Abstract
Carboxyl-functionalized poly(ionic liquid) (CFPIL) was easily synthesized from its starting material and characterized by Fourier transform infrared spectroscopy, thermal gravimetric analysis and elemental analysis. The synthesized CFPIL was used as an efficient heterogeneous catalyst for the synthesis of β-amino ketones by three-component one-pot Mannich reaction between various aromatic aldehydes with amines and acetophenone at room temperature under solvent-free condition. The effect of the amount of catalyst and reaction time were investigated. The results showed that CFPIL has good to moderate catalytic activity and reusability. The catalytic process was simple and the catalyst could be easily recovered by filtration and washed with ethanol to reuse for the next experiment without significant loss of catalytic activity.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mannich reaction is one of the most significant C–C bond=forming reactions in organic transformation for the synthesis of secondary and tertiary amine derivatives. These β-amino carbonyl compounds are found to be important building blocks for the synthesis of numerous biologically attractive natural products such as amino alcohols, peptides, and lactams, and as precursors to optically active amino acids as well as pharmaceuticals, alkaloids and polyketides [1].
In recent decades, various catalysts have been used for the preparation of β-amino carbonyl compounds including Lewis acids [2, 3], heteropoly acids [4, 5], Brønsted acids [6], rare metal salts [7, 8], lipase [9] proline [10] and hydrophobic polystyrene-supported sulfonic acids (PS-SO3H) [11].
Scandium tris(dodecylsulfate) and scandium tris(dodecanesulfonate); copper triflate [12] and HBF4 [13, 14] have also been recognized to catalyze these reactions in good yields with the help of just a surfactant. They normally suffer from the problems of long duration and harsh reaction conditions, toxicity and trproblemsouble in product separation which limit the process to obtain complex molecules. As there is an increasing demand for the improvement of organic reactions in environmentally friendly conditions, there is a need to minimize the use of hazardous chemicals by substituting the traditional organic solvents in reactions and their successive work-up with other non-toxic solvents like water or avoiding solvents during the synthesis.
In recent years, ionic liquids (ILs) have received widespread research interest as environmentally benign solvents and catalysts due to their promising properties such as high thermal stability, non-inflammability, negligible vapor pressure and reusability [15,16,17,18]. Acidic ionic liquids are widely used in various organic reactions such as esterification, alkylation, condensation and many more. Brønsted acidic ionic liquids have drawn special attention of researchers as efficient catalysts for the synthesis of valuable chemicals [19, 20]. In the past few years, ILs-based homogeneous catalysts have been plagued by a number of serious disadvantages like the leaching of the catalyst, side reactions and efforts in the isolation of products [21, 22]. These difficulties could be overcome by introducing the polymer moiety in ILs, as a heterogeneous catalyst [23]. Poly(ionic liquid)s (PILs) are a class of polymer compounds which is composed of ILs as repeating units and could be acting as a supporting material. Recently, PILs have been used as a catalyst for various catalytic transformations with better stability as well as better recoverability and recyclability [24,25,26]. Combining these unique properties of PILs, they are evolving as green heterogeneous catalysts.
To the best of our knowledge, there is no report on solvent-free synthesis of β-amino carbonyl compounds at room temperature (RT) via the Mannich reaction between aromatic aldehydes, aromatic ketone, and aromatic amines involving catalytic amounts of carboxyl-functionalized poly(ionic liquid). The reaction progressed with moderate yield of the preferred Mannich base using a catalytic amount of CFPIL-1. The effect of anions of the ionic liquid on the reaction rate has also been investigated. Furthermore, the optimizations of catalyst dose, duration and reusability have also been examined.
