Abstract
A series of mono- and di-cationic pyridinium salts with one or two promesogenic cyanobiphenyl groups and different counterions, bromide, dodecylsulfate or dioctyl sulfosuccinate, were designed and prepared while their thermal properties were investigated by means of differential scanning calorimetry (DSC) and thermogravimetric analysis. The monocationic bromide pyridinium salt 3 tethered with only one cyanobiphenyl group behaves as an ionic liquid crystal displaying monotropic nematic and smectic phases at low temperatures, evidenced by polarizing optical microscopy observations and DSC analysis. Replacement of the bromide ion with dodecylsulfate or DOSS− (dioctyl sulfosuccinate) ions gave pyridinium salts with no mesogenic properties. Monocationic pyridinium salt with a DOSS− ion behaves as an ionic liquid with glass transition near − 16 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ionic liquids (IL), salts with melting points below 100 °C, have been the subject of tremendous research over the last 15 years due to their remarkable potential for various applications, mainly because of their environmental friendly properties such as low volatility and nonflammability as well as other interesting properties like high-ionic conductivity [1–6]. The properties of these materials can be tuned by structural modifications of the anion or the cationic core, or by the changing the substituents of the anion or cation. Ionic liquids based on imidazolium or pyridinium salts are by far the most common salts investigated for their peculiar properties and for their liquid crystalline properties (LC). On the other hand, ionic liquid crystals (ILC) represent a special class of materials with unique properties resulting from the combination of LC and ILC properties. Several reviews covering this topic have recently been published [7–9]. The nature of ILC phases is influenced by a series of factors, such as the molecular shape, location and size of ionic groups, intermolecular interactions and microphase segregation. For instance, electrostatic interactions and ion–ion stacking in ILC give rise to a strong tendency to stabilize the lamellar phases. Moreover, depending on the combination of these factors, the LC properties range from typical calamitic materials to discotic materials. ILCs based on pyridinium salts have been known for long time, displaying very similar properties to the related imidazolium-based ILCs [10, 11].
Generally, dicationic ILs possess a higher melting point, wider liquid range, and better thermal stability compared to conventional monocationic ILs and, more recently, the same trend for the bis(cationic) ILCs has been proven [12–15]. Furthermore, LC dimers are well known to exhibit interesting mesomorphic behavior that can be, in many cases, different from the single mesogens [16, 17]. There are many examples of bis(pyridinium) salts with flexible spacers that have been employed as ionic liquids with various purposes (surfactants [18–23], catalytic [24, 25], lubricants [26], and biological [27–31]). Bis(pyridinium) salts with flexible spacers derived from 4-hydroxypyridine containing mesogenic 3,4,5-tris(alkyloxy)benzyl moieties (alkyl = dodecyl or tetradecyl) on each side and various counterions, such as bromide (Br−), hexafluorophosphate (PF6 −), tetrafluoroborate (BF4 −), and triflate (OTf−), have been reported recently [13]. While there are numerous examples of dicationic pyridinium ILC, these bis(pyridinium) salts represent the first example of this kind. However, it has to be noted that, compared to dimeric imidazolium dicationic ILs, bis(pyridinium) salts have been less commonly reported. In this work, we report the design, and a systematic investigation of the thermal properties, of a series of mono- and di-cationic pyridinium salts with promesogenic cyanobiphenyl groups and different counterions: bromide (Br−), dodecylsulfate (C12H25OSO3 −) or dioctyl sulfosuccinate (DOSS−). Previous reports have shown that the use of alkyl sulfate anions can lead to LC compounds with lower melting and clearing points [32–42]. Starting from these premises, a screening of the effect of anions on thermal properties was performed by replacing the bromide anion Br− with dodecylsulfate (C12H25OSO3 −), and dioctyl sulfosuccinate DOSS− ions. The thermal properties of the new compounds are discussed according to information provided by polarized optical microscopy (POM), differential scanning calorimetry (DSC) and thermogravimetric analysis (TG).
