Res. 41, 25 (2016)
Acknowledgements
The authors are grateful to Urmia University for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kafi-Ahmadi, L., Khademinia, S., Poursattar Marjani, A. et al. Fabrication of 5-aryl-1H-tetrazoles derivatives by solid-state synthesized MgFe2O4 and MgFe2ZnxO4+δ heterogeneous nanocatalysts. Res Chem Intermed 48, 2973–2986 (2022). https://doi.org/10.1007/s11164-022-04741-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04741-6