Experimental section
Materials and instruments
4-vinylbenzyl chloride (90%) and 3-bromopropionic acid (97%) were obtained from Sigma-Aldrich, India. Azobisisobutyronitrile (98%) was procured from Avra synthesis and recrystallized from methanol before use. Sodium hydride (60% suspension in paraffin oil), benzimidazole (LR) and all the substituted aromatic aldehydes, amines and acetophenone were procured from S D Fine Chemicals, India. Commercial grade solvents and reagents were used as received unless specified othterwise. NMR spectra were recorded in deuterated solvents such as DMSO-d6 and CDCl3 on a 400 MHz Bruker spectrometer with TMS as an internal reference. Thermal stability of the CFPIL was confirmed using thermogravimetric analyzer (TGA; (SDT Q600; USA). Fourier-transform Infrared (FT-IR) spectra of CFPIL-1 and synthesized β-amino ketones were recorded in a Shimadzu IR affinity-1 spectrometer with an attenuated total reflectance set-up in the range of 4000–400 cm−1 with a resolution of 4 cm−1. The elemental analysis (C, H and N) was performed on an Elementar Vario EL III instrument.
Synthesis of carboxyl-functionalized poly(ionic liquids) (CFPIL)
Synthesis of 1-(4-vinylbenyl)-1H-benzimidazole (VBBim)
1-(4-vinylbenyl)-1H-benzimidazole was synthesized according to our previously reported procedure [27] and the detailed process is given in the supporting information. Yield: 9.36 g, 80%. 1H NMR (400 MHz, CDCl3) δ: 7.92 (s, 1H), 7.83–7.81 (d, 2H), 7.36–7.34 (d, 2H), 7.25–7.23 (d, 2H), 7.12–7.10 (d, 2H) 6.70–6.63 (dd, 1H), 5.74–5.69 (d, 1H), 5.30 (s, 2H), 5.26–5.23 (d, 1H). 13C NMR (400 MHz, CDCl3) δ: 144.02, 143.26, 137.72, 136.09, 134.90, 133.98, 127.40, 126.88, 123.19, 122.39, 120.48, 114.70, 110.13, 48.68. GC–MS; Calcd. Mass 234.29, found: 234.30.
Synthesis of carboxyl-functionalized ionic liquid monomer (CFILM)
1-(4-vinylbenyl)-1H-benzimidazole (7.02 g, 30 mmol) and 3-bromopropionic acid (4.56 g, 30 mmol) were dissolved in 100 mL chloroform and the reaction mixture was stirred for 48 h at 70 °C. The solid mass of the product was filtered and washed with chloroform (3 × 50 mL) to obtain the pure, white amorphous carboxyl-functionalized ionic liquid monomer. Yield: 5.8 g, 50.08%.
1H NMR (400 MHz, DMSO-d6) δ: 12.67 (s, 1H), 9.99 (s, 1H), 8.15–8.13 (d, 2H), 7.94–7.92 (d, 2H),7.67–7.65 (d, 2H) 7.49–7.47 (d, 2H), 6.75–6.68 (dd, 1H), 5.87 (d, 1H), 5.83–5.78 (s, 2H), 5.29–5.27 (d, 1H), 4.73–4.70 (t, 2H), 3.04–3.01 (t, 2H).13C NMR (100 MHz, DMSO-d6) δ: 171.79, 143.06, 137.47, 135.90, 133.41, 131.18, 130.65, 128.62, 126.64, 126.59, 115.23, 114.00, 113.89, 49.57, 42.80, 32.75.
Synthesis of carboxyl-functionalized poly(ionic liquid) (CFPIL-1)
Synthesis of carboxyl-functionalized poly(ionic liquid) was prepared according to the published procedure [28] and spectral analysis data were found consistent with the proposed structure.
Yield: 3.56 g, 79.11%). 1H NMR (400 MHz, DMSO-d6) δ: 10.52 (s, 1H), 10.04 (s, 1H), 8.14 (s, 2H), 7.94 (s, 2H), 7.69 (s, 2H) 7.63 (s, 2H), 5.87 (s, 2H), 4.72 (s, 2H), 3.02 (s, 2H), 1.54 (s, 1H), 1.42 (s, 2H).13C NMR (100 MHz, DMSO-d6) δ: 171.83, 143.04, 135.90, 133.37, 131.20, 130.68, 128.58, 126.61, 115.27, 113.98, 51.79, 49.59, 42.78, 32.70, 22.03. Anal. Calcd for C21H25BrN2O2 (%): C, 60.44, H, 6.04, N, 6.71. Found: C, 55.84, H, 5.76, N, 6.93.
