Abstract
The current study deals with the applications of a surfactant-like Brønsted acidic ionic liquid (IL) 1-dodecyl-3-methylimidazolium hydrogen sulfate ([DMIm]HSO4) for Mannich reaction at room temperature. The reaction was efficiently preceded in water as solvent without using any harmful and expensive organic additives. Our findings showed that the reaction is selective for cyclohexanone and no Mannich product was observed when cyclopentanone was used as starting material. Density functional theory (DFT) calculations were performed to provide an evidence about the nature of reactivity of the cyclohexanone/cyclopentanone. The activity of the catalyst was also tested for biodiesel production of fatty acids with methanol and ethanol at mild thermal condition without applying additional water removal steps such as using additives or performing special methodologies like azeotropic distillation. In both reactions, the IL can be recycled and reused several times with relatively constant efficiency.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Elimination of organic solvent with hazardous environmental impacts and industrial cost is a crucial challenge in the field of chemical reaction engineering. In chemical reactions, “solvent free” or in the best case, selecting water as solvent is a desire goal in both economical and environmental point of view [1, 2]. Water is cheap, safe and releases no volatile harmful compounds. Unfortunately, in most cases organic substrates do not have desirable compatibility and miscibility with aqueous media. In some cases, modification of the surface of the inorganic solids is an alternative method for better diffusion of organic substrate on the surface of these materials in water [3]. In this condition, water “pushes” the organic substrates onto the hydrophobic surface of the catalyst. Despite successful achievements, the reaction kinetic on the surface of the heterogeneous catalyst is low and the desires yields only obtain in prolong reaction time.
Amphiphilic reagents which are miscible with water and can dissolve organic substrates are versatile selection for improving the reaction efficiency in water. Among them, surfactant-type Brønsted acids such as p-dodecylbenzenesulfonic acid (DBSA) may be a good selection with widespread applications. These compounds form hydrophobic cores in water and thus can conduct organic reactions in solution phase with high efficiency. However, these compounds still have very poor recovery and reusability as a catalyst. Therefore, the need for developing an efficient catalytic system in water with high efficiency and reusability is exist.
In recent year, the roles of the ionic liquids (ILs) have been moved beyond their applications as alternative solvents [4]. Several ILs have been designed, synthesized and applied as catalyst for organic and inorganic transformations [5]. Brønsted acidic ILs are a large group of compounds which attracts special attention for replacement of traditional homogeneous acid catalyst [6–8]. These compounds can be readily prepared, reused and separated from the reaction mixture without releasing noticeable acidic vapor. An interesting aspect of the ILs is attributed to the tunable properties. By incorporating a hydrocarbon chain with various lengths into the structure via simple chemical reactions, the hydrophobicity/hydrophilicity of the ILs can be controlled. The ionic nature of IL provides suitable solubility in water. Simultaneously, the hydrophobic nature of the IL facilitates approaching the organic groups toward IL.
In this paper, a Brønsted acidic IL, 1-dodecyl-3-methylimidazolium hydrogen sulfate ([DMIm]HSO4) has been introduced as an efficient catalyst for selective Mannich reaction in water. Mannich reaction is a useful synthetic method for carbon–carbon bond formation reactions for preparation of various drugs, natural products as well as biologically active compounds [9, 10]. These β-amino carbonyl derivatives are majorly synthesized by using either Brønsted [11, 12] or Lewis acid catalyst [13, 14]. A significant improvement of the reaction was reported by Sharma and co-workers which used an inorganic–organic hybrid silica-based tin(II) material as a reusable catalyst for this reaction at room temperature [15]. Despite introducing efficient synthetic protocol, these methods typically suffer from releasing hazardous metal wastes, using chlorinated solvent or employing highly toxic reagent during the catalyst preparation [16]. Moreover, despite extensive works which were reported for this reaction, no significant report focused on the selectivity of the reaction. In our method, high yield of β-amino carbonyl derivatives was obtained when cyclohexanone was used as ketone at room temperature. By replacing cyclopentanone as the starting material, no product was obtained at ambient temperature which suggests a meaningful selectivity for this reaction. Density functional theory (DFT) calculations were performed at B3LYP/6-311++G** to provide evidence about the reactivity of the starting material.