Experimental
Characterization methods
All the chemicals were used as supplied. The 4-pyridone intermediates 2a–c were prepared according to methods published earlier [43, 44]. C, H, and N analyses were carried out with an EuroEA 3300 instrument. IR spectra were recorded on a Bruker spectrophotometer using KBr discs or by using a Jasco FTIR 4200 spectrophotometer coupled to an ATR PIKE GladiATR device. 1H and 13C NMR spectra were recorded on a Bruker spectrometer operating at 500 MHz, using CDCl3 as solvent. 1H chemical shifts were referenced to the solvent peak position, δ 7.26 ppm. The phase assignments for all pyridinium salts were evaluated by POM, placed on untreated glass slides, using a Nikon 50iPol microscope equipped with a Linkam THMS600 hot stage and TMS94 control processor. Temperatures and enthalpies of transitions were recorded by using the DSC technique employing a Diamond DSC Perkin Elmer instrument. The mono- and di-cationic pyridinium salts were studied at 10°/min scanning rate after being encapsulated in aluminium pans. Three heating/cooling cycles were performed on each sample. TG analysis for selected samples was performed on a TA Q50 TGA instrument using alumina crucibles and nitrogen as purging gas. The heating rate employed was 10 °C min−1 from room temperature to 550 °C.
Synthesis of mono- and di-cationic pyridinium salts
Synthesis of compound 3
Compound 2b (0.2 g, 0.48 mmol) was dissolved in acetonitrile (40 ml). To this solution, 1-bromononane (0.29 g, 0.72 mmol) was added, and the reaction mixture was heated under reflux and in nitrogen atmosphere overnight. Then, the solution was concentrated via rotary evaporation to about half of its initial volume and ethyl ether (45 ml) was added to produce a white solid. The crude product was recrystallized twice from a mixture of dichloromethane/ethyl ether (1/2, v/v) and further dried in vacuum. Yield 35%, white solid. Anal. Calcd. for C36H49BrN2O2: C% 69.55, H% 7.94, N% 4.51. Found: C% 69.87, H% 8.21, N% 4.35.
1H-NMR (500 MHz, CDCl3, ppm): 9.15 (d, J = 7.0 Hz, 2H), 7.64 (m, 4H), 7.51 (d, J = 8.5 Hz, 2H), 7.44 (d, J = 8.8 Hz, 2H), 6.97 (d, J = 8.5 Hz, 2H), 4.73 (t, J = 7.5 Hz, 2H), 4.27 (t, J = 6.4 Hz, 2H), 3.97 (t, J = 6.4 Hz, 2H), 2.08–1.66 (m, 6H), 1.52–1.12 (m, 22H), 0.86 (t, J = 6.8 Hz, 3H).
13C-NMR (125 MHz, CDCl3, ppm): 170.26, 159.72, 146.22, 145.22, 132.51, 131.18, 128.27, 127.01, 119.07, 115.05, 113.87, 109.94, 71.58, 68.04, 60.09, 31.75, 31.52, 29.19, 28.94, 28.37, 25.94, 25.60, 22.58, 14.04.
IR (ATR, cm−1): 2920, 2851, 2224, 1642, 1600, 1523, 1496, 1465, 1327, 1314, 1289, 1248, 1216, 1179, 1046, 963, 861, 828, 726, 661, 601, 532.
Synthesis of compound 4
Compound 2c (1 g, 2.3 mmol) was dissolved in acetonitrile (50 ml). To this solution, 1,10-dibromodecane (0.23 g, 0.77 mmol) was added, and the reaction mixture was heated under reflux and in nitrogen atmosphere for 48 h. Then, the solvent was removed via rotary evaporation and the residue was purified by column chromatography on silica by using the CH2Cl2/CH3OH 9/1 v/v mixture as eluant. Yield 67%, white solid. Anal. Calcd. for C66H84Br2N4O4: C% 68.50, H% 7.32, N% 4.84. Found: C% 68.15, H% 7.67, N% 4.62.
1H-NMR (500 MHz, CDCl3, ppm): 9.15 (4H, d br); 7.63 (8H, m); 7.51–7.49 (8H, m); 6.95 (4H, m); 4.65 (4H, t, J = 7.4 Hz); 4.28 (4H, t, J = 6.4 Hz); 3.96 (4H, t, J = 6.5 Hz); 2.10–1.65 (12H, m); 1.51–1.12 (36H, m).
13C-NMR (125 MHz, CDCl3, ppm): 170.22; 159.73; 146.29; 145.19; 132.51; 131.10; 128.26; 127.00; 119.09; 115.05; 114.12; 109.87; 71.45; 68.07; 60.06; 31.49; 29.33; 29.22; 29.12; 28.99; 28.90; 28.65; 28.18; 26.02; 25.91; 25.38.
