Abstract
In the presented work, Fe3O4 bonded nicotinic acid-sulfonic acid chloride (Fe3O4@nicotinic acid @sulfonic acid chloride), as a magnetic reusable catalyst, was prepared by a simple procedure. The identification of Fe3O4@nicotinic acid @sulfonic acid chloride was studied by Fourier transform infrared spectroscopy, X-ray diffraction, thermal gravimetric analysis, scanning electron microscopy, energy dispersive X-ray analysis and vibrating sample magnetometry. Fe3O4@nicotinic acid @sulfonic acid chloride was employed as an efficient and heterogeneous catalyst for the one-pot synthesis of some 1-carbamato-alkyl-2-naphthol derivatives in high yields under solvent-free conditions. Simple work up, high yields, short reaction times and easy recovery of catalyst are some advantages of this work.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanoparticles are widely used in various pharmaceutical and electronic industries and in the coloring industry due to their different sizes, shapes and materials. Today, the application of nanoparticles has been much considered in catalytic applications. Nanoparticles are desirable substrates to replace homogeneous catalysts. When the size of the substrate is decreased to nanoscale, the surface-to-volume ratio is increased. As a result, the activity of stabilized catalysts on nanoparticles increases because of the interaction of these catalysts with high availability surfaces [1,2,3]. However, the easy separation and recovery of nano-catalysts from the reaction environment is still a challenge. Previous reported techniques such as straightening are not effective for separating these catalysts due to nanoscale particle size. It involves high cost, long time spent, and the use of special separation techniques such as centrifugation and chromatography.
In this case, Fe3O4 nanoparticles have been used as an efficient solid support for catalysts; due to their insoluble and magnetic properties. In addition, the separation of these compounds from the reaction mixture can be easily done with an external magnet, where the catalysis filtration is not necessary [4,5,6,7,8,9].
Recently, most of the chemists are interested in synthesis of organic compounds using multi component reactions (MCRs). Some advantages of this method are as follows: Preparing target compounds with high atomic economy and more efficiency by the reaction of three or more compounds together in one pot, increasing simplicity and synthetic efficiency of the conventional organic transformations [10,11,12,13,14,15,16].
1-Carbamato-alkyl-2-naphthols are interesting for chemists because of the biological activities of these compounds. The derivatives of these compounds have hypotensive and bradycardic effects [17, 18]. Many catalysts have been reported for the preparation of 1-carbamato-alkyl-2-naphthols by one-pot synthesis between alkyl carbamates, 2-naphthol and various aromatic aldehydes [19,20,21,22,23,24,25,26,27,28,29]. Some of these catalysts have some disadvantages such as hard reaction conditions, long reaction times, low yields, use of corrosive and expensive materials, boring work up and high reaction temperature. Having the above facts, finding an efficient method is still needed.
Herein, we have introduced Fe3O4@nicotinic acid @sulfonic acid chloride nanoparticles as a highly active catalyst for the one-pot synthesis of 1-carbamato-alkyl-2-naphthols (Scheme 1). Mild and solvent-free conditions, reusable processes, simple operation, short reaction times, easy recovery, and high yields are some advantages of this work. The separation of Fe3O4@nicotinic acid @sulfonic acid chloride from reaction mixture was done by an external magnet and reused for four times without a significant decrease of the yield.
Experimental
Materials and methods
All of the starting materials and solvents that have been used in this research were purchased from the Merck Company and were applied without any further purification. Fourier transform infrared (FT-IR) spectra were recorded using KBr Pill on a Perkin-Elmer Spectrum 65 .A FT-IR spectrometer in the range of 400–4000 cm−1 was used in order to identify the functional groups in synthesized materials. The 1H NMR and 13C-NMR spectrums of synthesized products were identified by 250 MHz Bruker Avance, Switzerland. The determination of the melting points was done on Büchi B‐545 melting points equipment. The crystalline structure of synthesized catalyst was investigated by X-ray diffraction (XRD) using an Ital structure APD 2000 with Cu Kα radiation in the 2θ range of 10.5–90° at ambient temperature. Thermal gravimetric analysis (TGA) was reported in the temperature range of 30–600 °C under nitrogen atmosphere and on a Perkin Elmer instrument (Model: Pyris 1). It should be noted that the rate of increase in the temperature is 10 °C/min in this apparatus. To provide elemental identification and quantitative compositional information of catalyst, the EDX was done using an Oxford scanning electron microscope instrument. To investigate the surface morphology of nano-catalyst, FE-SEM was carried out on a SIGMA VP-500 (ZEISS) instrument. The measurement of super magnetic properties of catalyst was performed using a vibrating sample magnetometer (MDKB instrument).