The second section of this paper deals with application of the surfactant-like [DMIm]HSO4 IL for acid-catalyzed esterification of fatty acids in both solvent-free and in aqueous media. Fatty acid methyl or ethyl esters are known as biodiesel which have received extensive attention for replacements of fossil fuel [17, 18]. It can traditionally be prepared via base-catalyzed transesterification of triglycerides with short-chain alcohols such as methanol or ethanol [19]. Unfortunately, in the case of less expensive feedstock such as waste oil (which contains high degree of free fatty acids) the process interrupted due to the soap formation by the reaction between fatty acid and the applied base catalyst. Additionally, excess amount of base is needed to neutralize the free fatty acids which cause releasing large volume of salty waste. Hence, acid catalyst esterification is the best solution for this reaction which has compatibility with free fatty acid-containing feedstock. Several methods based on supported IL [20–24] or homogeneous IL [25–28] systems have been developed for this reaction. A relatively good method for esterification reaction in water comprises using Brønsted acidic surfactant as catalyst [29, 30]. However, the most important challenge for conducting such reaction is attributed to the reversibility of the reaction and performing the esterification reaction in water since esters are metastable compounds and can readily hydrolyze in water. Additionally, when Brønsted acidic surfactant was used as catalyst, the isolation of the products is difficult and hence it is necessary to use ionic solution to breakdown the structure of the micelles. Consequently, the applied surfactant is not reusable in this condition.
Experimental
Preparation of [DMIm]HSO4
In a typical procedure, 20 mmol of 1-methylimidazol and 22 mmol of 1-bromododecane were refluxed in toluene for 8 h. After the termination of the reaction, the mixture was cooled to room temperature. The resulted white precipitate was collected and washed separately with toluene and diethyl ether to produce 1-dodecyl-3-methylimidazolium bromide. The anion exchange was performed by adding equimolar amount of H2SO4 in super dry toluene. After 16 h, the solvent was evaporated and the [DMIm]HSO4 was isolated as viscous oil. The IL was dried in oven in the presence of P2O5. 1H NMR (500 MHz, CDCl3): 0.73 (t, 3H), 1.09 (broad, 18 H), 1.69 (broad, 2H), 3.79 (s, 3H), 4.06 (t, 2H), 7.29 (s, 1H), 7.41 (s, 1H), 8.86 (s, 1H) and 10.01 (s, 1H). 13C NMR (125 MHz, CDCl3): 14.52, 23.09, 26.74, 29.53, 29.78, 29.91, 30.04, 30.07, 30.09, 30.58, 32.33, 36.97, 50.40, 122.36, 124.31, 137.03.
Mannich reaction in water
A mixture of benzaldehyde (4 mmol), cyclohexanone (4 mmol), aniline (4 mmol) and [DMIm]HSO4 (0.5 mmol) was added to a 25-mL round-bottom flask. Then 6 mL of distilled water was added, and the mixture was vigorously stirred (at 800 rpm) at room temperature. The reaction progress was monitored by thin layer chromatography (TLC). At the end of reaction, the mixture was filtrated and the solid product was collected and recrystallized in ethanol/acetone (v/v = 1:1). The remained solution can be reused for another reaction run.
Fatty acid esterification in water or solvent-free conditions
A mixture of fatty acid (2 mmol), methanol or ethanol (1.1–1.2 mL) and [DMIm]HSO4 was added to a round-bottom flask equipped with a reflex condenser and stirred in indicated times and temperatures. In the case of the reaction in water, 2 mL of distilled H2O was added to the reaction media. After the reaction termination, the biodiesel was extracted from the mixture by adding n-hexane (2 × 15 mL). The small amount of the residual fatty acid was neutralized with 5% aqueous solution of NaHCO3 (4 mL). Then the resulted mixture was dried over Na2SO4, and the n-hexane was evaporated under reduced pressure to afford the corresponding pure biodiesel.
Theoretical calculations
The initial structures were built and optimized at B3LYP/6-311++G** using Spartan software [31]. The imaginary frequencies were checked for all optimized structures. Both of the transition states have imaginary frequencies related to the corresponding vibrational modes for bond formation and bond dissociation. The imaginary frequencies confirmed that whether the structures are ground state or transition state.
Results and discussion
Mannich reaction
The [DMIm]HSO4 was simply prepared from the reaction between 1-methylimidazol and 1-bromododecane and subsequent anion exchange by H2SO4. In this condition, HBr was released during the reaction. The reaction was continued until the generation of HBr was finished. Then the [DMIm]HSO4 IL was isolated and was stored in a refrigerator. The schematic illustration of the synthesis of IL is shown in Scheme 1a.
