Abstract
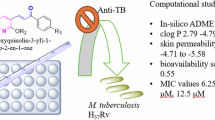
The present study reports the synthesis of a series of alkyl 4-(5/6-bromo-1H-indole-3-yl)-2,6,6/2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate derivatives by a simple, rapid and convenient modified Hantzsch condensation reaction under microwave irradiation. The structure elucidation of the target compounds was carried out by different spectral techniques including IR, 1H-NMR, COSY, 13C-NMR, and mass analysis. Additionally, the proposed structure of compound 3 was proved by single crystal X-ray analysis. In vitro anti-tubercular activity of the compounds was evaluated against Mycobacterium tuberculosis H37Rv. The obtained results indicated that some compounds exhibited moderate antimycobacterial activity with weak cytotoxicity. Among them, compounds carrying ethyl or isopropyl groups in their ester moiety were found to be the most active compounds in this series. Molecular modeling studies were carried out to gain an idea about the mechanism of action of the active compounds. According to the results, the interactions were found quite similar with the co-crystalized ligand of M. tuberculosis enoyl reductase (InhA).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) is a serious infectious diseases caused by Mycobacterium tuberculosis. Human TB has existed for years and still remains one of the most important global health-threatening problems [1]. The World Health Organization (WHO) has declared TB to be one of the leading causes of death from an infectious disease, and estimated that there were 10.4 million new TB cases and 0.4 million TB deaths in 2015 worldwide [2].
Effective drug treatments were first developed in the period 1945–1965 and the current treatment regimen for new cases of drug-susceptible TB consists of four first-line drugs: isoniazid, rifampicin, ethambutol and pyrazinamide [3]. The recent emergence of multidrug-resistant to these first-line drugs has made the treatment of tuberculosis more complicated and clearly indicates the urgent need for the development of new drugs with divergent structure and preferably novel mechanism of action for the successful clinical control of patients with TB [4, 5].
1,4-Dihydropyridines (1,4-DHP) represent the well-known class of calcium channel blockers that are employed for the treatment of cardiovascular diseases, particularly angina pectoris and hypertension [6]. Although DHPs were initially developed as cardiovascular drugs, the 1,4-DHP nucleus was modified by medicinal chemists, achieving different therapeutic applications [7]. 1,4-DHPs carrying lipophilic groups in combination with 3,5-dicarbamoyl or 3,5-dialkyloxycarbonyl moieties have been reported to possess considerable antitubercular activity [8,9,10]. These compounds presumably act as precursors and are activated by enzymatic hydrolysis to parent acids after penetration into the cell wall [11].
Nitrogen-containing heterocyclic compounds are valuable scaffolds in medicinal chemistry and have received considerable attention due to their various pharmacological activities such as antimicrobial, anti-inflammatory, antiviral, and antitumor over the past years [12, 13]. The indole moiety is the most widespread nitrogen heterocyclic ring present in both natural and synthetic compounds and attracts great interest because of its biological importance and versatile pharmacological properties [14, 15]. In particular, indole-containing compounds have been reported as anti-TB agents in recent years [16, 17].
Microwave irradiation (MW) has become an increasingly valuable tool in organic chemistry during the last decades for performing various chemical reactions. This technique offers a facile and versatile pathway with higher yields and short reaction times. Furthermore, MW also has the advantages of using a small amount or no organic solvents, making the reactions environmentally friendly with fewer side products [18]. Several successful applications of MW irradiation have been reported concerning the synthesis of nitrogen-containing heterocycles [19].
InhA, the enoyl acyl carrier protein reductase, from Mycobacterium tuberculosis, is a key regulatory enzyme employed in the biosynthesis pathway of type II fatty acids, which catalyzes the NADH-dependent stereospecific reduction of unsaturated fatty acids bound to the acyl carrier protein of M. tuberculosis [20]. Although there are no reported experimental studies of the mechanism of action of antitubercular 1,4-DHPs, these compounds are thought to exert their antitubercular activity through the inhibition of the InhA enzyme, based on the results of some theoretical studies, such as docking and molecular dynamic simulation methods with their binding affinity to InhA [21].
In the present study, we aimed to synthesize 20 hexahydroquinoline (HHQ) derivatives with the indole moiety and investigated how different ester groups and the position of the bromine atom attached to the indole ring affected the antitubercular activities of these compounds. Although the synthesis of compounds 1, 2, 5 and 6 has been previously described [22]; these compounds were also included in the study since their antimycobacterial activities have not been reported before.
Materials and methods
Experimental
All starting compounds were purchased from Sigma–Aldrich and were used without further purification. The purity of the synthesized compounds was initially checked on aluminium sheets coated with Silicagel 60 F254 (Merck) using ethyl acetate:hexane (1:1) as the mobile phase. Short-wavelength (254 nm) UV light (Camag UV Cabinet, Wiesloch, Germany) was used for visualizing the spots on thin layer chromatography (TLC) plates. Melting points were determined using Thomas Hoover Capillary Melting Point Apparatus (Philadelphia, PA, USA) and were uncorrected. Infrared spectra (IR) were obtained using Perkin Elmer Spectrum BX FT-IR (Beaconsfield, UK) equipped with the MIRacle ATR accessory (Pike Technologies) and were reported in cm−1. 1H-NMR, 13C-NMR and COSY spectra were recorded in dimethylsulfoxide (DMSO-d 6) solutions on a Varian Mercury 400, 400 MHz High Performance Digital FT-NMR Spectrometer (Palo Alto, CA, USA) using tetramethylsilane as the internal standard. Chemical shifts are reported in parts per million (ppm). The ESI-MS spectra were recorded on a micromass ZQ-4000 single quadrupole mass spectrometer (Waters, Eschborn, Germany). Elemental analyses (C, H, N) were carried out on a Leco CHNS-932 Elemental Analyzer (Philadelphia, PA, USA).
X-Ray crystallography
Crystal structure determination
Crystals suitable for single-crystal X-ray analysis for all the complexes were grown from the slow evaporation of the reaction mixtures at room temperature. Single crystals suitable for single-crystal X-ray of C22H23BrN2O3 were mounted on a glass fiber and used for data collection. X-ray measurements of the title compound was made at 100 K. Crystal data were collected on a Bruker APEX-II diffractometer using graphite monochromatized MoKα radiation (λ = 0.71073 Å). The crystal orientation, cell refinement and intensity measurements were made using the program CAD-4PC performing Ψ-scan measurements.
The title compound, C22H23BrN2O3, is a non-merohedrally twinned with the twin law in the reciprocal matrix of −1, 0, 0: 0, −1, 0: 0.802, 0.670, 1 and the twin component ratio of 0.5117(1) (1):0.4883(1).
The structure was solved using the nonoverlapping reflections and refined using all data in a SHELXTL HKLF5 format. In this format, reflections cannot be merged hence the data appear to be more than 100% complete. The structures were solved by direct method using the program SHELXT [23], and refined by full-matrix least-square techniques against F 2 using SHELXL2016/6 [23].
All hydrogen atoms were modeled with idealized geometry (N–H = 0.88 Å, C–H, 0.95–1.00 Å) and were included in the refinement in the riding-model approximation, with U iso(H) = 1.2U eq(C) or 1.5 U eq(C-methyl). A rotating-group model was applied for the methyl groups.