IR (ATR, cm−1): 3026, 2928, 2852, 2222, 1644, 1595, 1522, 1491, 1462, 1314, 1247, 1216, 1180, 943, 824, 727, 535.
Synthesis of compound 5
A solution of sodium dodecylsulfate (86 mg, 0.3 mmol) in methanol (20 ml) was added dropwise to a solution of compound 4 (116 mg, 0.1 mmol) in dichloromethane (10 ml). The mixture was stirred at room temperature for 1 h after which 50 ml of deionised water was added. The organic layer was separated and washed repeatedly with water until no reaction with silver nitrate for Br− was noticed. The organic phase was dried over sodium sulfate followed by solvent removal with a rotary evaporator. The product was recrystallized twice from a mixture of dichloromethane and ethyl ether to yield an off-white waxy solid. Yield 71%, white solid. Anal. Calcd. for C90H134N4O12S2: C% 70.74, H% 8.84, N% 3.67. Found: C% 70.31, H% 9.15, N% 3.51.
1H-NMR (500 MHz, CDCl3, ppm): 8.82 (4H, d br); 7.67 (8H, m); 7.51 (4H, d, J = 8.5 Hz); 7.44 (4H, d, J = 6.9 Hz); 6.98 (4H, d, J = 8.6 Hz); 4.48 (4H, t, J = 7.4 Hz); 4.27 (4H, t, J = 6.3 Hz); 4.03 (4H, t, J = 6.7 Hz); 3.99 (4H, t, J = 6.7 Hz); 2.04–1.72 (16H, m); 1.66 (4H, m); 1.53–1.13 (68H, m); 0.86 (6H, t, J = 6.8 Hz).
13C-NMR (125 MHz, CDCl3, ppm): 170.31, 159.77, 146.20, 145.25, 132.54, 131.21, 128.29, 127.04, 119.10, 115.07, 114.08, 109.98, 71.27, 68.10, 67.66, 60.22, 53.41, 31.90, 31.36, 29.66, 29.55, 29.09, 29.02, 28.65, 28.42, 28.14, 26.21, 25.86, 25.29, 22.67, 14.11.
IR (ATR, cm−1): 3050, 2922, 2850, 2227, 1642, 1604, 1523, 1497, 1467, 1315, 1244, 1220, 1183, 1040, 982, 826, 797, 581, 535.
Synthesis of compound 7
A solution of sodium dodecylsulfate (86 mg, 0.3 mmol) in methanol (20 ml) was added dropwise to a solution of compound 6 (146 mg, 0.2 mmol) in dichloromethane (10 ml). The mixture was stirred at room temperature for 1 h after which 50 ml of deionised water was added. The organic layer was separated and washed repeatedly with water until no reaction with silver nitrate for Br− was noticed. The organic phase was dried over sodium sulfate followed by solvent removal with a rotary evaporator. The product was recrystallized twice from a mixture of dichloromethane and ethyl ether to yield an off-white waxy solid. Yield 55%, white solid. Anal. Calcd. for C55H69N3O7S: C% 72.10, H% 7.59, N% 4.59. Found: C% 71.82, H% 7.93, N% 4.42.
1H-NMR (500 MHz, CDCl3, ppm): 8.86 (d, J = 7.0 Hz, 2H), 7.72–7.57 (m, 8H), 7.50 (t, J = 8.4 Hz, 4H), 7.40 (d, J = 7.0 Hz, 2H), 7.06–6.80 (m, 4H), 4.55 (t, J = 7.3 Hz, 2H), 4.27 (t, J = 6.3 Hz, 2H), 4.10–3.89 (m, 6H), 2.11–1.60 (m, 10H), 1.60–1.48 (m, 6H), 1.46–1.12 (m, 20H), 0.85 (t, J = 6.9 Hz, 3H).
13C-NMR (125 MHz, CDCl3, ppm): 170.22, 159.57, 146.24, 145.11, 132.51, 131.28, 128.28, 126.99, 119.04, 115.01, 113.95, 109.98, 71.24, 67.67, 60.00, 53.40, 31.85, 31.24, 29.61, 29.35, 29.00, 28.83, 28.35, 25.91, 25.66, 25.43, 22.62, 14.06.
IR (ATR, cm−1): 3049, 2923, 2850, 2228, 1640, 1603, 1518, 1495, 1467, 1322, 1293, 1247, 1224, 1179, 1057, 982, 857, 823, 774, 618, 577, 531.