The activity of the IL was investigated for three-component Mannich reaction of benzaldehyde, aniline and cyclohexanone as a bench reaction (Scheme 1b). At the first step, we started to optimize the reaction temperature and solvent which are two important parameters in chemical transformations. H2O was selected as an environmentally benign and cheap solvent. Surprisingly, we found that the reaction could be efficiently proceeded in water and at ambient temperature. In this condition, 91% of product was obtained when 0.5 mmol of the IL was used. Decreasing the amount of IL led to a significant drop of the reaction product.
Under this mild and clean condition, we then set up experiments to study the scope and limitation of our catalytic system. The results are summarized in Table 1.
Based on our finding, various available benzaldehydes undergo Mannich reaction with cyclohexanone and aniline in high yield at room temperature after 6 h (Table 1, entry 1–6). Moreover, 69% of the three-component Mannich derivative by using acetophenone as ketone was also obtained in this condition (Table 1, entry 7). It was found that the reaction with aniline derivative with electron-donating group was facilitated and 94% of product was obtained in our catalytic system (Table 1, entry 8).
For detailed study of the catalytic performance, we then set up experiment by using cyclopentanone as starting material (Table 1, entry 9). Our attempt to perform the reaction at room temperature led to achieve poor yield of Mannich product (8%). Having this interesting data in hand, in the next stage we investigated the possibility of chemoselective reaction of cyclohexanone in the presence of cyclopentanone (Scheme 2).
As shown in Scheme 2, a 1:1 mixture of cyclohexanone and cyclopentanone was allowed to react with aniline and benzaldehyde in the presence of [DMIm]HSO4 as catalyst. After 6 h stirring of the reaction mixture at room temperature and the product isolation, it was observed that only cyclohexanone participated in the reaction and the corresponding Mannich product of cyclopentanone was not obtained. This observation suggests an interesting selectivity toward cyclohexanone in the presence of cyclopentanone. On the other hand, by increasing the reaction temperature to 50 °C cyclopentanone was simply converted to its corresponding Mannich product. Consequently, the above catalytic system established the chemoselectivity at room temperature (22–25 °C).
The reaction mechanism comprises initial formation of an iminium ion from the reaction of aniline and the benzaldehyde. At the second step, the iminium ion attacked by the enol form of the applied ketone. Hence, the reactivity of the cyclic ketone is attributed to the rate of enol formation of cyclopentanone and cyclohexanone. Computational methods are fruitful techniques to provide evidence about reactivity and energetic of the reactions. To provide evidence about the origin of selectivity for cyclohexanone and cyclopentanone in Mannich reaction, we optimized the initial structure of cyclopentanone, cyclohexanone and their corresponding enol forms using B3LYP/6-311++G** level of calculation. Then the transition state of each enol–keto transformation was obtained at the same level of calculation (Scheme 3).
As shown in Scheme 3, in the transition state an H atom is moving from the α-carbon atom to the oxygen of carbonyl. Both of the imaginary frequencies are belong to this H-shift and occurred at 2249.7 cm−1 for cyclopentanone and at 2203.3 cm−1 for cyclohexanone. The higher value of the H-shift frequency for cyclopentanone may be interpreted that this tautomerization is more energetic than that in cyclohexanone. Moreover, the activation energy for conversion of cyclopentanone to its enol tautomer is approximately 6.7 kcal/mol higher than that of cyclohexanone. Accordingly, at lower temperature, the energy for cyclohexanone tautomerization could be better supplied than cyclopentanone. By increasing the reaction temperature to 50 °C, the energy for cyclopentanone was supplied, and thus, 79% of Mannich product from this ketone was obtained (Table 1, entry 9).
The possibility of the recovery for the IL for Mannich reaction was also investigated. For this purpose, the aqueous solution which was obtained from the first reaction run was subjected to another reaction at the identical condition for Mannich reaction from benzaldehyde, aniline and cyclohexanone (Table 1, entry 1). It was found that the catalytic system can be reused at least for four times with relatively constant activity.
Fatty acid esterification reaction
The high activity of the [DMIm]HSO4 encouraged us to turn our attention for esterification of fatty acid. The reaction of fatty acids with short-chain alcohol such as methanol or ethanol is one of the most important acid-catalyzed esterifications since the products are used as biodiesel. As discussed above, the limitations of this work are attributed to using organic solvent and utilizing dehydration techniques to remove water by-product to shift the reaction toward completion.
