Abstract
CoNi2S4/graphene nanocomposites were synthesized by a simple solvothermal method and used as supercapacitor electrodes. CoNi2S4 nanoparticles were doped in graphene layers, which is favorable for improving electrochemical performances of the as-prepared materials. The resulting electrochemical performance of CoNi2S4/graphene nanocomposite electrodes possessed pseudocapacitive behavior with high specific capacitance (1621 F g−1 at 0.5 A g–1), good rate capability (76.7% capacitance retention when reaching 10 A g–1) and outstanding cycling stability (no capacitance loss after 2500 charge–discharge cycles at 5 A g–1). In addition, as the positive electrode of an asymmetric supercapacitor, the material also shows a high specific capacitance of 126.6 F g–1 at 0.5 A g–1, good cycle stability with capacitance retention of 87.4% after 5000 cycles and the highest energy density of 39.56 W h kg–1 at the power density of 374.8 W kg–1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Supercapacitors have experienced a developing course for more than 30 years. Due to their excellent characteristics with long lifespan, strong environment adaptiveness, superior charge–discharge efficiency, high power density, flexible operating temperature and energy conservation [1,2,3], supercapacitors have become one of the most deserving areas of research in the world and are found in increasing numbers in many applications, such as electric vehicles, pulse laser techniques, military devices, wind energy equipment and memory storage systems [4,5,6].
Currently, commercial supercapacitors are, from a materials point of view, mostly made of high-surface area carbon-based and metal electrode materials. Carbonaceous materials are dominant mainly from the electric double-layer energy storage mechanism by the charge-transfer process between the electrode surface and electrolyte ions [7], such as carbon nanotubes [8], graphene [9, 10], activated carbon [11, 12], carbon nanofibers [13, 14] and organic carbide [15, 16]. Compared with other carbonaceous materials, graphene possesses intriguing properties with a unique structure, showing high electrical conductivity, good mechanical strength, high theoretical surface area and superior thermal stability [17, 18]. By virture of adjusting ionic charges easily through electrostatic interactions, graphene can serve as a current collector which delivers electrons rapidly from charge transfer reactions of anchored pseudo-capacitive materials [19]. Many reports have proved that graphene is an excellent substrate when combined with transition metal oxides/hydroxides in supercapacitors [20,21,22], in which graphene-based electroactive materials offer enormous potential. Despite its superior properties with excellent cycle stability and high conductivity, graphene shows a lower capacitance and lower energy density than pseudocapacitive materials.
Metal electrode materials, such as RuO2 and MnO2, store energy by a pseudocapacitance effect with fast reversible Faradaic redox reactions at the multi-electron surface [7, 23] to obtain high specific capacitance, but the inherent weakness of lower electrical conductivity and poor rate performance is caused by the instability of volume. Other pseudo-capacitive electrode materials of transition metal oxides with high oxidation states, such as Co3O4 [24], NiCo2O4 [25] and MnMo2O4 [26], have been researched to replace RuO2. Compared with these transition metal oxides, transition ternary metal sulfides, especially NiCo2S4 and CoNi2S4, have recently gained great attention due to their satisfactory structural features and higher efficiency electrochemical behavior in supercapacitors [27,28,29]. For example, CoNi2S4 shows higher electroconductivity than CoNi2O4 and is expected to provide better redox reactions than single cobalt sulfides or nickel sulfides [27]. The addition of graphene into metal materials can broaden the operating voltage window and further improve the conductivity of nanocomposites. It is thus necessary to design graphene-based metal electrode materials with electrochemical activities and microstructures.
Considering the characteristics of CoNi2S4 and graphene, we designed a novel nanocomposite called CoNi2S4/rGO, in which CoNi2S4 nanoparticles were successfully doped on graphene sheets through a simple solvothermal method. In the CoNi2S4/rGO nanocomposites, graphene acts as the substrate for the growth of CoNi2S4 nanoparticles and can anchor pseudocapacitive nanomaterials, and in addition, it accelerates electron and ion transport of the electrode materials. CoNi2S4/rGO nanocomposites with different additions of graphene were investigated and showed different microstructures. When used as electrode materials for supercapacitora, the CoNi2S4/rGO nanocomposites exhibit excellent electrochemical performances with high specific capacitance, excellent rate performance and good cycle stability.