Synthesis of carboxyl-functionalized poly(ionic liquid) containing mesylate/tosylate counteranion
The synthesis of carboxyl-functionalized poly(ionic liquid) containing mesylate/tosylate counter anion was carried out according to the published procedure [29] with slight modifications. Methane sulphonic acid (1.935 g, 5.5 mmol) and anhydrous methanol (10 mL) were charged into a 50-mL round-bottom flask and stirred at 0 °C for 10 min. The solid, CFPIL-1 (1.496 g, 5 mmol) was added gradually with constant stirring for 30 min at this temperature. The reaction mixture was stirred for 1 h at RT and stirring continued at 60 °C for 24 h until a negative silver nitrate test was confirmed. The methanol was removed using a rotational evaporator followed by washing with ethyl acetate to gobtainet desired pure product. Finally, CFPIL-2 was dried in vacuum at 45 °C for 24 h to obtain a yellowish solid compound.
Yield: 89%. 1H NMR (400 MHz, DMSO-d6) δ: 10.20 (s, 1H), 10 (s, 1H), 8.14 (s, 2H), 7.93 (s, 2H), 7.64 (s, 2H) 7.47 (s, 2H), 7.33 (s, 2H), 7.05 (s, 2H), 5.77 (s, 2H), 4.72 (s, 2H), 3.03 (s, 2H), 2.21 (s, 3H), 2.07 (s, 1H), 1.06 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ: 171.76, 147.94, 145.03, 143.11, 138.10, 135.94, 131.20, 128.62, 128.21, 128.01, 125.85, 125.48, 113.99, 67.70, 49.59, 42.78, 32.70, 25.87, 20.77.
The CFPIL-3 was synthesized using a similar procedure to that of CFPIL-2. CFPIL-3: yellowish solid. Yield: 87%. 1H NMR (400 MHz, DMSO-d6) δ: 10.02 (s, 1H), 10.00 (s, 1H), 8.14 (s, 2H), 7.94 (s, 2H), 7.65 (s, 2H) 7.63 (s, 2H), 5.77 (s, 2H), 4.72 (s, 2H), 3.85 (s, 2H), 2.37 (s, 3H), 2.07 (s, 1H), 1.22 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ: 172.27, 143.59, 131.67, 131.14, 130.44, 129.18, 129.09, 128.89, 127.14, 114.48, 56.50, 49.89, 43.26, 33.16, 21.55, 19.00, 14.38 (Scheme 1).
General synthesis procedure of β-amino ketones
In a typical procedure, aromatic aldehydes (2 mmol), acetophenone (2 mmol), aromatic amines (2 mmol) and the catalyst (15 wt% 32 mg) were stirred in a 50-mL single-neck round-bottom flask equipped with a magnetic stirrer under solvent-free condition at RT (Scheme 2). The reaction progress was monitored by TLC using ethyl acetate and n-hexane solvent (2:8) as a mobile phase and, upon completion; the product separated at ambient temperature. The resulting precipitate was dissolved in a minimum quantity of an ethanol and acetone (1:1) mixture by gentle heating. The separated catalyst was filtered off using a sintered funnel, washed with acetone and dried in an oven at 50 °C for 10 h to reuse for the next experiment. The filtrate was cooled to ice-cold temperature to afford the pure β-amino ketone derivatives (4a–4l) which did not required further purification. The structural elucidation of β-amino ketones was carried out using 1H, 13C NMR, and FT-IR, and their physical data (m.p.) were identified with those reported in literature.