To investigate the efficiency of the catalyst, the solvent-free esterification of stearic acid with methanol was tested as the sample reaction. At the first step, the amount of IL was optimized for this reaction. As shown in Table 2, by increasing the amounts of IL, the yield of ester was significantly increased.
However, increasing the amounts of IL did not show significant improvement in the reaction. For example, by increasing the amounts of IL from 0.20 to 0.30 g, a negligible improvement for the reaction was observed. To increase the reaction efficiency, the amounts of methanol decreased to 1.1 mL. Surprisingly, it was found that the yield of product reached 94% (isolated yield) in the presence of 0.1 g of IL. It can be concluded that reducing the amounts of alcohol has positive effect on the reaction efficiency.
Table 3 shows the effect of temperature on the esterification reaction of stearic acid with methanol.
As can be deduced from these experiments, the esterification was smoothly proceeds at room temperature and 73% of the corresponding biodiesel was obtained after 10 h. Performing the esterification reaction at room temperature without using water removal techniques is an interesting issue especially in the industrial point of view. By increasing the temperature, the yield of the reaction was reached 94% at 60 °C and even to its highest value at 70 °C.
The kinetic of the mentioned reaction was also investigated (Fig. 1). In this regard, several experiments for esterification of stearic acid and methanol were performed. The results showed that after 10 h, the amount of product was reached its maximum value and increasing the reaction time did not show a significant improvement of the reaction.
Performing the esterification in water is the most promising issue of the reaction. According to the Le Chatelier’s principle, by using water as solvent the reaction equilibrium tends to relief the effect of the additional H2O and readjusts to form initial acid and alcohol in the presence of the acidic catalyst. However, our screening about this issue revealed that even in the presence of water, high yield of biodiesel could be obtained. The yields of ester in the presence of various amounts of water are listed in Table 4.
The highest amount of the product (90%) was obtained when 2 mL of water was added to system. Worthy to note that in this condition the water may exist in the form of both solvent and impurity of the starting material. For example, in most esterification reaction it is necessary to use absolute ethanol or methanol which increases the reaction cost. However, our investigation showed that the current protocol is applicable with high efficiency even in the presence non-absolute alcohols.
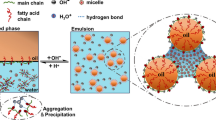
One plausible explanation for this phenomenon is attributed to the prevention of ester hydrolysis. The hydrophobic nature of the [DMIm]HSO4 led to formation of the micellar cores in water. The inner part of micelles has high degree of hydrophobicity which facilitates the transportation of organic substrates and simultaneous removal of water by-product. Schematic illustration for the proposed mechanism for the catalytic efficiency of IL is shown in Fig. 2.
The data for catalytic performance of the [DMIm]HSO4 for direct esterification of various fatty acids and ethanol in both solvent free and using water as solvent are shown in Table 5.
As shown in Table 5, various fatty acids efficiently reacted with methanol and ethanol to produce the corresponding biodiesel. In the case of acid-sensitive fatty acid such as oleic acid, esterification was occurred without hydration of the double bond. Moreover, in all cases the results of product in water are higher than those in solvent-free condition. To investigate whether the aqueous solution from the first reaction run of esterification is reusable or not, after the product isolation, the aqueous solution of the first run was subjected to other experiments with identical conditions to the first reaction. It was found that the catalytic system is reusable for four reaction runs without significant loss of its activity (Table 5).
Table 6 shows the comparisons among the catalytic efficiency of the [DMIm]HSO4 and some literature reports for direct esterification reactions. The results indicate that the catalytic activity of the [DMIm]HSO4 is comparable and even superior than those which were previously reported (Table 6).
Conclusion
In this study, we designed and introduced a highly efficient catalytic system based on surfactant-like IL for Mannich reaction and biodiesel production in water. High yields of Mannich products were obtained at room temperature. Despite the efficiency of [DMIm]HSO4 for Mannich reaction, the selectivity of the IL was also investigated between cyclopentanone and cyclohexanone. DFT calculation showed that cyclohexanone is more reactive than cyclopentanone in Mannich reaction. Furthermore, the activity of the catalyst for solvent-free biodiesel production was fully studied in various reaction conditions. It was shown that [DMIm]HSO4 has excellent activity in water and even was superior than solvent-free condition. Both catalytic systems (IL + H2O) are capable to reuse for four times without noticeable loss of activity.