Experimental
Materials
Cobalt nitrate hexahydrate, Co(NO3)2·6H2O, nickel nitrate hexahydrate, Ni(NO3)2· 6H2O, thioacetamide, CH3CSNH2, and anhydrous ethanol were purchased from Sinopharm Chemical Reagent (Shanghai, China), and all the chemicals reagent were of analytical grade and used without further purification. The deionized water was prepared in own laboratory.
Preparation of CoNi2S4/rGO nanocomposites
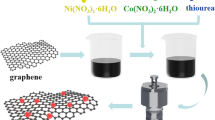
Graphene oxide (GO) was prepared through the modified Hummer′s method [30]. The CoNi2S4/rGO nanocomposites were synthesized by a one-step solvothermal method, and the fabrication process is illustrated in Fig. 1. The details are as follows: 1 mmol Co(NO3)2·6H2O, 2 mmol Ni(NO3)2·6H2O and 4 mmol CH3CSNH2 were dissolved in 30 mL absolute ethanol by ultrasonication. Then, GO (5 mol L–1) was added into the above solution and maintained with ultrasound for 1 h to form a uniform suspension. Finally, the mixture was transferred into a Teflon-lined autoclave and maintained at 180 °C for 12 h. The obtained products were washed with water and ethanol, and freeze-dried for 72 h. The GO additions were, respectively, 30, 50 and 70 mg, and the corresponding samples were labeled CoNi2S4/rGO-1, CoNi2S4/rGO-2 and CoNi2S4/rGO-3, respectively. In addition, reduced graphene (rGO) was also obtained by a solvothermal method without the addition of Co(NO3)2·6H2O, Ni(NO3)2·6H2O or CH3CSNH2.
Characterization
The morphology and structure of the obtained samples were characterized by scanning electron microscopy (SEM; Hitachi S-4800 N), and energy dispersive X-ray spectroscopy spectra (JEOL JSM 6700F). Fourier transform infrared spectroscopy (FT-IR; Bruker Vector 22) was measured at 400–4000 cm–1. X-ray diffraction (XRD; PANalytical PW3040/60) was conducted with Cu Kα radiation (γ = 0.1542 nm) with the 2θ ranging from 10 to 80°. Thermal gravimetric analysis (TGA; DTG- 60AH) were carried out from 25 to 1000 °C with a heating rate of 10 °C min–1 in nitrogen atmosphere. Raman spectra were visulaized on a SenterraR200L multichannel confocal microspectrometer with excitation from the 532-nm line of an Ar-ion laser. Nitrogen adsorption–desorption experiments were conducted using 3H-2000PM2 at 77.3 K. An X-ray photoelectron spectra (XPS; AXIS Ultra DLD system from Kratos) was performed using an X-ray source of Al Kα radiation.
Electrochemical measurement
The electrochemical measurements of all the as-prepared samples were investigated through a three-electrode system in 2 M KOH electrolyte solution. The working electrode was fabricated as follows: active materials (CoNi2S4/rGO nanocomposites), acetylene carbon black and a binder of polytetrafluoroethylene with a mass ratio of 8:1:1 in 1.0 mL anhydrous ethanol were stirred mechanically to form a homogeneous slurry and then pressed onto a foamed nickel sheet (1 cm × 3 cm), then dried at 60 °C in a vacuum -drying oven. A platinum electrode served as the counter electrode and a Ag/AgCl electrode as the reference electrode. Cyclic voltammetry (CV), galvanostatic charge–discharge (GCD) and electrochemical impedance spectroscopy (EIS) were measured in a CHI 760E electrochemical workstation (Shanghai Chenhua, China), and the cycle behavior was determined in a LAND-CT2001A system (Wuhan, China).