Computing details
The CrysAlis PRO 1.171.38.43 [24] was used for analytical numeric absorption correction using a multifaceted crystal model based on expressions derived by R.C. Clark and J.S. Reid [25]. Empirical absorption corrections using spherical harmonics were implemented in SCALE3 ABSPACK scaling algorithm.
Chemistry
The general procedure for the synthesis of 1,4-DHP derivatives was as follows: 2 mmol 1,3-cyclic diketone (4,4-dimethyl-1,3-cyclohexanedione or 5,5-dimethyl-1,3-cyclohexanedione), 2 mmol substituted indole carbaldehyde (5-bromo-1H-indole-3-carbaldehyde or 6-bromo-1H-indole-3-carbaldehyde), 2 mmol appropriate alkyl acetoacetate and 10 mmol ammonium acetate were dissolved in a 35-ml microwave pressure vial in ethanol and subjected to microwave irradiation (power 100 W, maximum temperature 150 °C) for 5 min. After completion of the reaction, monitored by TLC, the reaction mixture was poured into ice–water and the obtained precipitate was crystallized from ethanol–water. The route used to synthesize the title compounds is outlined in Fig. 1.
Methyl 4-(5-bromo-1H-indol-3-yl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (1)
Yield 69%. m.p. 130 °C. IR (ν, cm−1): 3295, 3215 (N–H), 1643 (C = O, ester), 1608 (C = O, ketone). 1H-NMR (δ) 0.87 (3H; s; 6-CH3), 0.97 (3H; s; 6-CH3), 1.60–1.80 (2H; m; H-7), 2.26 (3H; s; 2-CH3), 2.48–2.56 (2H; m; H-8), 3.33 (3H; s; OCH3), 5.07 (1H; s; H-4), 6.87 (1H; d; J: 2.4 Hz; indole H-2), 7.10 (H; dd; J: 8.4/2.0 Hz; indole H-6), 7.23 (1H; d; J: 8.4 Hz; indole H-7), 7.85 (1H; d; J: 2.0 Hz; indole H-4), 9.20 (1H; s; HHQ N–H), 10.85 (1H; s; indole N–H). ESI-MS (m/z): 465/467 [M + Na]+/[M + 2+Na]+, 248 (100%). Anal. Calcd. for C22H23BrN2O3 (MW: 443); C, 59.60; H, 5.23; N, 6.32. Found: C, 59.44; H, 5.30; N, 6.29.
Methyl 4-(5-bromo-1H-indol-3-yl-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (2)
Yield 79%. m.p. 194 °C. IR (ν, cm−1): 3351, 3304 (N–H), 1699 (C = O, ester), 1590 (C = O, ketone). 1H-NMR (δ) 0.79 (3H; s; 7-CH3), 1.00 (3H; s; 7-CH3), 1.95 (H; d; J: 16 Hz; H-8a), 2.15 (H; d; J: 16 Hz; H-8b), 2.27 (3H; s; 2-CH3), 2.29 (H; d; J: 17.2 Hz; H-6a), 2.46 (H; d; J: 17.2 Hz; H-6b), 3.33 (3H; s; OCH3), 5.07 (1H; s; H-4), 6.93 (1H; d; J: 2.0 Hz; indole H-2), 7.08 (1H; dd; J: 8.4/2.0 Hz; indole H-6), 7.22 (1H; d; J:8.4 Hz; indole H-7), 7.70 (1H; d; J: 2.0 Hz; indole H-4), 9.20 (1H; s; HHQ N–H), 10.88 (1H; s; indole N–H). ESI-MS (m/z): 465/467 [M + Na]+/[M + 2+Na]+, 248 (100%). Anal. Calcd. for C22H23BrN2O3 (MW: 443); C, 59.60; H, 5.23; N, 6.32. Found: C, 59.11; H, 5.42; N, 6.10.
Methyl 4-(6-bromo-1H-indol-3-yl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (3)
Yield 84%. m.p. 197 °C. IR (ν, cm−1): 3449, 3291 (N–H), 1690 (C = O, ester), 1641 (C = O, ketone). 1H-NMR (δ) 0.83 (3H; s; 6-CH3), 0.95 (3H; s; 6-CH3), 1.61–1.71 (2H; m; H-7), 2.24 (3H; s; 2-CH3), 2.45–2.48 (2H; m; H-8), 3.49 (3H; s; OCH3), 5.07 (1H; s; H-4), 6.83 (1H; d; J: 2.4 Hz; indole H-2), 7.01 (1H; dd; J: 8.4/1.6 Hz; indole H-5), 7.41 (1H; d; J:1.6 Hz; indole H-7), 7.48 (1H; d; J: 8.4 Hz; indole H-4), 9.14 (1H; s; HHQ N–H), 10.76 (1H; s; indole N–H). 13C-NMR (δ) 18.0, 22.8, 24.0, 25.2, 27.0, 34.1, 39.5, 50.5, 103.0, 109.0, 113.1, 113.6, 120.8, 121.1, 121.6, 123.6, 124.6, 137.0, 144.2, 149.1, 167.5, 199.4. ESI-MS (m/z): 465/467 [M + Na]+/[M + 2+Na]+, 248 (100%). Anal. Calcd. for C22H23BrN2O3 (MW:443); C, 59.60; H, 5.23; N, 6.32. Found: C, 59.32; H, 5.04; N, 6.52.
Methyl 4-(6-bromo-1H-indol-3-yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (4)
Yield 88%. m.p. 227 °C. IR (ν, cm−1): 3342 (N–H), 1682 (C = O, ester), 1608 (C = O, ketone). 1H-NMR (δ) 0.75 (3H; s; 7-CH3), 0.97 (3H; s; 7-CH3), 1.92 (H; d; J: 16.4 Hz; H-6a), 2.12 (H; d; J: 16.4 Hz; H-6b), 2.25 (3H; s; 2-CH3), 2.27 (H; d; J: 17.2 Hz; H-8a), 2.38 (H; d; J: 17.2 Hz; H-8b), 3.48 (3H; s; OCH3), 5.09 (1H; s; H-4), 6.88 (1H; d; J: 2.4 Hz; indole H-2), 7.00 (1H; dd; J: 8.4/1.6 Hz; indole H-5), 7.75 (1H; d; J: 1.6 Hz; indole H-7), 7.79 (1H; d; J: 8.4 Hz; indole H-4), 9.10 (1H; s; HHQ N–H), 10.76 (1H; s; indole N–H). 13C-NMR (δ) 18.1, 26.5, 27.0, 28.0, 29.7, 31.9, 50.3, 111.6, 113.1, 113.7, 115.7, 120.8, 121.2, 121.6, 123.7, 124.6, 137.0, 139.2, 144.2, 149.0, 167.5, 194.2. ESI-MS (m/z): 465/467 [M + Na]+/[M + 2+Na]+, 248 (100%). Anal. Calcd. for C22H23BrN2O3 (MW: 443); C, 59.60; H, 5.23; N, 6.32. Found: C, 59.26; H, 4.95; N, 5.90.