Synthesis of compound 8
A solution of sodium dioctyl sulfosuccinate (133 mg, 0.3 mmol) in methanol (20 ml) was added dropwise to a solution of compound 6 (146 mg, 0.2 mmol) in dichloromethane (10 ml). The mixture was stirred at room temperature for 1 h after which 50 ml of deionised water was added. The organic layer was separated and washed repeatedly with water until no reaction with silver nitrate for Br− was noticed. The organic phase was dried over sodium sulfate followed by solvent removal with a rotary evaporator to yield a yellowish viscous liquid. Yield 69%, yellow liquid. Anal. Calcd. for C63H81N3O10S: C% 70.56, H% 7.61, N% 3.92. Found: C% 70.22, H% 8.05, N% 3.73.
1H-NMR (500 MHz, CDCl3, ppm): 8.80 (d, J = 6.9 Hz, 2H), 7.66 (m, 8H), 7.52 (m, 4H), 7.41 (d, J = 6.9 Hz, 2H), 6.98 (t, J = 8.4 Hz, 4H), 4.53 (t, J = 7.3 Hz, 2H), 4.29 (t, J = 6.3 Hz, 2H), 4.17 (dd, J = 11.6, 3.4 Hz, 1H), 4.12–3.87 (m, 8H), 3.32–3.26 (m, 1H), 3.15 (m, 1H), 2.05–1.73 (m, 12H), 1.69–1.49 (m, 6H), 1.47–1.17 (m, 16H), 0.86 (m, 12H).
13C-NMR (125 MHz, CDCl3, ppm): 171.68, 170.29, 169.24, 159.61, 146.21, 145.18, 132.56, 131.38, 128.34, 127.05, 119.07, 115.05, 114.03, 110.07, 71.35, 67.89, 67.53, 67.06, 61.99, 60.11, 38.59, 34.30, 31.26, 30.18, 29.05, 28.86, 28.40, 25.72, 25.48, 23.63, 23.42, 22.95, 14.06, 10.88.
IR (ATR, cm−1): 3055, 2928, 2860, 2225, 1731, 1644, 1601, 1520, 1494, 1466, 1291, 1241, 1179, 1035, 997, 822, 661, 532.
Results and discussion
The synthesis of new mono- and di-cationic pyridinium salts with Br−, C12H25OSO3 − and DOSS−, anions along with their numbering scheme is presented in Scheme 1. In the first step, the reaction of 4-hydroxypyridine with 4′-(ω-bromoalkyloxy)-4-cyanobiphenyl 1a–c in THF, in the presence of NaOH and tetrabutylammonium bromide (TBABr) as phase transfer catalyst, produced the 4-pyridone derivative 2a–c as the main product (Scheme 1).
The conditions of the reaction and the whole synthetic procedure of these intermediates were described by us in a recent report [43, 44]. In the following step, the bromide mono- and di-cationic pyridinium salts were relatively easily prepared by reacting the 4-pyridone intermediates 2a–c with different bromide derivatives in acetonitrile under reflux: 1-bromononane to yield 3, 1,10-dibromodecane to give the dicationic salt 4 and the 4′-(6-bromohexyloxy)-4-cyanobiphenyl for 6 (Scheme 1). The monocationic salts 3 and 6 were crystallized from a mixture of dichloromethane and ethyl ether while the dicationic salt 4 was purified on silica by using a mixture of dichloromethane and methanol 9/1, v/v, as eluent. All these salts were isolated as white solids in relatively good yields. Furthermore, the metathesis reaction of the bromide anion with C12H25OSO3Na and DOSSNa produced the corresponding salts 5, 7 and 8. The final products were characterized by elemental analysis (C, H, N), IR, 1H- and 13C-NMR spectroscopy, supporting the proposed structure.