Results and discussion
The morphologies of rGO, CoNi2S4/rGO-1, CoNi2S4/rGO-2 and CoNi2S4/rGO-3, possessing different additions of graphene, spontaneously present different CoNi2S4 particle sizes and graphene microstructures. As can be seen from the SEM images in Fig. 2a–f, all the CoNi2S4/rGO materials take on CoNi2S4 nanoparticles located in the graphene. The nanocomposite of CoNi2S4/rGO-1 (Fig. 2a, b) with less graphene turns out to have a fragile cracked piece structure and exhibits more CoNi2S4 nanoparticles. In addition, the CoNi2S4 size is not concentrated but dispersed in a broad range of 5–60 nm. Figure 2c, d depicts the morphology of CoNi2S4/rGO-2 at different magnifications. The whole has a three-dimensional aerogel structure and the CoNi2S4 nanoparticles with a concentrated size of 15–20 nm have been well wrapped by the thin and wrinkled graphene layers. The well-designed three-dimensional aerogel structure implies favorable contact between the CoNi2S4 nanoparticles and the graphene layer, which is propitious for good conductivity as well as outstanding cycle stability. In addition, the three-dimensional body may provide more spaces between the graphene layer and the CoNi2S4 nanoparticles for improving the ion and electrolyte diffusion, which is crucial for further promoting the rate performance by increasing the availability of active sites in the electrode materials during the faradic redox reactions even with a high current density. As observed from the SEM images of the CoNi2S4/rGO-3 nanocomposite in Fig. 2e, f, the density of the CoNi2S4 nanoparticles in CoNi2S4/rGO-3 is significantly lower than that in CoNi2S4/rGO-1 and CoNi2S4/rGO-2. Furthermore, the CoNi2S4 size in CoNi2S4/rGO-3 has a range of 100–500 nm, obviously larger than that of CoNi2S4/rGO-1 and CoNi2S4/rGO-2.
The FT-IR spectrum has been analyzed to obtain some information about functional groups and chemical bonds of the samples. As clearly observed in Fig. 3a, the rGO exhibits several peaks within the range of 1100–1700 cm–1, and the peak at about 1580 cm–1 is attributed to a typical C=C stretching vibration due to the sp2 mode. and the peak located at 1370 cm–1 is attributed to the C–OH of graphene [31]. Moreover, the broad band at approximately 3400 cm–1 represents γO–H, implying the existence of rGO [21]. For CoNi2S4/rGO-1, CoNi2S4/rGO-2 and CoNi2S4/rGO-3, new peaks at 550–1100 cm–1 are found, which are typical peaks of CoNi2S4, demonstrating the successful formation of CoNi2S4 in the rGO layers [31].
The composition and structure of rGO, CoNi2S4/rGO-1, CoNi2S4/rGO-2 and CoNi2S4/rGO-3 have been investigated by XRD patterns, as shown in Fig. 3b. For the rGO sheets, an apparent intense peak at around 25° is well matched to the (002) plane of graphene, which can be attributed to the overlap of the graphene layers (JCPDS PDF#22-1086) [32]. For the CoNi2S4/rGO-1, CoNi2S4/rGO-2 and CoNi2S4/rGO-3 nanocomposites, the peaks exhibited at 16.06°, 26.55°, 31.60°, 38.18°, 46.73°, 50.31°, 54.42°, 59.21°, 61.72° and 77.20° corresponding to the (111), (220), (311), (400), (422), (511), (440), (442), (620) and (642) planes (JCPDS PDF#73-1297), respectively, demonstrate the successful formation of CoNi2S4. As can also be seen from the spectra, the more the addition of graphene, the weaker is the CoNi2S4 peak displayed in CoNi2S4/rGO. Moreover, the peak of rGO disappears in the CoNi2S4/rGO-1, CoNi2S4/rGO-2 and CoNi2S4/rGO-3 nanocomposites, which is because the introduction of CoNi2S4 weakened or avoided the overlap of rGO sheets, further reducing the graphitizing crystallization [33]. In addition, the absence of peaks of NiS2, CoS2 or CoNi2O4, indicate relatively purer CoNi2O4 generation.
Raman spectra were examined to analyze the structure of the samples. In Fig. 4a, two obvious primary graphitic peaks at about 1350 and 1588 cm–1 belong to the D and G bands, respectively. The D band arises from the imperfections of the rGO sheets, which is an indication of defects in the graphene, and the G band is because of the splitting of the E2g in-plane vibrational mode of graphene, illustrating the sp2 hybridization of C atoms in the rGO [21]. In addition, the ID/IG value denotes the degree of graphitization, the defects or the domain size of graphitization. Generally, the ID/IG value for amorphous carbon is about 1.0. The decreased ID/IG values suggest disordered carbon coupled with a partial graphite layer [34, 35]. Compared with rGO, the nanocomposites of CoNi2S4/rGO-1, CoNi2S4/rGO-2 and CoNi2S4/rGO-3 have higher values of ID/IG, implying more detects in the rGO sheets after combined with CoNi2S4. Several peaks recorded within the range in 200–700 cm–1 reveal a valid demonstration of typical Raman vibration modes for the metal-containing compound CoNi2S4 [28].