Ethyl 4-(5-bromo-1H-indol-3-yl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (5)
Yield 80%. m.p. 183 °C. IR (ν, cm−1): 3339, 3283 (N–H), 1657 (C = O, ester), 1598 (C = O, ketone). 1H-NMR (δ) 0.87 (3H; s; 6-CH3), 0.98 (3H; s; 6-CH3), 1.15 (3H; t; J: 6.8 Hz; CH2CH 3), 1.62–1.76 (2H; m; H-7), 2.25 (3H; s; 2-CH3), 2.46–2.54 (2H; m; H-8), 3.98 (2H; q; J: 6.8 Hz; OCH2), 5.08 (1H; s; H-4), 6.83 (1H; d; J: 2.4 Hz; indole H-2), 7.09 (1H; dd; J: 8.4/2.0 Hz; indole H-6, 7.22 (1H; d; J: 8.4 Hz; indole H-7), 7.73 (1H; d; J: 2.0 Hz; indole H-4), 9.16 (1H; s; HHQ N–H), 10.85 (1H; s; indole N–H). ESI-MS (m/z): 479/481[M + Na]+/[M + 2+Na]+ (100%). Anal. Calcd. for C23H25BrN2O3 (MW: 457); C, 60.40; H, 5.51; N, 6.13. Found: C, 60.20; H, 5.30; N, 5.95.
Ethyl 4-(5-bromo-1H-indol-3-yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (6)
Yield 82%. m.p. 195 °C. IR (ν, cm−1): 3301 (N–H), 1706 (C = O, ester), 1587 (C = O, ketone). 1H-NMR (δ) 0.80 (3H; s; 7-CH3), 1.00 (3H; s; 7-CH3), 1.12 (3H; t; J: 7.2 Hz; CH2CH 3), 1.94 (H; d; J: 16 Hz; H-8a), 2.14 (H; d; J: 16 Hz; H-8b), 2.26 (3H; s; 2-CH3), 2.30 (H; d; J: 16.8 Hz; H-6a), 2.42 (H; d; J: 16.8 Hz; H-6b), 3.95 (2H; q; J: 7.2 Hz; OCH2), 5.07 (1H; s; H-4), 6.93 (1H; d; J: 2.4 Hz; indole H-2), 7.08 (1H; dd; J: 8.4/1.6 Hz; indole H-6), 7.22 (1H; d; J: 8.4 Hz; indole H-7), 7.70 (1H; d; J: 1.6 Hz; indole H-4), 9.17 (1H; s; HHQ N–H), 10.88 (1H; s; indole N–H). ESI-MS (m/z): 479/481 [M + Na]+/[M + 2+Na]+ (100%). Anal. Calcd. for C23H25BrN2O3 (MW: 457); C, 60.40; H, 5.51; N, 6.13. Found: C, 60.64; H, 5.09; N, 5.77.
Ethyl 4-(6-bromo-1H-indol-3-yl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (7)
Yield 76%. m.p. 190 °C. IR (ν, cm−1): 3281, 3222 (N–H), 1676 (C = O, ester), 1644 (C = O, ketone). 1H-NMR (δ) 0.84 (3H; s; 6-CH3), 0.96 (3H; s; 6-CH3), 1.05 (3H; t; J: 6.8 Hz; CH2CH 3), 1.60–1.71 (2H; m; H-7), 2.24 (3H; s; 2-CH3), 2.45–2.50 (2H; m; H-8), 3.95 (2H; q; 6.8 Hz; OCH2), 5.08 (1H; s; H-4), 6.84 (1H; d; J: 2.0 Hz; indole H-2), 7.01 (1H; dd; J: 8.4/2.0 Hz; indole H-5), 7.41 (1H; d; J: 2.0 Hz; indole H-7), 7.49 (1H; d; J: 8.4 Hz; indole H-4), 9.08 (1H; s; HHQ N–H), 10.73 (1H; s; indole N–H). 13C-NMR (δ) 14.2, 18.1, 18.5, 22.8, 24.1, 25.2, 27.2, 34.2, 39.5, 56.0, 58.9, 109.1, 113.1, 120.8, 121.2, 121.9, 123.7, 124.8, 137.0, 143.8, 149.1, 167.2, 199.4. ESI-MS (m/z): 479/481 [M + Na]+/[M + 2+Na]+ (100%). Anal. Calcd. for C23H25BrN2O3 (MW: 457); C, 60.40; H, 5.51; N, 6.13. Found: C, 60.43; H, 5.76; N, 6.01.
Ethyl 4-(6-bromo-1H-indol-3-yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (8)
Yield 69%. m.p. 147 °C. IR (ν, cm−1): 3468, 3210 (N–H), 1679 (C = O, ester), 1606 (C = O, ketone). 1H-NMR (δ) 0.77 (3H; s; 7-CH3), 0.99 (3H; s; 7-CH3), 1.10 (3H; t; J: 7.2 Hz; CH2CH 3), 1.93 (H; d; J: 16 Hz; H-8a), 2.13 (H; d; J: 16 Hz; H-8b), 2.26 (3H; s; 2-CH3), 2.28 (H; d; J: 16.8 Hz; H-6a), 2.40 (H; d; J: 16.8 Hz; H-6b), 3.94 (2H; q; J: 7.2 Hz; OCH2), 5.10 (1H; s; H-4), 6.91 (1H; d; J: 2.0 Hz; indole H-2), 7.02 (1H; dd; J: 8.4/2.0 Hz; indole H-5), 7.43 (1H; d; J: 2.0 Hz; indole H-7), 7.48 (1H; d; J: 8.4 Hz; indole H-4), 9.08 (1H; s; HHQ N–H), 10.78 (1H; s; indole N–H). 13C-NMR (δ) 14.2, 18.1, 26.5, 27.1, 29.1, 32.0, 39.5, 50.3, 58.9, 103.7 109.7, 113.1, 113.7, 120.8, 121.2, 121.8, 123.8, 124.8, 137.0, 143.9, 148.9, 167.1, 194.3. ESI-MS (m/z): 479/481 [M + Na]+/[M + 2+Na]+ (100%). Anal. Calcd. for C23H25BrN2O3 (MW: 457); C, 60.40; H, 5.51; N, 6.13. Found: C, 60.83; H, 5.55; N, 6.00.
Isopropyl 4-(5-bromo-1H-indol-3-yl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (9)
Yield 63%. m.p. 121 °C. IR (ν, cm−1): 3223 (N–H), 1676 (C = O, ester), 1607 (C = O, ketone). 1H-NMR (δ) 0.75 (3H; s; 6-CH3), 0.95 (3H; s; 6-CH3), 0.97 (3H; d; J: 6.4 Hz; CH(CH 3)2), 1.17 (3H; d; J: 6.4 Hz; CH(CH 3)2, 1.62–1.76 (2H; m; H-7), 2.27 (3H; s; 2-CH3), 2.51–2.57 (2H; m; H-8), 4.76–4.81 (H; m; OCH), 5.01 (1H; s; H-4), 6.94 (1H; d; J: 2.4 Hz; indole H-2), 7.08 (1H; dd; J: 8.8/2.0 Hz; indole H-6), 7.22 (1H; d; J: 8.8 Hz; indole H-7), 7.71 (1H; d; J: 2.0 Hz; indole H-4), 9.13 (1H; s; HHQ N–H), 10.85 (1H; s; indole N–H). 13C-NMR (δ) 18.1, 22.9, 24.1, 24.9, 31.4, 38.8, 40.0, 50.3, 104.8, 110.7, 122.4, 125.0, 125.8, 126.0, 126.2, 126.5, 127.6, 129.7, 136.2, 142.2, 144.0, 149.3, 167.4, 199.5. ESI-MS (m/z): 493/495 [M + Na]+/[M + 2+Na]+, 234 (100%). Anal. Calcd. for C24H27BrN2O3 (MW: 471); C, 61.15; H, 5.77; N, 5.94. Found: C, 61.36; H, 5.73; N, 6.12.