Spectral data for selected β-amino ketone
1,3-Diphenyl-3-phenylamino-propan-1-one (4a): white solid; Yield: 70%; mp 132–136 °C. 1H NMR (400 MHz, CDCl3): δ 3.47 (m, 2H), 4.96 (d, 1H), 6.48 (t, 1 H, J = 7.27 Hz), 6.61 (d, 2H, J = 8.02 Hz), 7.24 (t, 1H, J = 7.35 Hz), 7.37 (t, 2H, J = 7.6 Hz), 7.39 (t, 2H, J = 7.80 Hz), 7.47 d, (2H, J = 7.64 Hz), 7.51 (t, 1H, J = 7.2 Hz), 7.85 (d, 2H, J = 7.49 Hz). 13C NMR (100 MHz, CDCl3): 198.29, 147.01, 142.99, 136.72, 133.44, 129.12, 128.84, 128.72, 128.22, 127.37, 126.39, 117.80, 113.83, 54.82, 46.33. (KBr, ν/cm−1): 3383, 3022, 1741, 1668. HRMS (EI): m/z calculated for [M]+ [C21H19NO]+ 301.1467, Found 301.1465.
Results and discussion
Characterization of the CFPIL-1
Figure 1 shows the 1H NMR spectrum of synthesized VBBim, CFILM monomer and CFPIL-1 polymer. The 1H NMR spectrum of VBBim shows all the expected peaks of the corresponding structure. The peak at 7.92 ppm is attributed to the C2-H of the benzimidazole ring in the VBBim (Fig. 1A).
The 1H NMR spectrum of CFILM (Fig. 1B) exhibited the one unique absorption at 12.67 (broad) expected for the –OH group. Also, the peak has shifted from 7.92 to 10.11 ppm to the deshielding region, confirming the successful formation of the CFILM monomer.
After polymerization, the absorption at 0.5–1.5 ppm can be attributed to the alkyl end-group of the polymer. Moreover, broadening of peaks can be endorsed the formation of CFPIL-1 polymer (Fig. 1C).
Figure 2 shows the comparative FT-IR spectra of VBBim, CFILM and CFPIL-1. The –OH stretching frequency of the –COOH group in CFILM (B) and CFPIL-1 (C) appeared at 2821 and 2978 cm−1, respectively.
Also, the carbonyl group (C=O) of the CFILM and CFPIL-1 was found at 1712 and 1724 cm−1, respectively. This confirms the successful formation of the CFPIL-1 polymer. However, –OH and –COOH stretching frequencies are absent in the VBBim (A) spectrum.
Initially, we optimized the catalytic efficacy of different CFPIL catalysts for solvent free one-pot three-component reaction of aromatic aldehydes, acetophenone, and aromatic amines with a catalyst (5 wt%) at RT as a model reaction with benzaldehyde (0.212 g, 2 mmol), acetophenone (0.240 g, 2 mmol) and aniline (0.186 g, 2 mmol).
Among them, CFPIL-1 showed the best catalytic activity toward the reaction when compared to other polymeric catalysts (Table 1, entries 1–3).
First, we performed the reaction without a catalyst at RT for 5 h. There was little yield indicated that, the catalyst played a key role in the reaction (Table 2, entry 1). We tested the reaction with 5 wt% of the CFPIL-1 to obtain 25% yield (Table 2, entry 2). By increasing the loading of the CFPIL-1 from 10 to 15 wt%, an improvement in yield up to 70% in 5 h of reaction time was observed (Table 2, entries 3, 4). It is significant that no progress was perceived in the case of the reaction rate and yield by increasing the amount of the catalyst up to 20 wt% (Table 2, entry 5). Based on these results, 15 wt% of the CFPIL-1 is appropriate for the reaction.
The duration played an important role in the Mannich reaction catalyzed by CFPIL-1. The reaction was carried out at different intervals, with the reaction at 5 h affording the products in high selectivity with nearly complete conversion with a maximum yield of 70% (Table 3, entries 1–5).
Increasing the period of the reaction from 6 to 7 h (Table 3, entries 6, 7) produced no substantial increase in the yield of the reaction. Therefore, 5 h was selected as the time for the further investigations.
Having recognized the optimal reaction conditions, we sought to assess the possibility and efficacy of the reaction. For this purpose, a wide range of aldehydes, substituted anilines and acetophenone were selected to accomplish the Mannich reaction, and the results were displayed in Table 4.