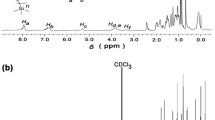
The metathesis reaction of the bromide anion with dodecylsulfate and dioctyl sulfosuccinate ions was unambigously confirmed by IR spectroscopy. These new anions produce characteristic strong frequencies found in the typical wavenumber ranges for such groups in the IR spectra of the new pyridinium salts (1220–1224 cm−1 for dodecysulfate and 1730, 1214 and 1035 cm−1 for DOSS−, respectively). A comparison between the IR spectrum of bromide salt 4 and its related salt with the dodecylsulfate ion 5 is presented in Fig. 1a, while the differences between the IR spectrum of the bromide salt 6 and its related salt with the dioctyl sulfosuccinate ion 8 can be seen in Fig. 1b. The exchange of bromide ion for all the pyridinium salts was supported by 1H- and 13C- NMR spectroscopy data. The chemical shifts of the two protons located in the vicinity of nitrogen atom of the pyridinium ring were the most affected by the anion exchange. While for the bromide salts 4 and 6 these signals were found at 9.15 ppm, upfield shifts were observed for both dodecylsulfate and dioctyl sulfosuccinate anions (up to 8.82 and 8.86 ppm for 5 and 7, respectively, X − = C12H25OSO3 −, and to 8.80 ppm for 8, X = DOSS−). The effect of counterion exchange on the chemical shifts of pyridinium doublets in the 1H-NMR spectra of compounds 4 and 5 is presented in Fig. 2. The NMR chemical shifts are significantly influenced by the anion–cation interaction specific to ILCs due to the presence of hydrogen-bonding interactions [45, 46].
These hydrogen-bonding interactions produced a downfield chemical shift of the related H atoms for pyridinium-based ILC, and as a consequence much stronger interactions with anions resulted in further downfield chemical shifts [47–50]. Moreover, the 1H-NMR data found for the mono- and di-cationic pyridinium salts investigated in this study are in good agreement with previous results reported for other pyridinium salts [10, 43, 51].
Thermal behavior
The thermal behavior of new mono- and di-cationic pyridinium salts was investigated by differential scanning calorimetry combined with thermogravimetrical analysis. The thermal parameters are summarized in Table 1 together with the enthalpy associated with these processes. Monocationic pyridinium bromide salt 3 with only one cyanobiphenyl group shows monotropic nematic and smectic LC phases on the cooling run from the isotropic state. It is important to mention here that the nematic phase is quite rare in the case of ILCs; several examples have been reported so far for ammonium [52, 53], imidazolium [54–56], pyridinium-based ILCs [57], or miscellaneous types of ILCs [58–62]. The nematic phase for such systems can be seen rather as an exception. The smectic phases are the most common phases for ILCs, especially due to electrostatic interactions that exist in ILCs.
The POM observations made for compound 3 on cooling the isotropic phase to the transition temperature to mesophase revealed regions with a marbled texture or thread-like texture that flashed brightly under mechanical disturbance. Some regions with poorly developed Schlieren texture have also been seen (Fig. 3). By shearing the sample, homeotropic dark areas could be noticed. A very fine change of texture was seen by POM around 33 °C, and, in conjunction with the information provided by DSC, this transition was assigned to a N–Sm phase change. Unfortunately, the narrow temperature domain of LC phases together with their monotropic character prevented additional X-ray diffractions measurements to be performed. A definite phase assignment could not be properly made. However, the POM observations together with the DSC data are good premises for such phase assignment taking into account that the related pyridinium salts with two cyanobiphenyl groups on each side showed a nematic phase.
The texture of compound 3 was preserved at a lower temperatures due to a glass transition seen around 19 °C in the DSC. On the following heating run, after partial clearing seen at 36 °C, a cold crystallization process to another crystalline phase was noticed followed by a transition between two crystalline phases around 47 °C (Fig. 4a).
The following heating–cooling cycle for 3 reproduced the same transitions with the same values for the transition temperatures.
Considering the structural features of compound 3 and of nematic pyridinium salts tethered with two cyanobiphenyl groups (compound 6 and its related analogues with spacers having nine or ten methylene groups) reported elsewhere [43], it would be reasonable to expect compound 4 to exhibit mesogenic properties. In fact, the latter compound could be regarded as a dimeric analogue of bromide pyridinium salt 3. Unexpectedly, compound 4 shows no LC properties. Only one transition was seen during the first heating run corresponding to melting from the crystalline state into the isotropic state. Cooling the isotropic liquid back to room temperature resulted in a glass transition around 27 °C. Subsequent heating–cooling cycles displayed only the glass transitions with no sign of crystallization, meaning that this salt behaves as an ionic liquid.
A drop of melting temperature of ca. 30 °C was observed for compound 5, when the bromide ion was exchanged with the dodecylsulfate ion. The dicationic salt 5 shows one transition during the first heating run at 120 °C assigned to melting to the isotropic state and one transition during the first cooling run at 21 °C corresponding to crystallization to a second crystalline phase (Cr′). This latter crystalline phase (Cr′) is not thermodynamically stable at higher temperatures and goes to the first crystalline state (Cr) at temperatures above 30 °C during the second heating run (Fig. 4b).