The TGA curves shown in Fig. 4b reflect the thermal stabilities of all the materials. For all the samples, the weight loss before 120 °C is due to the evaporation of the physical water adsorbed on the material surfaces. The weight loss at 120–730 °C is due to the thermal degradation of rGO; the mass degradation values are 15.5, 20.3 and 30.5% for CoNi2S4/rGO-3, CoNi2S4/rGO-2 and CoNi2S4/rGO-1, respectively, showing that CoNi2S4/rGO-2 and CoNi2S4/rGO-3 have relatively high thermallstability. As can clearly be seen, the almost complete lack of weight loss when the temperature is higher than 730 °C and the residual mass indicate the thermally stable CoNi2S4.
The N2 adsorption–desorption isotherms of CoNi2S4/rGO-2 are explored in Fig. 5a for the specific surface area and pore size distribution. This curve exhibits a clear adsorption–desorption hysteresis loop, which is in accordance with a type IV isotherm on the basis of the IUPAC classification [36]. Moreover, as evaluated by the Barrett–Joyner–Halenda method, the pore sizes (inset of Fig. 5a) concentrate at 2.82 and 3.77 nm, indicating the mesoporous structure of CoNi2S4/rGO-2. The calculated specific Brunauer–Emmett–Teller surface (75.08 m2 g–1) and the mesoporous structure can together provide more active sites for the electrolyte and provide easy permeation to the mesoporous structure [37, 38].
XPS was further investigated to analyze the elemental composition and chemical states of the CoNi2S4/rGO-2 nanocomposites, as shown in Fig. 5b–f and Fig. S3. The representative survey in Fig. 5b reveals the existence of Co, Ni, S, C and O elements. In the Co 2p spectrum of Fig. 5c, the peaks with binding energies around 781.92 and 798.29 eV can be well assigned to Co 2p3/2 and Co 2p1/2, respectively, and peaks at 777.83 and 803.45 eV can be attributed to Co3+ and Co2+, respectively [39, 40]. With regard to the Ni 2p spectrum in Fig. 5d, the peaks at 856.71 and 861.52 eV are, respectively, Ni 2p3/2 and its shake-up satellite peak, while the peaks located at 874.55 and 880.62 eV can be ascribed to Ni 2p1/2 and its shake-up satellite peak, respectively. Furthermore, the two spin–orbit doublets and two shake-up satellites fit well with Ni2+ and Ni3+ [32, 41]. In the S 2p spectrum (Fig. 5e), the obvious peak centered on the binding energy of 169.29 eV is caused by the sulfur ions with a higher oxide state of S4O62− at the surface due to the partial oxidation of CoNi2S4 [29, 32, 42], and the binding energy at 162.73 eV agrees with the metal-sulfur bonds (CO–S, Ni–S) in the ternary metal sulfides [40]. In the C 1s spectrum (Fig. 5f), the distinct peak at the binding energy of 287.82 eV can be traced back to the C–C, C=C and C–H bonds, which also illustrates the successful formation of honeycomb-like graphene [42, 43]. To evaluate the electrochemical performance in supercapacitors, the CV, GCD and EIS measurements were carried out in 2 M KOH electrolytes. Figure 6a exhibits CV curves of the CoNi2S4/rGO-1, CoNi2S4/rGO-2 and CoNi2S4/rGO-3 electrodes with the potential window at 0–0.55 V at a scan rate of 10 mV s–1. Clearly, the CoNi2S4/rGO-2 material holds the largest integral area, indicating the maximum specific capacitance when compared with CoNi2S4/rGO-1 and CoNi2S4/rGO-2. All the nanocomposites take on distinct redox peaks rather than rectangular shapes, implying a pseudo-capacitance energy storage mechanism, which is dominated by CoNi2S4 through the reversible Faradaic redox reactions of Co2+/Co3+/Co4+ and Ni2+/Ni3+ at the electrolyte/electrode interface by the following reactions [41]:
CV curves of the CoNi2S4/rGO-2 electrode at various scan rates from 5 to 100 mV s–1 are shown in Fig. 6b. As can be clearly seen, the anodic peak shifts into higher potentials and the corresponding cathodic peak shifts toward lower potentials as the scan rates increase, which indicates fast redox reactions and a relatively small resistance for this electrode material [39]. In addition, when the scan rate iis ncreased to 100 mV s–1, the redox peaks can still maintain a distinct form, clearly indicating a good rate performance. Furthermore, the redox peak current and the square root of the scanning rate illustrates a linear correlation with the result in Fig. 6c, which is due to the fast redox reactions between the electrode and the electrolyte interface [44].