With regard to the various aldehydes bearing both electron-donating and -withdrawing substituents, they can be competently converted into β-amino ketones in good to moderate yields as shown in Table 4, entries 1–12. All the Mannich bases were characterized by 1H, 13C NMR and IR spectroscopy. Moreover, the yields obtained for CFPIL-1 towards the Mannich reactions is better than or comparable to the literature reports with respect to catalyst loading, temperature and duration (Table 5).
Recyclability and stability of the CFPIL-1
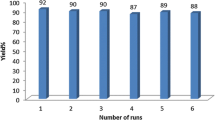
In addition, we have examined the reusability of CFPIL-1 for the model reaction under optimized conditions. At the end of the reaction, the catalyst was simply filtered in hot conditions from an acetone–ethanol mixture, washed with ethanol, and dried at 50 °C for 12 h in an oven. Catalyst reutilizing experiments were carried out with repeated use for further three consecutive reactions between benzaldehyde, aniline and acetophenone to yield the Mannich base, i.e. 1, 3-diphenyl-3-phenylamino-propan-1-one. As shown in Fig. 3, the first reaction using recovered CFPIL-1 afforded a yield similar to that achieved in the first run. In the second and third runs, the yields steadily decreased.
TGA of the CFILM monomer as well as the CFPIL-1 polymer have been studied to discover the thermal stability (Fig. 4a, b). TGA of the CFILM and CFPIL-1 was studied in the range of 30–800 °C with the temperature increasing at the rate of 10 °C min−1 in a N2 atmosphere. The thermogram of CFILM shows an initial weight loss of 64.07% which started from 180 to 380 °C. Another weight loss of 24.12% started from 380 to 548 °C. However, the thermogram of CFPIL-1 shows an initial weight loss of 50.41% which started from 239 to 403 °C. Another weight loss of 30.85% started from 380 to 640 °C.
From the above TGA results, it can be concluded that the weight loss of the monomer starts from 180 °C, but this initial weight loss in the polymer was exhibited at 239 °C and hence it clearly indicates that the stability of the CFPIL-1 polymer is better as compared to the CFILM monomer.
Conclusions
We have developed a CFPIL-1 catalyst and used it in a Mannich reaction between aromatic aldehydes, aromatic ketone and aromatic amines to prepare β-amino carbonyl compounds in good to moderate yieldsof up to 70%. Owing to the combination of –COOH functional groups and the poly(ionic liquid), the Mannich reactions can be conducted effectively. In addition, mild reaction conditions, easy work-up, low catalyst loading, non-requirement of column chromatography purification, and the recoverability and reusability of the catalyst up to three cycles are the prominent features of this method. Besides the synthesis of β-amino carbonyl compounds, such an environmentally benign catalyst should find a wider application in various acid-catalyzed reactions, which is a continuing project. Furthermore, the good yields, mild conditions and suitable operation, as well as straightforward path, confirmed that our method would play a vital role in synthesis of corresponding pharmaceuticals and natural products.