Synthesis of nano Fe3O4@Nicotinic acid
Fe3O4@Nicotinic acid was prepared using the previous method [30] as follows: In a three-necked round-bottom flask, FeCl3·6H2O (0.0216 mol, 5.89 g), FeCl2·4H2O (0.011 mol, 2.17 g) and nicotinic acid (0.043 mol, 5.29 g) were dissolved in 100 mL of distilled water at room temperature. The homogeneous solution is stirred using a magnetic stirrer under nitrogen atmosphere at 60 °C for 30 min. Then, by adding 25% solution of NH3 (15 mL) to this mixture, a black colored suspension appeared. Then the mixture was refluxed under N2 atmosphere at 90 °C for 6 h. In the end, the resulting black precipitate was separated by an external magnetic field, and it was washed with distilled water for five times. The obtained nanoparticles were dried at 60 °C for 12 h.
Synthesis of Fe3O4@nicotinic acid @sulfonic acid chloride nanoparticles
Nano Fe3O4@Nicotinic acid (0.5 g) was added to 6 mL of dry dichloromethane (under sonication for 30 min) and then added slowly in a 50 mL round-bottomed flask containing a solution of chlorosulfonic acid (15 mmol) in dichloromethane (10 mL) and stirred for 3 h. Then, a brown powder was separated by a permanent magnet, washed thoroughly with dichloromethane for several times and dried at 60 °C for 12 h.
General procedure for solvent free synthesis of 1-carbamato-alkyl-2-naphthols
A mixture of 2-naphthol (2.0 mmol), benzyl carbamate (2.2 mmol), aromatic aldehyde (2.0 mmol) and Fe3O4@nicotinic acid @sulfonic acid chloride nanoparticles (0.02 g) as a nanostructured magnetic catalyst was stirred magnetically at 80 ºC in an oil bath for an appropriate time. The completion of the reaction was monitored by TLC. Hot ethyl acetate was added to reaction vessel in order to dissolve the reaction mixture. The catalyst was separated easily by an external magnet. After recrystallization, pure crystals of 1-carbamato-alkyl-2- naphthol derivatives were obtained. The products were identified by spectral and physical data. The recovered catalyst was washed with hot ethyl acetate for several times, and dried to reuse for another reaction. The recovered catalyst was used again for four times on the synthesis of 1-carbamato-alkyl-2-naphthol derivatives without a significant decrease in the yield of products. The obtained products were identified by 1H NMR, 13C NMR and FT-IR analysis.
Analytical data of selected products
Benzyl ((2-hydroxynaphthalen-1-yl)(phenyl)methyl)carbamate (1a)
M. P. 185–187 °C. IR (KBr, ν, cm−1): 3424, 3210, 3067, 1676, 1631, 1516, 1439, 1328, 1271, 1229, 1131, 1041, 944, 808, 753, 697; 1HNMR (250 MHz DMSO‐d6, δ, ppm): δ 5.046 (s, 2H, CH2), 6.881 (s, 1H, CH), 7.218–7.767 (m, 16H, ArH), 7.882 (s, 1H, NH,), 10.111 (s,1H,OH); 13CNMR (62.9 MHz DMSO‐d6, δ, ppm): 50.858, 66.131, 118.888, 119.249, 122.960, 123.530, 126.446, 126.814, 126.950, 128.221, 128.545, 128.772, 129.001, 129.777, 132.489, 137.425, 142.758, 153.341, 156.491.
Benzyl ((3-chlorophenyl)(2-hydroxynaphthalen-1-yl)methyl)carbamate (2a)
M. P. 182–186 °C. IR (KBr, ν, cm−1): 3436, 3245, 3069, 1675, 1629, 1596, 1508, 1439, 1328, 1272, 1228, 1066, 948, 892, 813, 702; 1HNMR (250 MHz DMSO‐d6, δ, ppm): δ 5.023 (d, 1H, CH, J = 12.5 Hz), 5.104 (d, 1H, CH, J = 12.5 Hz) 6.89 (d, 1H, CH, J = 7.75 Hz), 7.150–7.779 (m, 15H, ArH), 7.894 (s, 1H, NH), 10.189 (s, 1H, OH); 13CNMR (62.9 MHz DMSO‐d6, δ, ppm): 50.489, 66.228, 118.648, 118.864, 123.060, 123.341, 125.246, 126.201, 126.818, 127.149, 128.233, 128.777, 129.080, 130.114, 130.463, 132.378, 133.367, 137.373, 145.521, 153.462, 156.548.