Electrochemical performances: a CV curves of CoNi2S4/rGO-1, CoNi2S4/rGO-2 and CoNi2S4/rGO-3 electrodes at 10 mV s−1; b CV curves of CoNi2S4/rGO-2 at various scan rates of 5, 10, 20, 40, 50 and 100 mV s−1; c the linear relationship of the CoNi2S4/rGO-2 electrode between the anodic peak and the square root of various scan rates, and the cathodic peak and the square root of various scan rates; d GCD curves of the CoNi2S4/rGO-2 electrode at current densities of 0.5, 1, 2, 5 and 10 A g−1
To further estimate the capacitive performance of the CoNi2S4/rGO-2 nanocomposite electrode, GCD measurements were conducted with the voltage window ranging from 0 to 0.47 V at a series of current densities from 0.5 to 10 A g–1. As shown in Fig. 6d, the obtained GCD curves take on nonlinear profiles, which results from the reversible redox reactions and demonstrates typical pseudocapacitive behavior, matching well with the CV curves in Fig. 6b. In addition, the symmetrical GCD curves hold good proof of the fast reversibility of the CoNi2S4/rGO-2 electrode material.
The specific capacitances can be obtained from the GCD curves and Eq. (3) [45]:
where \( C_{s} \), \( I \), \( \Delta t \) and \( m \) are the specific capacitance (F g–1), discharge current density (A g–1), discharge time (s), potential window (V) during the charge–discharge process and the mass (g) of the active materials, respectively. The calculated specific capacitances of the CoNi2S4/rGO-1, CoNi2S4/rGO-2 and CoNi2S4/rGO-3 electrodes are shown in Fig. 7a. At a current density of 0.5 A g–1, the specific capacitances of CoNi2S4/rGO-1, CoNi2S4/rGO-2 and CoNi2S4/rGO-3 are 1308, 1621 and 1216 F g–1, respectively, proving the excellent electrochemical performance of CoNi2S4/rGO-2. In addition, the specific capacitances of CoNi2S4/rGO-2 electrode are 1539, 1466, 1321 and 1243 F g–1 at 1, 2, 5 and 10 A g–1, respectively, and the specific capacitance retentions are calculated to be 56.04, 76.7 and 62.7% of CoNi2S4/rGO-1, CoNi2S4/rGO-2 and CoNi2S4/rGO-3, respectively, even at 10 A g–1. It is well known that specific capacitances decay by degrees in pace with the increase of discharge current densities, and that this phenomenon can be interpreted as the diffusion and migration of electrolyte ions OH− being restricted because of the diffusion effect at higher current densities [39, 46].
a Specific capacitances with various discharge current densities; b cycle stability on the charge–discharge numbers measured at the current density of 10 A g−1; c Nyquist plots for CoNi2S4/rGO-1, CoNi2S4/rGO-2 and CoNi2S4/rGO-3 electrodes; d equivalent circuit of the as-prepared CoNi2S4/rGO-2 electrode
The good supercapacitive behavior of the CoNi2S4/rGO nanocomposite can be explained as follows: (1) the well-coated CoNi2S4 nanoparticles in the rGO layers improved the electrical contact between the active materials and the electrolytes; (2) the aerogel structure offers more electroactive spots for electroactive ions and provides more efficient pathways for charge transfer [39, 47]; and (3) compared with Co–Ni oxide, CoNi2S4 exhibits good electrical conductivity.
As a key criterion of electrochemical characteristics for electrode materials, cycle stability tests were examined through repeated charge–discharge procedures. Figure 7b shows the cycle performances of the CoNi2S4/rGO-1, CoNi2S4/rGO-2 and CoNi2S4/rGO-3 electrodes at a current density of 5 A g–1. It can clearly be seen that there is almost no capacitance loss for the CoNi2S4/rGO-2 material, and 97.2 and 98.8% capacitance retention for CoNi2S4/rGO-2 and CoNi2S4/rGO-3, respectively, after 2500 cycles, indicating excellent cycle stability. The good cycle stability for the materials may be attributed to their stable combination between CoNi2S4 and rGO. In addition, the good reversible redox reaction of CoNi2S4 can also ensure good cycling stability.