References
N.R. Candeias, F. Montalbano, P.M.S.D. Cal, P.M.P. Gois, Chem. Rev. 110, 6169–6193 (2010)
S. Saikia, P. Gogoi, A.K. Dutta, P. Sarma, R. Borah, J. Mol. Catal. A Chem. 416, 63–72 (2016)
S. Ramalingam, P. Kumar, Catal. Commun. 9, 2445–2448 (2008)
H.G.O. Alvim, G.A. Bataglion, L.M. Ramos, A.L. de Oliveira, H.C.B. de Oliveira, M.N. Eberlin, J.L. de Macedo, W.A. da Silva, B.A.D. Neto, Tetrahedron 70, 3306–3313 (2014)
W.Y. Li, Y.X. Zong, J.K. Wang, Y.Y. Niu, Chin. Chem. Lett. 25, 575–578 (2014)
T. Chang, L. He, L. Bian, H. Han, M. Yuan, X. Gao, RSC Adv. 4, 727–731 (2014)
W.B. Yi, C. Cai, J. Fluorine Chem. 127, 1515–1521 (2006)
L. Wang, J. Han, J. Sheng, H. Tian, Z. Fan, Catal. Commun. 6, 201–204 (2005)
K. Li, T. He, C. Li, X.W. Feng, N. Wang, X.Q. Yu, Green Chem. 11, 777–779 (2009)
B. List, P. Pojarliev, W.T. Biller, H. Martin, J. Am. Chem. Soc. 124, 827–833 (2002)
S. Iimura, D. Nobutou, K. Manable, S. Kobayashi, Chem. Commun. 14, 1644–1645 (2003)
S. Kobayashi, T. Busujima, S. Nagayama, Syn. Lett. 5, 545–546 (1999)
T. Akiyama, J. Takaya, H. Kagoshima, Syn. Lett. 9, 1426–1428 (1999)
T. Akiyama, J. Takaya, H. Kagoshima, Syn. lett. 7, 1045–1048 (1999)
T. Welton, Chem. Rev. 99, 2071–2084 (1999)
P. Wasserscheid, W. Keim, Angew. Chem. Int. Ed. 39, 3772–3789 (2000)
A.G. Khiratkar, P.N. Muskawar, P.R. Bhagat, RSC Adv. 6, 105087–105093 (2016)
G.M.Z.N. Lashgari, A. Badiei, J. Mol. Catal. A: Chem. 397, 166–191 (2015)
M. Vafaeezadeh, H. Alinezhad, J. Mol. Liq. 218, 95–105 (2016)
M. Vafaeezadeh, M. Karbalaie-Reza, M.M. Hashemi, K.Q. Soleimany, J. Iran. Chem. Soc. 14, 907–914 (2017)
Dennis J.M. Snelders, P.J. Dyson, Org. Lett. 13, 4048–4051 (2011)
T. Joseph, S. Sahoo, S.B. Halligudi, J. Mol. Catal. A: Chem. 234, 107–110 (2005)
Y. Leng, J. Liu, P. Jiang, J. Wang, Catal. Commun. 40, 84–87 (2013)
J. Yuan, M. Antonietti, Polymer 52, 1469–1482 (2011)
Q. Zhao, P. Zhang, M. Antonietti, J. Yuan, J. Am. Chem. Soc. 134, 11852–11855 (2012)
A. Pourjavadia, S.H. Hosseinia, R. Soleyman, J. Mol. Catal. A: Chem. 365, 55–59 (2012)
A.G. Khiratkar, S.S. Kumar, P.R. Bhagat, RSC Adv. 6, 37757–37764 (2016)
K.R. Balinge, A.G. Khiratkar, M. Krishnamurthy, D.S. Patle, Cheralathan K.K.P.R. Bhagat, Resource-Efficient Technol. 2, S105–S113 (2016)
P.N. Muskawar, S.S. Kumar, P.R. Bhagat, J. Mol. Catal. A Chem. 380, 112–117 (2013)
S. Iimura, D. Nobutou, K. Manabe, S. Kobayashi, Chem. Commun. 0, 1644–1645 (2003)
N. Azizi, L. Torkiyan, M.R. Saidi, Org. Lett. 8, 2079–2082 (2006)
S. Ramalingam, P. Kumar, Catal. Commun. 9, 2445–2448 (2008)
B.M. Reddy, M.K. Patil, B.T. Reddy, Catal. Lett. 125, 97–103 (2008)
Acknowledgements
We gratefully acknowledge SIF DST-VIT-FIST, VIT University, Vellore for providing NMR, FT-IR as well as other required facilities. The authors are also grateful for financial help provided by RGEMS, VIT University, Vellore and other facilities provided by “Smart Materials Laboratory for Bio-sensing and Catalysis.”
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khiratkar, A.G., Balinge, K.R., Bhansali, K.J. et al. Solvent-free synthesis of β-amino ketones using carboxyl-functionalized poly(ionic liquid) at room temperature. Res Chem Intermed 44, 787–798 (2018). https://doi.org/10.1007/s11164-017-3134-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3134-x