Isopropyl 4-(5-bromo-1H-indol-3-yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (10)
Yield 65%. m.p. 198 °C. IR (ν, cm−1): 3303 (N–H), 1701 (C = O, ester), 1650 (C = O, ketone). 1H-NMR (δ) 0.81 (3H; s; 7-CH3), 0.97 (3H; d; J: 6.4 Hz; CH(CH 3)2), 1.00 (3H; s; 7-CH3), 1.17 (3H; d; J: 6.4 Hz; CH(CH 3)2), 1.93 (H; d; J: 16.4 Hz; H-8a), 2.14 (H; d; J: 16.4 Hz; H-8b), 2.25 (3H; s; 2-CH3), 2.29 (H; d; J: 17.2 Hz; H-6a), 2.42 (H; d; J: 17.2 Hz; H-6b), 4.76–4.81 (1H; m; OCH), 5.05 (1H; s; H-4), 6.94 (1H; d; J: 2.4 Hz; indole H-2), 7.08 (1H; dd; J: 8.8/2.0 Hz; indole H-6), 7.22 (1H; d; J:8.8 Hz; indole H-7), 7.71 (1H; d; J: 2.0 Hz; indole H-4), 9.13 (1H; s; HHQ N–H), 10.85 (1H; s; indole N–H). 13C-NMR (δ) 18.1, 21.5, 21.8, 26.5, 27.0, 29.1, 32.0, 39.5, 50.3,66.0, 104.3, 109.8, 110.8, 113.1, 121.8, 122.0, 122.6, 124.5, 127.6, 134.6, 143.5, 149.0, 166.7, 194.3. ESI-MS (m/z): 493/495 [M + Na]+/[M + 2+Na]+ (100%). Anal. Calcd. for C24H27BrN2O3 (MW: 471); C, 61.15; H, 5.77; N, 5.94. Found: C, 61.02; H, 5.82; N, 6.23.
Isopropyl 4-(6-bromo-1H-indol-3-yl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (11)
Yield 70%. m.p. 202 °C. IR (ν, cm−1): 3275, 3217 (N–H), 1676 (C = O, ester), 1576 (C = O, ketone). 1H-NMR (δ) 0.83 (3H; s; 6-CH3), 0.97 (3H; s; 6-CH3), 0.98 (3H; d; J: 6.0 Hz; CH(CH 3)2), 1.15 (3H; d; J: 6.0 Hz; CH(CH 3)2), 1.63–1.70 (2H; m; H-7), 2.25 (3H; s; 2-CH3), 2.47–2.50 (2H; m; H-8), 4.75–4.82 (1H; m; OCH), 5.08 (1H; s; H-4), 6.88 (1H; d; J: 2.4 Hz; indole H-2), 7.04 (1H; dd; J: 8.8/1.6 Hz; indole H-5), 7.43 (1H; d; J: 1.6 Hz; indole H-7), 7.51 (1H; d; J: 8.8 Hz; indole H-4), 9.08 (1H; s; HHQ NH), 10.76 (1H; s; indole N–H). 13C-NMR (δ) 18.1, 21.6, 21.8, 22.9, 24.1, 25.2, 27.4, 34.2, 39.5, 48.6, 65.9, 103.8, 109.0, 113.1, 113.7, 120.7, 121.3, 122.0, 123.8, 124.9, 137.0, 143.5, 166.8, 199.4. ESI-MS (m/z): 493/495 [M + Na]+/[M + 2+Na]+ (100%). Anal. Calcd. for C24H27BrN2O3 (MW: 471); C, 61.15; H, 5.77; N, 5.94. Found: C, 60.97; H, 5.81; N, 6.16.
Isopropyl 4-(6-bromo-1H-indol-3-yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (12)
Yield 64%. m.p. 150 °C. IR (ν, cm−1): 3420, 3220 (N–H), 1678 (C = O, ester), 1649 (C = O, ketone). 1H-NMR (δ) 0.76 (3H; s; 7-CH3), 0.96 (3H; d; J: 6.0 Hz; CH(CH 3)2), 0.99 (3H; s; 7-CH3), 1.14 (3H; d; J: 6.0 Hz; CH(CH 3)2), 1.92 (H; d; J: 16 Hz; H-8a), 2.12 (H; d; J: 16 Hz; H-8b), 2.25 (3H; s; 2-CH3), 2.28 (H; d; J: 16.8 Hz; H-6a), 2.40 (H; d; J: 16.8 Hz; H-6b), 4.78 (1H; m; OCH), 5.08 (1H; s; H-4), 6.92 (1H; d; J: 2.4 Hz; indole H-2), 7.02 (1H; dd; J: 8.0/2.4 Hz; indole H-5), 7.42 (1H; s; J: 2.4 Hz; indole H-7), 7.47 (1H; d; J: 8.0 Hz; indole H-4), 9.08 (1H; s; HHQ N–H), 10.79 (1H; s; indole N–H). 13C-NMR (δ) 18.1, 21.5, 21.8, 26.5, 27.2, 29.1, 32.0, 39.5, 50.3, 65.9, 104.0, 109.6, 113.0, 113.7, 120.7, 121.3, 121.8, 123.9, 124.8, 136.9, 143.5, 148.9, 166.6, 194.3. ESI-MS (m/z): 493/495 [M + Na]+/[M + 2+Na]+ (100%). Anal. Calcd. for C24H27BrN2O3 (MW: 471); C, 61.15; H, 5.77; N, 5.94. Found: C, 60.65; H, 5.98; N, 5.97.
Isobutyl 4-(5-bromo-1H-indol-3-yl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (13)
Yield 59%. m.p. 157 °C. IR (ν, cm−1): 3320 (N–H), 1614 (C = O, ester), 1572 (C = O, ketone). 1H-NMR (δ) 0.73 (3H; d; J: 6.8 Hz; CH(CH 3)2), 0.75 (3H; d; J: 6.8 Hz; CH(CH 3)2), 0.75 (3H; s; 6-CH3), 0.95 (3H; s; 6-CH3), 1.58–1.65 (1H; m; CH2CH(CH3)2), 1.62–1.76 (2H; m; H-7), 2.25 (3H; s; 2-CH3), 2.51–2.57 (2H; m; H-8), 3.30 (2H; m; OCH2), 5.11 (1H; s; H-4), 6.83 (1H; d; J: 2.4 Hz; indole H-2), 7.00 (H; dd; J: 8.4/1.6 Hz; indole H-6), 7.40 (H; d; J:1.6 Hz; indole H-7), 7.51 (H; d; J: 8.4 Hz; indole H-4), 9.13 (1H; s; HHQ N–H), 10.74 (1H; s; indole NH). 13C-NMR (δ) 18.1, 22.9, 24.1, 24.9, 31.4, 38.8, 40.0, 50.3, 104.8, 110.7, 113.4, 117.2, 122.4, 125.0, 125.8, 126.2, 126.5, 127.6, 129.7, 136.2, 142.2, 144.0, 149.3, 167.4199.5. ESI-MS (m/z): 507/509 [M + Na]+/[M + 2+Na]+ (100%). Anal. Calcd. for C25H29BrN2O3 (MW: 485); C, 61.86; H, 6.02; N, 5.77. Found: C, 61.65; H, 5.98; N, 5.97.