The EIS analysis was executed within the range of 0.01–100 kHz at an open-circuit potential with the amplitude of 5 mV for further research on the conductivity of the materials with the Nyquist plots shown in Fig. 7c and the corresponding equivalent circuit in Fig. 7d. The Nyquist plots include high-, low- and intermediate-frequency regions. All electrodes take on similar semi-circle arc shapes in the high-frequency region, implying charge-transfer resistance (Rct), which is controlled by reaction kinetics. Compared with the CoNi2S4/rGO-1 (1.32 Ω) and CoNi2S4/rGO-3 (2.33 Ω) electrodes, the Rct of CoNi2S4/rGO-2 (0.99 Ω) is smaller, which is consistent with this law: the larger the semicircle, the larger the Rct. The bulk resistances (Rs) were obtained by the intercept in the real part (Z′) in the high-frequency region with their values of 1.37, 0.92 and 1.28 Ω for CoNi2S4/rGO-1, CoNi2S4/rGO-2 and CoNi2S4/rGO-3, respectively. This finding illustrates that the proper combination between CoNi2S4 and rGO could promote electroconductivity.
Resent research has shown that the two-electrode cell is more suitable than the three-electrode cell in simulating a packaged supercapacitor, which is because of the similar physical architecture and the charge transfer condition for the two-electrode cell and a packaged supercapacitor [48]. Therefore, an asymmetric two-electrode system has been assembled in Fig. 8a. To balance the charge in the positive and negative electrodes, the CoNi2S4/rGO-2 and AC masses were in accord with Eq. (4):
where m represents the mass of the positive (m+) or negative electroactive material (m−), C represents the specific capacitance for the positive (Cs+) or negative material (Cs−), and (\( \Delta V_{-} \)) represents the operating voltage window for positive (\( \Delta V_{ + } \)) or negative material (\( \Delta V_{ - } \)).
a Diagram of the AC//CoNi2S4/rGO-2 asymmetric electrode device; b CV curves of AC and CoNi2S4/rGO-2 at 10 mV s−1; c CV curves of the AC//CoNi2S4/rGO-2 asymmetric electrode at different voltage windows at the scan rate of 50 mV s−1; d CV curves of AC//CoNi2S4/rGO-2 asymmetric supercapacitor at various scan rates of 5, 10, 20, 50, 80 and 100 mV s−1
Figure 8b exhibits the CV curves at 10 mV s–1 of the CoNi2S4/rGO-2 from 0 to 0.55 V and AC from − 1 to 0 V, respectively. For the CoNi2S4/rGO-2 and AC, the specific capacitance were calculated as 1539 and 330 F g–1, and, accordingly, the masses were 0.8 and 2.0 mg, respectively. From the CV curves of the CoNi2S4/rGO-2 asymmetric electrode at 50 mV s–1 in various voltage windows (Fig. 8c), the appropriate stable window was chosen as 0–1.5 V for further investigation. As can be seen from Fig. 8d, the asymmetric GCD curves take on a rectangular-like shape, representative of both the electrical double-layer capacitor and the pseudocapacitance capacitor.
The GCD measurement was carried out at different current densities (Fig. 9a) and the obtained GCD curves take on sawtooth-like lines, which is attributed to the reversible Faraday reactions arising from the pseudocapacitive materials of CoNi2S4/rGO-2. In addition, the AC//CoNi2S4/rGO-2 asymmetric supercapacitor exhibited specific capacitances of 126.6, 121.2, 117.1, 111.2 and 100.5 F g–1 with current densities of 0.5, 1, 2, 5 and 10 A g–1, respectively, having the high capacitance retention of 79.4%.
The GCD curves (a) and corresponding specific capacitances (b) of the AC//CoNi2S4/rGO-2 asymmetric electrode at different current densities; c cycling stability of AC//CoNi2S4/rGO-2 asymmetric supercapacitor at a current density of 5 A g−1; d Ragone plot of the AC//CoNi2S4/rGO-2 asymmetric supercapacitor
Promoting energy density has been studied very actively. A Ragone plot was fabricated (Fig. 9c) and the energy densities and power densities were received through GCD curves at various densities by Eqs. (5) and (6), respectively. According to the Ragone plot, the energy density possesses the highest value of 39.56 W h kg–1 at the power density of 374.8 W kg–1.