Replacement of the bromide ion with the dodecylsulfate ion has the same effect on the thermal behavior of the related salts 6 and 7. The transition to the isotropic state occurred at 144 °C for 6 and at 120 °C for 7. While compound 6 shows a strongly monotropic nematic phase, the related salt with dodecylsulfate ion 7 has no LC properties. The first DSC heating run for compound 7 shows only one transition corresponding to the melting process. The liquid state was preserved on cooling to 9 °C when a glass transition was noticed. This phase was not stable during the heating run and the cold crystallization process to a second crystalline phase occurred around 50 °C (Fig. 4c). The transition from the second crystalline phase to the first crystalline phase could be seen at 103 °C during the second heating run. The subsequent DSC heating–cooling runs were perfectly reproducible, preserving the number of transitions and their corresponding temperatures.
The related salt 8 with the dioctyl sulfosuccinate ion was isolated as a liquid at room temperature. A glass transition was evidenced at − 16 °C which is perfectly reversible. No further signs of crystallization were seen on cooling. Generally, the presence of additional alkyl chains contained by anions should bring the fluidity necessary to allow the formation of LC phases. In this case, it seems that the dioctyl sulfosuccinate ion has a large contribution to the disruption of packing forces, leading to the absence of mesogenic properties and the formation of an ionic liquid.
The thermal stability of these mono- and di-cationic pyridinium salts was analyzed in the 25–550 °C range, and the TG curves for the selected samples are shown in Fig. 5. The values for T onset corresponding to the decomposition stages are shown in Table 1. All the pyridinium salts mainly show a single decomposition step. A higher thermal stability was evidenced for pyridinium salts with either dodecylsulfate or dioctyl sulfosuccinate ions, with a decomposition temperature around 250 °C, compared to their related bromide-containing pyridinium salts. The latter salts have T onset values below 200 °C. The thermal behavior of these pyridinium salts resembles the one displayed by the pyridinium salts related to the monocationic salt 6, when replacement of bromide ion with bulkier anions such as hexafluorphosphate (PF6 −) or tetrafluoroborate (BF4 −) led to higher decomposition temperatures. The imidazolium or pyridinium derivatives with weakly coordinating anions, such as tetrafluoroborate and hexafluorophosphate, are well known for their high thermal stabilitiy [43, 51, 63, 64].
Conclusions
A series of mono- and di-cationic pyridinium salts with promesogenic cyanobiphenyl groups and different counterions, bromide, dodecylsulfate or dioctyl sulfosuccinate, were designed and prepared while their thermal properties were investigated by means of DSC and TG. The monocationic pyridinium salt 3 tethered with only one cyanobiphenyl group behaves as an ionic liquid crystal displaying monotropic nematic and smectic phases at low temperatures, evidenced by POM and DSC analysis. Surprisingly, the dicationic pyridinium salts 4 and 5 that could be seen as the dimeric analogues of 3 do not show LC properties, no matter the counterion employed, either bromide or dodecylsulfate ions. On the other hand, the monocationic pyridinium salt 8 with the dioctylsulfosuccinate ion behaves as an ionic liquid at room temperature with a corresponding glass transition at − 16 °C. The effect of bromide ion replacement with dodecylsulfate or dioctyl sulfosuccinate ions resulted in lower melting temperatures, ca. 40 °C for the dodecylsulfate ion and around 160 °C for the dioctyl sulfosuccinate ion. Pyridinium salts with dodecylsulfate and dioctyl sulfosuccinate ions proved to be more thermally stable than their bromide-related pyridinium salts. The decomposition step of the latter salts start at temperatures below 200 °C, while the decomposition of related dodecylsulfate and DOSS pyridinium salts begins at higher temperatures, near 250 °C.