Isobutyl 4-(5-bromo-1H-indol-3-yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (14)
Yield 49%. m.p. 213 °C. IR (ν, cm−1): 3308 (N–H), 1702 (C = O, ester), 1648 (C = O, ketone). 1H-NMR (δ) 0.76 (3H; d; J: 6.8 Hz; CH(CH 3)2), 0.77 (3H; s; 7-CH3), 0.80 (3H; d; J: 6.8 Hz; CH(CH 3)2), 0.99 (3H; s; 7-CH3), 1.76–1.82 (H; m; CH2CH(CH3)2), 1.95 (H; d; J: 16 Hz; H-8a), 2.14 (H; d; J: 16 Hz; H-8b), 2.28 (H; d; J: 16.8 Hz; H-6a), 2.29 (3H; s; 2-CH3), 2.41 (H; d; J: 16.8 Hz; H-6b), 3.66–3.73 (2H; m; OCH2), 5.09 (1H; s; H-4), 6.93 (1H; d; J: 2.4 Hz; indole H-2), 7.08 (1H; dd; J: 8.4/2.0 Hz; indole H-6), 7.21 (1H; d; J: 8.4 Hz; indole H-7), 7.74 (1H; d; J: 2.0 Hz; indole H-4), 9.18 (1H; s; HHQ N–H), 10.87 (1H; s; indole N–H). 13C-NMR (δ) 18.2, 18.9, 19.0, 26.4, 26.9, 27.3, 29.1, 32.0, 39.5, 50.3, 69.1, 103.7, 109.9, 110.7, 113.0, 121.6, 122.0, 122.7, 124.4, 127.5, 134.7, 144.1 148.9, 167.1, 194.3. ESI-MS (m/z): 507/509 [M + Na]+/[M + 2+Na]+ (100%). Anal. Calcd. for C25H29BrN2O3 (MW: 485); C, 61.86; H, 6.02; N, 577. Found: C, 61.86; H, 6.08; N, 6.05.
Isobutyl 4-(6-bromo-1H-indol-3-yl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (15)
Yield 64%. m.p.164 °C. IR (ν, cm−1): 3293 (N–H), 1666 (C = O, ester), 1609 (C = O, ketone). 1H-NMR (δ) 0.73 (3H; d; J: 6.8 Hz; CH(CH 3)2), 0.75 (3H; d; J: 6.8 Hz; CH(CH 3)2), 0.81 (3H; s; 6-CH3), 0.96 (3H; s; 6-CH3), 1.58–1.65 (1H; m; CH2CH(CH3)2), 1.67–1.76 (2H; m; H-7), 2.27 (3H; s; 2-CH3), 2.45–2.51 (2H; m; H-8), 3.30 (2H; m; OCH2), 5.10 (1H; s; H-4), 6.83 (1H; d; J: 2.4 Hz; indole H-2), 7.00 (1H; dd; J: 8.4/1.6 Hz; indole H-5), 7.40 (1H; d; J:1.6 Hz; indole H-7), 7.51 (1H; d; J: 8.4 Hz; indole H-4), 9.13 (1H; s; HHQ N–H), 10.74 (1H; s; indole NH). 13C-NMR (δ) 18.2, 18.9, 19.0, 22.8, 24.1, 25.3, 27.2, 27.3, 34.1, 39.5, 69.1, 103.1, 109.2, 113.1, 113.6, 120.8, 121.3, 121.8, 123.8, 124.7, 137.1, 144.2, 149.0, 167.2, 199.4. ESI-MS (m/z): 507/509 [M + Na]+/[M + 2+Na]+ (100%). Anal. Calcd. for C25H29BrN2O3 (MW: 485); C, 61.86; H, 6.02; N, 5.77. Found: C, 61.63; H, 6.16; N, 6.06.
Isobutyl 4-(6-bromo-1H-indol-3-yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8 hexahydroquinoline-3-carboxylate (16)
Yield 68%. m.p. 209 °C. IR (ν, cm−1): 3302 (N–H), 1595 (C = O, ester), 1528 (C = O, ketone). 1H-NMR (δ) 0.72 (3H; d; J: 7.2 Hz; CH(CH 3)2), 0.75 (3H; d; J: 7.2 Hz; CH(CH 3)2), 0.77 (3H; s; 7-CH3), 0.97 (3H; s; 7-CH3), 1.72–1.79 (1H; m; CH2CH(CH3)2), 1.92 (H; d; J: 16 Hz; H-8a), 2.12 (H; d; J: 16 Hz; H-8b), 2.25 (H; d; J: 16.8 Hz; H-6a), 2.28 (3H; s; 2-CH3), 2.38 (H; d; J: 16.8 Hz; H-6b), 3.68 (2H; d; OCH2), 5.11 (1H; s; H-4), 6.88 (1H; d; J: 2.0 Hz; indole H-2), 7.00 (1H; dd; J: 8.4/2.0 Hz; indole H-5), 7.41 (1H; d; J: 2.0 Hz; indole H-7), 7.50 (1H; d; J: 8.4 Hz; indole H-4), 9.12 (1H; s; HHQ N–H), 10.77 (1H; s; indole N–H). 13C-NMR (δ) 18.3, 18.9, 26.5, 27.1, 27.3, 28.0, 29.1, 32.0, 39.5, 50.3, 69.1, 103.4, 109.8, 113.1, 113.7, 120.7, 121.3, 121.6, 123.8, 123.8, 137.0, 144.2, 148.8, 167.1, 194.3. ESI-MS (m/z): 507/509 [M + Na]+/[M + 2+Na] + (100%). Anal. Calcd. for C25H29BrN2O3 (MW:485); C, 61.86; H, 6.02; N, 5.77. Found: C, 60.89; H, 5.85; N, 5.77.
Tert-butyl 4-(5-bromo-1H-indol-3-yl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (17)
Yield 48%. m.p. 179 °C. IR (ν, cm−1): 3303 (N–H), 1676 (C = O, ester), 1599 (C = O, ketone). 1H-NMR (δ) 0.86 (3H; s; 6-CH3), 0.97 (3H; s; 6-CH3), 1.31 (9H; s; C(CH3)3), 1.62–1.76 (2H; m; H-7), 2.19 (3H; s; 2-CH3), 2.46–2.52 (2H; m; H-8), 5.01 (1H; s; H-4), 6.85 (1H; d; J: 2.0 Hz; indole H-2), 7.07 (H; dd; J: 8.4/2.0 Hz; indole H-6), 7.21 (H; d; J:8.4 Hz; indole H-7), 7.76 (H; d; J: 2.0 Hz; indole H-4), 9.08 (1H; s; HHQ N–H), 10.87 (1H; s; indol N–H). 13C-NMR (δ) 18.1, 18.9, 25.2, 27.3, 27.9, 34.3, 38.9, 39.3, 40.1, 48.6, 78.7, 105.2, 108.8, 110.7, 113.1, 121.6, 122.6, 124.2, 127.6, 134.8, 142.5149.4, 166.9, 172.7, 199.4. ESI-MS (m/z): 507/509 [M + Na]+/[M + 2+Na]+ (100%). Anal. Calcd. for C25H29BrN2O3 (MW: 485); C, 61.86; H, 6.02; N, 5.77. Found: C, 61.95; H, 5.73; N, 5.85.