As a significant factor in investigating the practical application of asymmetric supercapacitors, the cycle stability was measured, as shown in Fig. 9d. After 5000 charge–discharge cycles at the current density of 5 A g–1, the AC//CoNi2S4/rGO-2 asymmetric electrode demonstrated an outstanding cycle stability of 87.4% capacitance retention. All these electrochemical performances confirm the potential application of CoNi2S4/rGO materials for supercapacitors.
Conclusions
CoNi2S4/rGO nanocomposites with various amounts of graphene loading were prepared by a facile one-step solvothermal method. The well-coated CoNi2S4 nanoparticles in the rGO layers can improve the electrochemical performance of active materials. The electrodes possess excellent performances with high specific capacitance, good rate capability and outstanding cycling stability. In addition, the electrodes show high energy densities as asymmetric supercapacitors. Hence, the CoNi2S4/rGO nanocomposite materials are potential candidates for energy storage applications.
References
P. Simon, Y. Gogotsi, Nat. Mater. 7, 845 (2008)
X. Wei, X. Jiang, J. Wei, S. Gao, Chem. Mater. 28, 445 (2016)
L. Wang, Y. Shao, J. Zhang, M. Anpo, Res. Chem. Intermed. 34, 267 (2008)
Y. Liu, L. Wang, J. Zhang, F. Chen, M. Anpo, Res. Chem. Intermed. 37, 949 (2011)
F. Zhang, T. Zhang, X. Yang, L. Zhang, K. Leng, Y. Huang, Energ. Environ. Sci. 6, 1623 (2013)
Y. Jiang, X. Cui, L. Zu, Z. Hu, J. Gan, J. Nanosci. Nanotechno. 17, 507 (2017)
M. Winter, R.J. Brodd, Cheminform 35, 4245 (2004)
D.P. Dubal, N.R. Chodankar, Z. Caban-Huertas, F. Wolfart, M. Vidotti, R. Holze, C.D. Lokhande, J. Power Sourc. 308, 158 (2016)
J. Yu, J. Wu, H. Wang, A. Zhou, C. Huang, H. Bai, L. Li, A.C.S. Appl, Mater. Inter. 8, 4724 (2016)
B. Dong, X. Zhang, X. Xu, G. Gao, S. Ding, J. Li, B. Li, Carbon 80, 222 (2014)
G. Godillot, P.L. Taberna, B. Daffos, P. Simon, C. Delmas, L. Guerlou-Demourgues, J. Power Sour. 331, 277 (2016)
B. Li, F. Dai, Q. Xiao, L. Yang, J. Shen, C. Zhang, M. Cai, Energ. Environ. Sci. 9, 102 (2015)
F. Miao, C. Shao, X. Li, K. Wang, Y. Liu, J. Mater. Chem. A 4, 4180 (2016)
C. Ma, J. Sheng, C. Ma, R. Wang, J. Liu, Z. Xie, J. Shi, Chem. Eng. J. 304, 587 (2016)
L. Wei, M. Sevilla, A.B. Fuertes, R. Mokaya, G. Yushin, Adv. Energy Mater. 1, 356 (2011)
M. Oschatz, S. Boukhalfa, W. Nickel, J.P. Hofmann, C. Fischer, G. Yushin, S. Kaskel, Carbon 113, 283 (2017)
F. Yang, H. Yang, B. Tian, J. Zhang, D. He, Res. Chem. Intermed. 39, 1685 (2013)
S. Li, D. Wu, C. Cheng, J. Wang, F. Zhang, Y. Su, Angew. Chem. 52, 12105 (2013)
H. Li, L. Wang, Y. Liu, J. Lei, J. Zhang, Res. Chem. Intermed. 42, 3979 (2016)
W. Ma, S. Chen, S. Yang, W. Chen, W. Weng, Y. Cheng, M. Zhu, Carbon 113, 151 (2017)
X. Yan, K. Gan, B. Tian, J. Zhang, L. Wang, D. Lu, Res. Chem. Intermed. 44, 1 (2018)
N. Li, K. Gan, D. Lu, J. Zhang, L. Wang, Res. Chem. Intermed. 44, 1105 (2018)
M. Li, Z. Mei, J. Yu, H. Cheng, Q. Li, Small 13, 1602994 (2017)