References
W. Qian, J. Texter, F. Yan, Chem. Soc. Rev. 46, 1124 (2017)
A.N. Masri, M.I. Abdul Mutalib, J.M. Leveque, Ind. Eng. Manag. 5, 197 (2016)
R. Ratti, Adv. Chem. 2014, 16 (2014)
J.P. Hallett, T. Welton, Chem. Rev. 111, 3508 (2011)
R.D. Rogers, K.R. Seddon, Science 302, 792 (2003)
F. D’Anna, R. Noto, Eur. J. Org. Chem. 2014, 4201 (2014)
K. Goossens, K. Lava, C.W. Bielawski, K. Binnemans, Chem. Rev. 116, 4643 (2016)
K.V. Axenov, S. Laschat, Materials 4, 206 (2011)
V. Cîrcu, Ionic liquid crystals based on pyridinium salts, in Progress and Developments in Ionic Liquids, ed. by S. Handy (InTech, New York, 2017). https://doi.org/10.5772/65757
G. Kohnen, M. Tosoni, S. Tussetschlager, A. Baro, S. Laschat, Eur. J. Org. Chem. 2009, 5601 (2009)
G.A. Knight, B.D. Shaw, J. Chem. Soc. 141, 682 (1938)
Y. Gao, J.M. Slattery, D.W. Bruce, New J. Chem. 35, 2910 (2011)
A. Pană, M. Iliş, T. Staicu, I. Pasuk, V. Cîrcu, Liq. Cryst. 43, 381 (2016)
A. Pană, M. Ilis, M. Micutz, F. Dumitrascu, I. Pasuk, V. Cîrcu, RSC Adv. 4, 59491 (2014)
J.L. Anderson, R.F. Ding, A. Ellern, D.W. Armstrong, J. Am. Chem. Soc. 127, 593 (2005)
C.T. Imrie, P.A. Henderson, Curr. Opin. Colloid Interface Sci. 7, 298 (2002)
C.T. Imrie, P.A. Henderson, Chem. Soc. Rev. 36, 2096 (2007)
P. Patial, A. Shaheen, I. Ahmad, J. Surfactants Deterg. 17, 929 (2014)
P. Quagliotto, G. Viscardi, C. Barolo, E. Barni, S. Bellinvia, E. Fisicaro, C. Compari, J. Org. Chem. 68, 7651 (2003)
V. Chauhan, S. Singh, R. Kamboj, R. Mishra, G. Kaur, J. Colloid Interface Sci. 417, 385 (2014)
A. Bhadani, H. Kataria, S. Singh, J. Colloid Interface Sci. 361, 33 (2011)
L. Zhou, X. Jiang, Y. Li, Z. Chen, X. Hu, Langmuir 23, 11404 (2007)
V. Chauhan, S. Singh, T. Kaur, G. Kaur, Langmuir 31, 2956 (2015)
H.F. Liu, F.X. Zeng, L. Deng, B. Liao, H. Pang, Q.X. Guo, Green Chem. 15, 81 (2013)
A. Chinnappan, H. Kim, RSC Adv. 3, 3399 (2013)
M. Mahrova, F. Pagano, V. Pejakovic, A. Valea, M. Kalin, A. Igartua, E. Tojo, Tribol. Int. 82(A), 245 (2015)
T. Yabuhara, T. Maeda, H. Nagamune, H. Kourai, Biocontrol Sci. 9, 95 (2004)
H. Kourai, T. Yabuhara, A. Shirai, T. Maeda, H. Nagamune, Eur. J. Med. Chem. 41, 437 (2006)
A. Shirai, T. Sumitomo, M. Kurimoto, H. Maseda, H. Kourai, Biocontrol Sci. 14, 13 (2009)
A. Shirai, S. Ueta, H. Maseda, H. Kourai, T. Omasa, Biocontrol Sci. 17, 77 (2012)
A. Bhadani, S. Singh, Langmuir 25, 11703 (2009)
D.W. Bruce, S. Estdale, D. Guillon, B. Heinrich, Liq. Cryst. 19, 301 (1995)
C. Cruz, B. Heinrich, A.C. Ribeiro, D.W. Bruce, D. Guillon, Liq. Cryst. 27, 1625 (2000)
K. Lava, Y. Evrard, K. Van Hecke, L. Van Meervelta, K. Binnemans, RSC Adv. 2, 8061 (2012)
T. Cardinaels, K. Lava, K. Goossens, S.V. Eliseeva, K. Binnemans, Langmuir 27, 2036 (2011)
D. Haristoy, D. Tsiourvas, Liq. Cryst. 31, 697 (2004)
T. Mihelj, J. Popovic, Ž. Skoko, V. Tomašic, Thermochim. Acta 591, 119 (2014)
A. Nikokavoura, D. Tsiourvas, M. Arkas, Z. Sideratou, C.M. Paleos, Liq. Cryst. 29, 1547 (2002)