Tert-butyl 4-(5-bromo-1H-indol-3-yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8–hexahydroquinoline-3 carboxylate (18)
Yield 56%. m.p. 168 °C. IR (ν, cm−1): 3303 (N–H), 1677 (C = O, ester), 1600 (C = O, ketone). 1H-NMR (δ) 0.80 (3H; s; 7-CH3), 0.99 (3H; s; 7-CH3), 1.29 (9H; s; C(CH3)3), 1.94 (H; d; J: 16.4 Hz; H-8a), 2.12 (H; d; J: 16.4 Hz; H-8b), 2.21 (3H; s; 2-CH3), 2.29 (H; d; J: 17.2 Hz; H-6a), 2.39 (H; d; J: 17.2 Hz; H-6b), 5.01 (1H; s; H-4), 6.92 (1H; d; J: 2.4 Hz; indole H-2), 7.08 (1H; dd; J: 8.4/1.6 Hz; indole H-6), 7.22 (1H; d; J:8.4 Hz; indole H-7), 7.72 (1H; d; J: 1.6 Hz; indole H-4), 9.03 (1H; s; HHQ N–H), 10.83 (1H; s; indole N–H). 13C-NMR (δ) 18.1, 26.5, 27.3, 27.9, 29.1, 32.0, 39.3, 39.5, 39.7,50.3, 78.6, 105.5, 109.4, 110.8, 113.1, 121.6, 122.1, 122.6, 124.3, 127.6, 134.7, 142.4, 149.1, 166.8, 194.2. ESI-MS (m/z): 507/509 [M + Na]+/[M + 2+Na]+, 256 (100%). Anal. Calcd. for C25H29BrN2O3 (MW: 485); C, 61.86; H, 6.02; N, 5.77. Found: C, 60.17; H, 5.63; N, 5.89.
Tert-butyl 4-(6-bromo-1H-indol-3-yl)-2,6,6-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (19)
Yield 55%. m.p. 207 °C. IR (ν, cm−1): 3352, 3281 (N–H), 1673 (C = O, ester), 1578 (C = O, ketone). 1H-NMR (δ) 0.84 (3H; s; 6-CH3), 0.96 (3H; s; 6-CH3), 1.28 (9H; s; C(CH3)3), 1.62–1.69 (2H; m; H-7), 2.20 (3H; s; 2-CH3), 2.44–2.50 (2H; m; H-8), 5.03 (1H; s; H-4), 6.85 (1H; d; J: 2.0 Hz; indole H-2), 7.02 (1H; dd; J: 8.8/2.0 Hz; indole H-5), 7.42 (1H; d; J: 2.0 Hz; indole H-7), 7.53 (1H; d; J: 8.8 Hz; indole H-4), 8.98 (1H; s; HHQ N–H), 10.73 (1H; s; indole N–H). 13C-NMR (δ) 18.1, 18.3, 22.9, 24.1, 25.3, 27.6, 27.9, 34.2, 39.3, 39.5, 39.7, 78.5, 105.0, 108.8, 111.1, 113.0, 113.6, 120.6, 121.4, 123.6, 137.1, 142.5, 149.2, 165.7, 196.1. ESI-MS (m/z): 507/509 [M + Na]+/[M + 2+Na]+, 256 (100%). Anal. Calcd. for C25H29BrN2O3 (MW: 485); C, 61.86; H, 6.02; N, 5.77. Found: C, 61.44; H, 5.96; N, 5.90.
Tert-butyl 4-(6-bromo-1H-indol-3-yl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (20)
Yield 40%. m.p. 166 °C. IR (ν, cm−1): 3363, 3283 (N–H), 1678 (C = O, ester), 1580 (C = O, ketone). 1H-NMR (δ) 0.76 (3H; s; 7-CH3), 0.98 (3H; s; 7-CH3), 1.27 (9H; s; (CH3)3), 1.91 (H; d; J: 16 Hz; H-8a), 2.10 (H; d; J: 16 Hz; H-8b), 2.21 (3H; s; 2-CH3), 2.33 (H; d; J: 16.8 Hz; H-6a), 2.37 (H; d; J: 16.8 Hz; H-6b), 5.03 (1H; s; H-4), 6.90 (1H; d; J: 2.0 Hz; indole H-2), 7.00 (1H; dd; J: 8.8/2.0 Hz; indole H-5), 7.43 (1H; d; J: 2.0 Hz; indole H-7), 7.49 (1H; d; J: 8.8 Hz; indole H-4), 8.99 (1H; s; HHQ N–H), 10.76 (1H; s; indole N–H). 13C-NMR (δ) 18.1, 18.3, 26.6, 27.5, 27.9, 29.0, 31.9, 39.5, 48.6, 50.3, 78.5, 105.2, 109.4, 113.0, 113.7, 120.6, 121.3, 121.7, 123.7, 124.8, 137.0, 142.5, 149.0, 166.7, 194.1. ESI-MS (m/z): 507/509 [M + Na]+/[M + 2+Na]+, 256 (100%). Anal. Calcd. for C25H29BrN2O3 (MW: 485); C, 61.86; H, 6.02; N, 5.77. Found: C, 61.44; H, 5.96; N, 5.89.
Antitubercular activity and cytotoxicity screening
All newly synthesized compounds were screened for their in vitro antimycobacterial activity against M. tuberculosis H37Rv (ATCC 27294 strain) in Middlebrook 7H9 medium supplemented with OADC growth supplement by the MABA (Microplate Alamar Blue Assay) method similar to that recommended by the National Committee for Clinical Laboratory Standards for the determination of the minimum inhibitory concentration (MIC) in duplicate [26]. The MABA assay is based on oxidation–reduction of alamar blue as a function of cell growth. The blue, nonfluorescent, oxidized form of the dye becomes pink and fluorescent upon reduction in the presence of growing cells. This change in color is measured, and the MIC is defined as the minimum concentration of compounds required to inhibit 99% of bacterial growth. The MIC values of the synthesized compounds determined were compared with that of the standard drugs, isoniazid, ethambutol and ciprofloxacin.
The in vitro cytotoxicity of the privileged antitubercular active analogues with lower MIC values were assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay against growth inhibition of RAW 264.7 cells at 25 μg/mL concentration [27].
Molecular docking studies
The chemical formulas of the compounds were drawn in Chembiodraw Ultra 12.0 and saved as a Simplified Molecule Input Entry System (SMILES) file. The file was transfered to LigandScout 3.1. [28] in order to prepare the appropriate file needed for the docking study. For this purpose, the structures were geometrically optimized and energy minimized to a 3D structure using the MMFF94x force field in LigandScout 3.1. The molecular docking studies were performed using the published crystal structure of the M. tuberculosis enoyl reductase (InhA) complexed with 1-cyclohexyl-N-(3,5-dichlorophenyl)-5-oxopyrrolidine-3-carboxamide (Protein Data Bank code: 4TZK) [29]. All docking runs were performed using GOLD 5.4 (CCDC; Cambridge, UK) [30] with default parameters and GoldScore as scoring function. Hydrogen atoms were added, and the co-crystallized ligand and all water molecules were removed from the structure. The selection of the active site was based on the position of the co-crystallized ligand and all residues around the original ligand were chosen in a circle of 10 Å. The software LigandScout was used for the visualization and the 3D-pharmacophores analysis.