Benzyl ((2,4-dichlorophenyl)(2-hydroxynaphthalen-1-yl)methyl)carbamate (3a)
M. P. 205–207 °C. IR (KBr, ν, cm−1): 3417, 3344, 3069, 1686, 1628, 1585, 1513, 1436, 1341, 1271, 1244, 1054, 845, 820, 744; 1HNMR (250 MHz DMSO‐d6, δ, ppm): δ 5.011(s, 2H, CH2), 6.810 (s, 1H, CH), 7.074–7.747 (m, 14H, ArH), 7.965–8.053 (d, 1H, NH, J = 22 Hz), 9.899 (s, 1H,OH); 13CNMR (62.9 MHz DMSO‐d6, δ, ppm): 49.893, 65.885, 116.671, 118.982, 122.833, 123.096, 126.918, 127.075, 127.905, 128.137, 128.712, 129.020, 129.081, 130.178, 131.666, 132.416, 132.998, 133.619, 137.532, 139.209, 154.016, 156.070.
Benzyl ((2-hydroxynaphthalen-1-yl)(3-nitrophenyl)methyl)carbamate (4a)
M. P. 201–203 °C. IR (KBr, ν, cm−1): 3414, 3306, 3064, 1697, 1630, 1528, 1516, 1441, 1347, 1281, 1048, 811, 696; 1HNMR (250 MHz DMSO‐d6, δ, ppm): δ 5.03(d, 1H, CH, J = 12.5 Hz), 5.11 (d, 1H, CH, J = 12.5 Hz), 6.97 (d, 1H, CH, J = 8.25 Hz), 7.185–8.053 (m, 15H, ArH), 8.121 (s, 1H, NH), 10.241 (s, 1H, OH); 13CNMR (62.9 MHz DMSO‐d6, δ, ppm): 50.497, 66.278, 118.236, 118.838, 120.944, 121.945, 123.129, 123.177, 127.287, 128.193, 128.261, 128.790, 129.130, 130.138, 130.395, 132.340, 133.259, 137.322, 145.428, 148.197, 153.601, 156.641.
Benzyl ((2-hydroxynaphthalen-1-yl)(4-nitrophenyl)methyl)carbamate (5a)
M. P. 203–205 °C. IR (KBr, ν, cm−1): 3411, 3306, 3064, 1687, 1628, 1606, 1515, 1347, 1276, 1238, 1049, 852, 824, 745; 1HNMR (250 MHz DMSO‐d6, δ, ppm): δ 5.070 (t, 2H, CH2), 6.97 (d, 1H, CH, J = 7.75 Hz), 7.194–7.963 (m, 15H, ArH), 8.11 (d, 1H, NH, J = 7.75 Hz), 10.195 (s, 1H, OH); 13CNMR (62.9 MHz DMSO‐d6, δ, ppm): 50.724, 66.329, 118.349, 118.820, 123.089, 123.268, 123.762, 127.226, 127.586, 128.248, 128.769, 129.095, 130.355, 132.361, 137.271, 146.529, 151.075, 153.571, 156.635.
Benzyl ((4-bromophenyl)(2-hydroxynaphthalen-1-yl)methyl)carbamate (8a)
M. P. 180–182 °C. FT-IR (KBr, ν, cm−1): 3425, 3196, 3069, 1682, 1628, 1517, 1486, 1329, 1273, 1069, 1009, 817, 748; 1HNMR (250 MHz DMSO‐d6, δ, ppm): δ 5.00 (d, 1H, CH, J = 12.75 Hz), 5.07 (d, 1H, CH, J = 12.75 Hz), 6.831 (d, 1H, CH, J = 8.5 Hz), 7.128–7.801 (m, 15H, ArH), 7.843 (s, 1H, NH), 10.140 (s, 1H, OH); 13CNMR (62.9 MHz DMSO‐d6, δ, ppm): 50.389, 66.183, 118.75, 118.830, 119.851, 123.002, 123.412, 127.038, 128.216, 128.733, 129.035, 129.990, 131.390, 132.364, 137.349, 142.311, 153.397, 156.485.