E. Rettenmeier, A. Tokarev, C. Blanc, P. Dieudonné, Y. Guari, P. Hesemann, J. Colloid Interface Sci. 356, 639 (2011)
Y. Wang, E.F. Marques, J. Phys. Chem. B 110, 1151 (2006)
Y. Wang, E.F. Marques, J. Therm. Anal. Calorim. 100, 501 (2010)
E. Westphal, D. Henrique da Silva, F. Molin, H. Gallardo, RSC Adv. 3, 6442 (2013)
A. Pană, I. Pasuk, M. Micutz, V. Cîrcu, CrystEngComm 18, 5066 (2016)
A. Pană, F.L. Chiriac, M. Secu, I. Pasuk, M. Ferbinteanu, M. Micutz, V. Cîrcu, Dalton Trans. 44, 14196 (2015)
P.A. Hunt, C.R. Ashworth, R.P. Matthews, Chem. Soc. Rev. 44, 1257 (2015)
K. Dong, S. Zhang, J. Wang, Chem. Commun. 52, 6744 (2016)
S. Men, D.S. Mitchell, K.R.J. Lovelock, P. Licence, ChemPhysChem 16, 2211 (2015)
A. Wulf, K. Fumino, D. Michalik, R. Ludwig, ChemPhysChem 8, 2265 (2007)
H. He, H. Chen, Y. Zheng, S. Zhang, Z. Yu, Chem. Eng. Sci. 121, 169 (2015)
S. Chen, R. Vijayaraghavan, D.R. MacFarlane, E.I. Izgorodina, J. Phys. Chem. B 117, 3186 (2013)
A. Pana, F.L. Badea, M. Ilis, T. Staicu, M. Micutz, I. Pasuk, V. Cîrcu, J. Mol. Struct. 1083, 245 (2015)
W. Li, J. Zhang, B. Li, M. Zhang, L. Wu, Chem. Commun. 35, 5269 (2009)
L. Lu, N. Sharma, G.A.N. Gowda, C.L. Khetrapal, R.G. Weiss, Liq. Cryst. 22, 23 (1997)
K. Goossens, P. Nockemann, K. Driesen, B. Goderis, C. Görller-Walrand, K. Van Hecke, L. Van Meervelt, E. Pouzet, K. Binnemans, T. Cardinaels, Chem. Mater. 20, 157 (2008)
S. Ahn, S. Yamakawa, K. Akagi, J. Mater. Chem. C 3, 3960 (2015)
X.H. Cheng, X.Q. Bai, S. Jing, H. Ebert, M. Prehm, Chem. Eur. J. 16, 4588 (2010)
X. Liu, J.L. Liu, B. Cai, X.M. Ren, Inorg. Chem. Commun. 14, 1428 (2011)
M. Ghedini, D. Pucci, J. Organomet. Chem. 395, 105 (1990)
A. Liebmann, C. Mertesdorf, T. Plesnivy, H. Ringsdorf, J.H. Wendorff, Angew. Chem. Int. Ed. Engl. 30, 1375 (1991)
J.W. Goodby, G.H. Mehl, I.M. Saez, R.P. Tuffin, G. Mackenzie, R. Auzely-Velty, T. Benvegnu, D. Plusquellec, Chem. Commun. 19, 2057 (1998)
B. Ringstrand, A. Jankowiak, L.E. Johnson, P. Kaszynski, D. Pociecha, E. Gorecka, J. Mater. Chem. 22, 4874 (2012)
A. Jankowiak, J. Kanazawa, P. Kaszynski, R. Takita, M. Uchiyama, J. Organomet. Chem. 747, 195 (2013)
C.M. Gordon, J.D. Holbrey, A.R. Kennedy, K.R. Seddon, J. Mater. Chem. 8, 2627 (1998)
G.H. Min, T. Yim, H.Y. Lee, D.H. Huh, E. Lee, J. Mun, S.M. Oh, Y.G. Kim, Bull. Korean Chem. Soc. 27, 847 (2006)
Acknowledgement
This work was supported by a grant of the Romanian Authority for Scientific Research, CNCS-UEFISCDI, Project Number PN-II-ID-PCE-2011-3-0384.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pană, A., Panait, A.L. & Cîrcu, V. Simple and double pyridinium salts with cyanobiphenyl groups as ionic liquids and ionic liquid crystals: synthesis and investigation of thermal behavior. Res Chem Intermed 44, 2025–2038 (2018). https://doi.org/10.1007/s11164-017-3212-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3212-0