L. Bao, T. Li, S. Chen, C. Peng, L. Li, Q. Xu, Y. Chen, E. Ou, W. Xu, Small 13, 1602077 (2017)
Z. Wu, Y. Zhu, X. Ji, J. Mater. Chem. A 2, 14759 (2014)
Y. Johnbosco, V. Elumalai, M. Bhagavathiachari, A.S. Samuel, E. Elaiyappillai, P.M. Johnson, J. Electroanal. Chem. 797, 78 (2017)
Z. Ai, Z. Hu, Y. Liu, M. Yao, ChemPlusChem 81, 322 (2016)
J. Shen, J. Wu, L. Pei, M.T.F. Rodrigues, Z.Q. Zhang, F. Zhang, X. Zhang, P.M. Ajayan, M. Ye, Adv. Energy Mater. 6, 314 (2016)
J. Zhao, Z. Li, M. Zhang, A. Meng, Q. Li, J. Power Sour. 332, 355 (2016)
J. Shen, X. Xu, P. Dong, Z. Zhang, R. Baines, J. Ji, Y. Pei, M. Ye, Ceram. Int. 42, 8120 (2016)
S. Liu, J. Xie, C. Fang, G. Cao, T. Zhu, X. Zhao, J. Mater. Chem. 22, 19738 (2012)
J. Tang, J. Shen, N. Li, M. Ye, Ceram. Int. 41, 6203 (2015)
W.S.H. Jr, R.E. Offeman, J. Am. Chem. Soc. 80, 1339 (1958)
M. Liu, X. Ma, L. Gan, J. Mater. Chem. A 2, 17107 (2014)
L. Miao, D. Zhu, Y. Zhao, Micropor. Mesopor. Mat. 253, 1 (2017)
K. Kailasam, A. Fischer, G. Zhang, J. Zhang, M. Schwarze, M. Schröder, X. Wang, R. Schomäcker, A. Thomas, Chemsuschem 8, 1404 (2015)
X. Wang, Y. Lin, Y. Su, B. Zhang, C. Li, H. Wang, L. Wang, Electrochim. Acta 225, 263 (2017)
M. Qorbani, N. Naseri, A.Z. Moshfegh, A.C.S. Appl, Mater. Inter. 7, 11172 (1944)
C. Yuan, J. Li, L. Hou, X. Zhang, L. Shen, X.W. Lou, Adv. Funct. Mater. 22, 4592 (2012)
J. Pu, T. Wang, H. Wang, Y. Tong, C. Lu, W. Kong, Z. Wang, ChemPlusChem 79, 577 (2014)
L. Mei, T. Yang, C. Xu, Nano Energy 3, 36 (2014)
J. Yang, C. Yu, X. Fan, S. Liang, S. Li, H. Huang, Z. Ling, C. Hao, J. Qiu, Energ. Environ. Sci. 9, 1299 (2016)
L.G. Beka, X. Li, X. Xia, W. Liu, Diam. Relat. Mater. 73, 169 (2017)
H. Chen, Y. Ai, F. Liu, X. Chang, Y. Xue, Q. Huang, C. Wang, H. Lin, S. Han, Electrochim. Acta 213, 55 (2016)
S. Zhang, D. Li, S. Chen, X. Yang, X. Zhao, Q. Zhao, S. Komarneni, D. Yang, J. Mater. Chem. A 5, 12453 (2017)
B. Liu, B. Liu, Q. Wang, X. Wang, Q. Xiang, D. Chen, G. Shen, A.C.S. Appl, Mater. Inter. 5, 10011 (2013)
G. Zhang, X.W. Lou, Adv. Mater. 25, 976 (2013)
R.B. Rakhi, W. Chen, D. Cha, H.N. Alshareef, Nano Lett. 12, 2559 (2012)
Acknowledgements
This work was supported from the National Natural Science Foundation of China (Project Numbers 51641208 and 21606151), Shanghai Association for Science and Technology Achievements Transformation Alliance Program (Project Numbers LM201680 and LM201559), Shanghai Excellent Technology Leaders Program (Project Number 17XD1424900), Shanghai Leading Talent Program (Project Number 017), Science and Technology Commission of Shanghai Municipality Project (Project Numbers 14520503200 and ZX2016-7). Collaborative Innovation Fund of SIT, XTCX2015-9.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lin, J., Yan, S., Liu, P. et al. Facile synthesis of CoNi2S4/graphene nanocomposites as a high-performance electrode for supercapacitors. Res Chem Intermed 44, 4503–4518 (2018). https://doi.org/10.1007/s11164-018-3400-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3400-6