Results and discussion
Synthesis and identification of Fe3O4@nicotinic acid @sulfonic acid chloride
In this work, we report the fabrication of Fe3O4@nicotinic acid @sulfonic acid chloride nanoparticles, and its catalytic application on the synthesis of 1-carbamato-alkyl-2-naphthols. The structure of the catalyst was studied by IR, XRD, SEM, TGA, VSM and EDX analysis. According to the procedure that is shown in Scheme 2, the catalyst was synthesized in two steps. Firstly, Fe3O4@nicotinic acid was prepared by refluxing method of iron (II), iron (III) ions and nicotinic acid and then adding to an aqueous solution of ammonia. Secondly, Fe3O4@nicotinic acid was functionalized by ClSO3H to produce Fe3O4@nicotinic acid @sulfonic acid chloride as reusable magnetic catalyst.
The FT-IR spectra of the nicotinic acid (a), Fe3O4@nicotinic acid (b), and Fe3O4@ nicotinic acid @sulfonic acid chloride nanoparticles (c) were recorded and shown in Fig. 1. The comparison between FT-IR spectra of nicotinic acid, and Fe3O4@ nicotinic acid showed that the C=O stretching bond of the carboxyl group, which appeared at 1711 cm–1 in the pure nicotinic acid, was not present in the spectrum of Fe3O4@nicotinic acid because of the binding of the nicotinic acid to the surface of Fe3O4. In Fig. 1b, the observed peak at 1403 cm–1 is due to symmetrical connection of carboxylic acid oxygen atoms to the surface of Fe3O4 nanoparticles [31, 32] and also the peak that appeared at 1628 cm–1 corresponded to C=N vibration of the pyridine ring. Thus, Fe–O bond vibration of iron oxide at 593 cm−1 is shown in Fig. 1b. In the spectrum of Fe3O4@ nicotinic acid@ sulfonic acid chloride (c), the assigned peak at 3415 cm–1 was related to OH stretching of the SO3H group. Additionally, spectrum (c) showed the bonds at 1032 and 1133 cm−1 correspond to N-SO2 and O-SO2 vibrations, respectively.
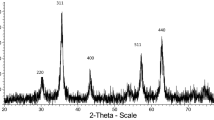
To investigate the crystalline nature and surface state of Fe3O4@ nicotinic acid@ sulfonic acid chloride nanoparticles, X-ray diffraction analysis (XRD) was studied in a domain of 10.5–90°, and the results are shown in Fig. 2. According to Scherer’s formula, the size of the crystals is calculated from 15 to 90 nm. X-ray diffraction pattern and FT‐IR spectrum of Fe3O4 in comparison with other species of the catalyst are given in the supporting information (Figures S1 and S2).
Thermal gravimetric analysis (TGA) curves of Fe3O4@ nicotinic acid (a) and Fe3O4@ nicotinic acid@ sulfonic acid chloride (b) are shown in Fig. 3. According to Fig. 3a, the weight losses of Fe3O4@nicotinic acid was done in two steps. The first weight loss up to 100 °C could be related to exit solvent from the catalyst. The thermal decomposition of the organic moiety established on the Fe3O4 surface, appeared at 260–481 °C at about 5.5%. Also, the Fig. 3b shows a very severe weight loss in the range of 242–680 °C which represents the stability of the nano-catalyst in this temperature range.
The measurement of magnetic properties of Fe3O4@nicotinic acid (a), and Fe3O4@ nicotinic acid@ sulfonic acid chloride nanoparticles (b) was investigated by VSM at room temperature (Fig. 4). The evaluation of these two curves showed that the saturation magnetization was reduced from 55 to 12 emu g−1 due to the functionalization of nicotinic acid by ClSO3H.
The presence of elements such as carbon (C), nitrogen (N), oxygen (O), sulfur (S), chlorine (Cl) and iron (Fe) was confirmed by an energy-dispersive X-ray (EDX) spectrum (Fig. 5). The EDX data well demonstrated the synthesis of Fe3O4@ nicotinic acid@ sulfonic acid chloride nanoparticles.