Results and dıscussıon
Chemistry
In this study, 20 condensed 4-indolyl-1,4-DHP derivatives was obtained via a general Hantzsch procedure by the condensation of 4,4/5,5-dimethyl-1,3-cyclohexanedione, appropriate alkyl acetoacetate, 5/6-bromoindole-3-carboxaldehyde and excess ammonium acetate under microwave heating. Ethanol was employed as the solvent in this reaction due to its excellent microwave-absorbing property [31]. Compared to conventional heating, it is obvious that microwave irradiation shortens the reaction time, which was determined as only 5 min. [32]. Structures of the synthesized compounds are reported in Table 1.


Various spectral methods (IR, 1H-NMR, 13C-NMR, COSY, X-ray analysis and mass spectra) and elemental analysis were carried out to elucidate the structures of the synthesized compounds. In the IR spectra, characteristic stretching bands were observed around 3300 cm−1 due to N–H bonds. Two different C = O bands were also seen, indicating the presence of ester and ketone groups. In the 1H-NMR spectra, the signal of two methyl substituents at the 6/7-position of HHQ ring were separately observed at 0.75–1.00 ppm and as singlets. H-4 protons of the HHQ ring were seen as singlets at 5.01–5.11 ppm. The aromatic protons belonging to the indole ring were at 6.83–7.85 ppm. The N–H protons of the HHQ ring and the indole ring were observed at 8.98–9.20 and 10.73–10.88 ppm, respectively. The methyl groups of the isopropyl and isobutyl moieties produced different signals as separate doublets, because they are no longer equivalent and not in the same environment due to the chiral center at C-4 of the HHQ ring.
In the 13C-NMR spectra, the appropriate number of resonances that exactly fitted the number of carbon atoms was displayed. The signal of the carbonyl group at C-5 of the HHQ ring appeared at about 195 ppm, while the ester carbonyl groups were seen close to 165 ppm. The correlations between the interacting protons of compound 7 were determined by correlation spectroscopy (COSY). The correlations between the H-7 and H-8 protons of HHQ ring, also methylene and methyl protons in the ester side chain were observed. The N–H signal, observed at around 11 ppm, was definitely determined to belong to the N–H proton of the indole ring due to the correlation between N–H and indole H-2. Based on the obtained data; the structure of compound 7 was proved to be ethyl 4-(6-bromo-1H-indole-3-yl)-2,6,6–trimethyl-5-oxo-1,4,5,6,7,8–hexahydroquinoline-3-carboxylate. These correlations are depicted in Fig. 2 and the COSY spectrum is provided as supplementary data.
The mass spectra of the compounds further confirmed the structures of the synthesized compounds by the peaks belonging to molecular ions created by the addition of a sodium ion. As all the target molecules contain one bromine atom, two approximately equal peaks are present in the molecular ion region, depending on which bromine isotope (79Br or 81Br) the molecular ion contains.
X-Ray analysis of the compound 3
The three-dimensional structure of compound 3, evaluated by X-ray crystallography, is shown in Fig. 3. Crystallographic data, molecular structure refinement, the relevant bond lengths and bond angles are presented as supplementary data. In compound 3, the cyclohexene ring is in a sofa conformation [Q T = 0.486(1) Å, θ = 117.3(1)°, φ = 295.0(1)°] and the 1,4-DHP ring is in a slight boat conformation. In the indole ring system, the pyrrole and benzene rings form a dihedral angle of 4.06° (Fig. 3). The molecular structure is stabilized by intra- and intermolecular hydrogen bonds. In the crystal, molecules are linked by N–H•••Br, N–H•••O and C–H•••O hydrogen bonds is shown in Fig. 4. The data belonging to intra- and intermolecular hydrogen bonds is given as supplementary data. The values of the bond lengths and bond angles are accordance with those of the related structures previously reported [33,34,35].
Antitubercular activity and cytotoxicity
The 20 synthesized 4-indolyl-1,4-dihydropyridine derivatives were subjected to an in vitro anti-tubercular activity test against M. tuberculosis H37Rv (ATCC 27294 strain). All the synthesized compounds showed MIC values in the micromolar range of 13.27 to >51.55 µM. Seven compounds exhibited MIC at 13.27–14.11 µM. The in vitro cell viability of the compounds with MIC ≤ 14.11 µM were evaluated against HEK-293T (human embryonic kidney) cell lines at 25 µg/mL concentration by using the MTT assay. The % inhibitory cytotoxicity data are summarized in Table 2, along with the MIC values of the respective compounds. In general, most of the active compounds were found to be non-toxic (<50% inhibition), and compounds 2, 5, 6, 7, 10, 11 and 12 turned out to be the active compounds and anti-tubercular leads for further studies from this series.
As the primary difference between the synthesized compounds is their ester moiety, it is obvious that this group plays the most important role in the ability of these compounds to possess the mentioned activity. The most active derivatives carry ethyl or isopropyl esters; therefore, it can be concluded that increasing the alkyl chain length of the ester group from isopropyl to isobutyl or introducing a bulky structure (tert-butyl) at the same locus mediated a decrease in the activity. When the obtained results are analyzed, no significant differences were observed between the derivatives and the position of the dimethyl groups on the HHQ ring, suggesting that it is not the most critical modification for the preferential activity. The position of the bromine atom on the indole ring at 4-position of the 1,4-DHP ring did not show a clear correlation with the antimycobacterial activity.
Molecular docking studies
The inhibition of the InhA enzyme was suggested as a possible mechanism for the antitubercular properties of 1,4-DHP derivatives after theoretical studies [21]. In order to investigate the mechanism of anti-TB activity and detailed interactions between the synthesized compounds, molecular docking studies were carried out on the crystal structure of M. tuberculosis enoyl reductase (InhA) complexed with 1-cyclohexyl-N-(3,5-dichlorophenyl)-5-oxopyrrolidine-3-carboxamide. All compounds were docked into the active site of InhA.
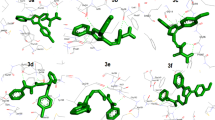
Binding conformation of compound 11 in the binding pocket of InhA and the pharmacophore features and 3D interactions of compound 11 with the binding site of the enzyme are shown in Fig. 5.
The carbonyl oxygen of the hexahydroquinoline ring forms two key hydrogen bonds in the ligand binding pocket with Tyr158 and NAD500 in the same manner as the co-crystalized ligand. The hydrophobic pocket formed by the residues Ile215, Leu218, Ala157 and Met155 is important for hosting the alkyl chain of the ester group. The methyl group is not able to fill the hydrophobic site compared to the ethyl and isopropyl groups, resulting in the loss of hydrophobic interaction. The bulkier groups in the ester chain (isobutyl, t-butyl) cannot be hosted by this hydrophobic pockets. This situation describes the activity differences between various ester alkyl chains. Additional hydrophobic interactions were observed between the 2-CH3 group and the Met103 and Ile 202 and Leu207. Also, the indole ring and the bromine atom were employed for hydrophobic interactions with Met199, Trp222, Leu218 and Phe149. The dimethyl groups on the hexahydroquinoline ring do not interact with the enzyme; therefore the position of them is unlikely to be the factor determining antitubercular activity. Common interactions of the co-crystallized ligand and compound 11 with the binding pocket of 4TZK have been depicted in Fig. 6.