References
P.A. Grieco (ed.), Organic Synthesis in Water (Blackie Academic and Professional, London, 1998)
C.-J. Li, T.-H. Chan, Organic Reactions in Aqueous Media (Wiley, New York, 1997)
S. Minakata, M. Komatsu, Chem. Rev. 109, 711 (2009)
R.D. Rogers, K.R. Seddon, Science 302, 792 (2003)
C. Yue, D. Fang, L. Liu, T. Yi, J. Mol. Liq. 163, 99 (2011)
M. Olkiewicz, N.V. Plechkova, M.J. Earle, A. Fabregat, F. Stüber, A. Fortuny, J. Font, C. Bengo, Appl. Catal. B Environ. 181, 738 (2016)
A.A. Jafari, F. Moradgholi, F. Tamaddon, Eur. J. Org. Chem. 2009, 1249 (2009)
T. Chang, L. He, L. Bian, H. Han, M. Yuan, X. Gao, RSC Adv. 4, 727 (2014)
S. Kobayashi, H. Ishitani, Chem. Rev. 99, 1069 (1999)
A. Cordova, Acc. Chem. Res. 37, 102 (2004)
R.O. Duthaler, Angew. Chem. Int. Ed. 42, 975 (2003)
S. Iimura, D. Nobutou, K. Manable, S. Kobayashi, Chem. Commun. 9, 1644 (2003)
I. Komoto, S. Kobayashi, Chem. Commun. 18, 1842 (2001)
T.P. Loh, S.L. Chen, Org. Lett. 4, 3647 (2002)
R.K. Sharma, D. Rawat, G. Gaba, Catal. Commun. 19, 31 (2012)
F. Dong, F. Zhenghao, L. Zuliang, Catal. Commun. 10, 1267 (2009)
D.E. López, J.G. Goodwin Jr., D.A. Bruce, S. Furuta, Appl. Catal. A Gen. 339, 76 (2008)
K. Suwannakarn, E. Lotero, K. Ngaosuwan, J.G. Goodwin Jr., Ind. Eng. Chem. Res. 48, 2810 (2009)
F. Maa, M.A. Hanna, Bioresour. Technol. 70, 1 (1999)
A. Chinnappan, H. Kim, Chem. Eng. J. 187, 283 (2012)
D. Zhao, M. Liu, J. Zhang, J. Li, P. Ren, Chem. Eng. J. 221, 99 (2013)
D. Fang, J. Yang, C. Jiao, ACS Catal. 1, 42 (2011)
X. Liang, J. Yang, Green Chem. 12, 201 (2010)
L. He, S. Qin, T. Chang, Y. Sun, X. Gao, Catal. Sci. Technol. 3, 1102 (2013)
B. Karimi, M. Vafaeezadeh, Chem. Commun. 48, 3327 (2012)
M. Ghiaci, B. Aghabarari, A. Gil, Fuel 90, 3382 (2011)
B. Zhen, H. Li, Q. Jiao, Y. Li, Q. Wu, Y. Zhang, Ind. Eng. Chem. Res. 51, 10374 (2012)
B. Zhen, Q. Jiao, Q. Wu, H. Li, J. Energy Chem. 23, 97 (2014)
K. Manabe, X. Sun, S. Kobayashi, J. Am. Chem. Soc. 123, 10101 (2001)
K. Manabe, S. Iimura, X. Sun, S. Kobayashi, J. Am. Chem. Soc. 124, 11971 (2002)
Spartan’10V102’ (Wavefunction Inc., Irvine, 2010)
M. Barbero, S. Cadamuro, S. Dughera, Tetrahedron Asymmetry 26, 1180 (2015)
H. Xing, T. Wang, Z. Zhou, Y. Dai, Ind. Eng. Chem. Res. 44, 4147 (2005)
J. Miao, H. Wan, G. Guan, Catal. Commun. 12, 353 (2011)
Y. Leng, P. Jiang, J. Wang, Catal. Commun. 25, 41 (2012)
H. Du, X. Zhang, Y. Kuang, Z. Tan, L. Song, X. Han, J. Taiwan Inst. Chem. Eng. 49, 51 (2015)
D. Forbes, K. Weaver, J. Mol. Catal. A Chem. 214, 129 (2004)
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vafaeezadeh, M., Karbalaie-Reza, M., Hashemi, M.M. et al. Surfactant-like Brønsted acidic ionic liquid as an efficient catalyst for selective Mannich reaction and biodiesel production in water. J IRAN CHEM SOC 14, 907–914 (2017). https://doi.org/10.1007/s13738-016-1036-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-016-1036-2