The morphology and the size of the nanostructured catalyst were evaluated by SEM analysis. These results showed that Fe3O4@ nicotinic acid@ sulfonic acid chloride nanoparticles appeared uniform, and the size of them is between 11 and 34 nm (Fig. 6).
Catalytical application of Fe3O4 bonded nicotinic acid-sulfonic acid chloride
We have checked the efficacy of the catalyst for the synthesis of 1-carbamato-alkyl-2-naphthols. The reaction between benzyl carbamate, benzaldehyde and 2-naphthol under solvent-free conditions was selected as a model reaction. At first, the reaction between benzyl carbamate, benzaldehyde and 2-naphthol was done in the absence of the catalyst over a period of 60 min, but the yield of product was low (Table 1, Entry 1). The high yield of product was obtained with 0.02 g of catalyst at 80 ºC under solvent-free conditions (Table 1-Entry 6).
The comparisons between this catalyst and the other previous reported catalysts for the preparation of compound 1a are depicted in Table 2. According to Table 2, the presented catalyst is more effective than the other reported catalysts in order to secure shorter reaction time, higher yield, more suitable temperature and easy recovery.
The generality of the catalyst was investigated by the reaction of various aromatic aldehydes with 2-naphthol and benzyl carbamate using 0.02 g of the catalyst under mild reaction conditions (Table 3). The synthesis of 1-carbamato-alkyl-2-naphthol derivatives with different aldehydes in the presence of Fe3O4@ nicotinic acid@ sulfonic acid chloride had good and acceptable results.
According to previous literature [20, 22, 24, 28, 29], we suggest a possible mechanism for the synthesis of 1-carbamato-alkyl-2-naphthols catalyzed by Fe3O4@ nicotinic acid@ sulfonic acid chloride nanoparticles (Scheme 3). In the initial step, the intermediate (A) was formed by the reaction of 2-naphthol with an activated aldehyde by the catalyst. Then, by leaving one molecule of H2O from the intermediate (A), ortho-quinone methide (o-QM) (B) was prepared. Finally, the desired 1-carbamato-alkyl-2-naphthol was prepared by the Michael reaction between benzyl carbamate and orthoquinone methide.
Recovery and reusability of nano-catalyst
To investigate the stability of the catalyst, the reaction between benzaldehyde (2 mmol), benzyl carbamate (2.2 mmol) and 2-naphthol (2 mmol) was studied as a model reaction using 0.02 g catalyst at 80 °C under solvent-free conditions. After completion of the reaction, hot ethyl acetate was added to the reaction mixture and stirred for 5 min. Then, the catalyst was easily separated by the external magnet. The separated catalyst was washed with hot ethyl acetate for several times, dried and reused for another reaction. The catalyst was successfully reused for four times without any remarkable reducing of catalytic activity (Fig. 7). The FT-IR spectrum of a reused catalyst was compared with fresh catalyst for which they have high compliance with each other (Fig. 8).
Conclusions
In this study, we have synthesized Fe3O4 bonded nicotinic acid-sulfonic acid chloride (Fe3O4@nicotinic acid @sulfonic acid chloride) as a novel heterogeneous catalyst and fully characterized by various techniques. The catalytic activity of this catalyst was checked by the one-pot synthesis of 1-carbamato-alkyl-2-naphthol derivatives under solvent-free conditions. This work showed notable advantages such as short reaction times, high yields, easily separation and retrievable of the catalyst.