2D interactions of 1-cyclohexyl-N-(3,5-dichlorophenyl)-5-oxopyrrolidine-3-carboxamide with the binding site of the enzyme (a) and common interactions of compound 11 with the co-crystallized ligand (b). Color-coded pharmacophore features: hydrophobic feature (yellow sphere), and hydrogen bond acceptor (red vector). (Color figure online)
Conclusion
A new series of condensed 1,4-dihydropyridine derivatives with indole moiety has been reported as antimycobacterial agents . The compounds were obtained by an easy, very rapid and convenient method under microwave irradiation. Among them, seven compounds were found to be moderate antitubercular agents with a good safety profile. It is important to note that the introduction of ethyl or isopropyl groups to the ester moiety significantly improved the preferential activity. The docking results showed that the compounds are likely to bind InhA as their possible target enzyme. Our data suggest that a condensed dihydropyridine-based scaffold with the indole ring may serve as a new pharmacophore for antimycobacterial activity.
References
P.R. Donald, P.D. van Helden, N. Engl, J. Med. 360, 2393 (2009)
World Health Organisation, Global tuberculosis report 2016, http://www.who.int/tb/publications/global_report/en/
W. H. World Health Organization and Global Tuberculosis Programme, WHO Treatment Guidelines for Drug-Resistant Tuberculosis: 2016 Update. (World Health Organization, 2016)
J.C. Palomino, A. Martin, J. Antimicrob. Chemother. 68, 275 (2013)
L. Nguyen, M.R. Jacobs, Expert Rev. Anti. Infect. Ther. 10, 959 (2012)
C. Bladen, M.G. Gündüz, R. Şimşek, C. Şafak, G.W. Zamponi, Pflügers Arch.Eur. J. Physiol. 466, 1355 (2014)
E. Carosati, P. Ioan, M. Micucci, F. Broccatelli, G. Cruciani, B.S. Zhorov, A. Chiarini, R. Budriesi, Curr. Med. Chem. 19, 4306 (2012)
A. Trivedi, D. Dodiya, B. Dholariya, V. Kataria, V. Bhuva, V. Shah, Chem. Biol. Drug Des. 78, 881 (2011)
K. Sirisha, D. Bikshapathi, G. Achaiah, V.M. Reddy, Eur. J. Med. Chem. 46, 1564 (2011)
A.R. Trivedi, D.K. Dodiya, B.H. Dholariya, V.B. Kataria, V.R. Bhuva, V.H. Shah, Bioorg. Med. Chem. Lett. 21, 5181 (2011)
A. Fassihi, Z. Azadpour, N. Delbari, L. Saghaie, H.R. Memarian, R. Sabet, A. Alborzi, R. Miri, B. Pourabbas, J. Mardaneh, Eur. J. Med. Chem. 44, 3253 (2009)
R. Danac, I.I. Mangalagiu, Eur. J. Med. Chem. 74, 664 (2014)
D. Mantu, V. Antoci, C. Moldoveanu, G. Zbancioc, I.I. Mangalagiu, J. Enzyme Inhib. Med. Chem. 31, 96 (2016)
V. Sharma, P. Kumar, D. Pathak, J. Heterocycl. Chem. 47, 491 (2010)
D.F. Taber, P.K. Tirunahari, Tetrahedron 67, 7195 (2011)
A. Nakhi, B. Prasad, U. Reddy, R.M. Rao, S. Sandra, R. Kapavarapu, D. Rambabu, G. Rama Krishna, C.M. Reddy, K. Ravada, P. Misra, J. Iqbal, M. Pal, Medchemcomm 2, 1006 (2011)
H. Shen, F. Wang, Y. Zhang, Q. Huang, S. Xu, H. Hu, J. Yue, H. Wang, FEBS J. 276, 144 (2009)
A. Loupy (ed.), Microwaves in Organic Synthesis (Wiley-VCH, Weinheim, 2006)
G. Zbancioc, V. Bejan, M. Risca, C. Moldoveanu, I.I. Mangalagiu, Molecules 14, 403 (2009)
S.L. Parikh, G. Xiao, P.J. Tonge, Biochemistry 39, 7645 (2000)
K. Mahnam, A. Sadeghi, M. Mohammadpour, A. Fassihi, Monats Chem. 143, 19 (2012)
A. El-Khouly, M. Gündüz, Ç. Çengelli, R. Şimşek, K. Erol, C. Şafak, S. Yıldırım, R. Butcher, Drug Res. 63, 579 (2013)
G.M. Sheldrick, Acta Crystallogr. Sect. C Struct. Chem. 71, 3 (2015)
O. D. Rigaku, CrysAlis PRO 1.171.38.43 (Oxford Diffraction Ltd, Yarnton, 2015)
R.C. Clark, J.S. Reid, Acta Crystallogr. Sect. A 51, 887 (1995)
L. Collins, S.G. Franzblau, Antimicrob. Agents Chemother. 41, 1004 (1997)
D. Gerlier, N. Thomasset, J. Immunol. Methods 94, 57 (1986)
G. Wolber, T. Langer, J. Chem. Inf. Model. 45, 160 (2005)
X. He, A. Alian, R. Stroud, P.R. Ortiz de Montellano, J. Med. Chem. 49, 6308 (2006)
G. Jones, P. Willett, R.C. Glen, A.R. Leach, R. Taylor, J. Mol. Biol. 267, 727 (1997)
C.O. Kappe, Angew. Chemie Int. Ed. 43, 6250 (2004)
C. Şafak, M.G. Gündüz, S.Ö. İlhan, R. Şimşek, F. İşli, Ş. Yıldırım, G.S.Ö. Fincan, Y. Sarıoğlu, A. Linden, Drug Dev. Res. 73, 332 (2012)
M. Gündüz, E. Albayrak, F. İşli, G. Fincan, Ş. Yildirim, R. Şimşek, C. Şafak, Y. Sarioğlu, S. Yidirim, R. Butcher, J. Serbian Chem. Soc. 81, 729 (2016)
M.G. Gündüz, R.J. Butcher, S. Öztürk Yildirim, A. El-Khouly, C. Şafak, R. Şimşek, Acta Crystallogr. Sect. E. 68, o3404 (2012)
S. Öztürk Yildirim, R.J. Butcher, M.G. Gündüz, A. El-Khouly, R. Şimşek, C. Şafak, Acta Crystallogr. Sect. E. 69, o40 (2013)
Acknowledgement
The authors acknowledge the financial support provided by Scientific Research Fund of Hacettepe University, Turkey through Project THD-2016-9674.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baydar, E., Gündüz, M.G., Krishna, V.S. et al. Synthesis, crystal structure and antimycobacterial activities of 4-indolyl-1,4-dihydropyridine derivatives possessing various ester groups. Res Chem Intermed 43, 7471–7489 (2017). https://doi.org/10.1007/s11164-017-3087-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3087-0