References
D. Astruc, F. Lu, J.R. Aranzaes, Angew. Chem. Int. Ed. 44, 7852 (2005)
D. Astruc, Nanoparticles and Catalysis (Wiley, Weinheim, 2008)
G.A. Somorjai, H. Frei, J.Y. Park, J. Am. Chem. Soc. 131, 16589 (2009)
A. Taher, J.B. Kim, J.Y. Jung, W.S. Ahn, M.J. Jin, Synlett 15, 2477 (2009)
Q. Zhang, H. Su, J. Luo, Y. Wei, Green Chem. 14, 201 (2012)
L. Lin, C. Zou, J. Chem. Eng. Data 62, 762 (2017)
M.A. Zolfigol, A.R. Moosavi-Zare, P. Moosavi, V. Khakyzadeh, A. Zare, C. R. Chimie 16, 962 (2013)
M.A. Zolfigol, V. Khakyzadeh, A.R. Moosavi-Zare, A. Rostami, A. Zare, N. Iranpoor, M.H. Beyzavi, R. Luque, Green Chem. 15, 2132 (2013)
M.A. Zolfigol, V. Khakyzadeh, A.R. Moosavi-Zare, A. Zare, P. Arghavani-Hadi, Z. Mohammadi, M.H. Beyzavi, S. Afr, J. Chem. 65, 280 (2012)
A. Khazaei, A.R. Moosavi-Zare, H. Afshar-Hezarkhania, V. Khakyzadeh, RSC Adv. 4, 32142 (2014)
A. Khazaei, F. Gholami, V. Khakyzadeh, A.R. Moosavi-Zare, J. Afsar, RSC Adv. 5, 14305 (2015)
A. Khazaei, A. Ranjbaran, F. Abbasi, M. Khazaei, A.R. Moosavi-Zare, RSC Adv. 5, 13643 (2015)
A. Khazaei, M.A. Zolfigol, F. Karimitabar, I. Nikokar, A.R. Moosavi-Zare, RSC Adv. 5, 71402 (2015)
A. Khazaei, F. Abbasi, A.R. Moosavi-Zare, New J. Chem. 38, 5287 (2014)
A.R. Moosavi-Zare, M.A. Zolfigol, M. Daraei, Synlett 25, 1173 (2014)
A.R. Moosavi-Zare, M.A. Zolfigol, S. Farahmand, A. Zare, A.R. Pourali, R. Ayazi-Nasrabadi, Synlett 25, 193 (2014)
H.R. Shaterian, H. Yarahmadi, M. Ghashang, Bioorg. Med. Chem. Lett. 18, 788 (2008)
A.Y. Shen, C.T. Tsai, C.L. Chen, Eur. J. Med. Chem. 34, 877 (1999)
Z. Song, X. Sun, L. Liu, Y. Cui, Res. Chem. Intermed. 39, 2123 (2012)
D. Kundu, A. Majee, A. Hajra, Catal. Comm. 11, 1157 (2010)
N. Tavakoli-Hoseini, M.M. Heravi, F.F. Bamoharran, A. Davoodnia, Bull. Korean Chem. Soc. 32, 787 (2011)
H.R. Shaterian, A. Hosseinian, M. Ghashang, Tetrahedron Lett. 49, 5804 (2008)
M. Wang, Q. Wang, S. Zhao, X. Wan, Monatsh. Chem. 144, 975 (2013)
A. Khazaei, F. Abbasi, A.R. Moosavi-Zare, RSC Adv. 4, 1388 (2014)
M.R. Mohammad Shafiee, R. Moloudi, M. Ghashang, J. Chem. Res. 35, 622 (2011)
J.M. Yang, ChN Jiang, H. Dong, D. Fang, J. Chem. Res. 37, 257 (2013)
H.R. Shaterian, A. Hosseinian, Res. Chem. Intermed. 40, 3011 (2014)
A. Zare, T. Yousofi, A.R. Moosavi-Zare, RSC Adv. 2, 7988 (2012)
M.A. Zolfigol, A. Khazaei, A.R. Moosavi-Zare, A. Zare, V. Khakyzadeh, Appl. Catal. A 400, 70 (2011)
A. Ghorbani-Choghamarani, G. Azadi, RSC Adv. 5, 9752 (2015)
Y.T. Tao, J. Am. Chem. Soc. 115, 4350 (1993)
K.V.P.M. Shafi, A. Ulman, X.Z. Yan, N.L. Yang, C. Estournes, H. White, Rafailovich M. Langmuir 17, 5093 (2001)
A. Khazaei, F. Abbasi, A.R. Moosavi-Zare, J. Sulfur Chem. 36, 364 (2015)
Acknowledgements
We thank Bu-Ali Sina University and Iran National Science Foundation (INSF) for financial support given to our research groups.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khazaei, A., Tavasoli, M. & Moosavi-Zare, A.R. Fabrication, identification and application of Fe3O4 bonded nicotinic acid-sulfonic acid chloride as a retrievable magnetic nanostructured catalyst for the one-pot synthesis of 1-carbamato-alkyl-2-naphthols. Res Chem Intermed 44, 5893–5910 (2018). https://doi.org/10.1007/s11164-018-3462-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3462-5