Abstract
Tuberculosis (TB) is a contagious disease caused by M. tuberculosis (Mtb) affecting people across the globe. Quinoline and chalcone cores have good anti-tubercular properties; thus, we have designed a hybrid scaffold containing quinoline and chalcone. A series of 3-(quinolin-3-yl)-1-phenylprop-2-en-1-one analogs 7a-p and 8a-k were synthesized through different reactions involving nucleophilic substitution, Vilsmeier Haack formylation, Claisen Schmidt condensation, and demethylation. Spectroscopic methods, including 1H NMR, 13C NMR, IR, and HRMS, were used to characterize all synthesized compounds. The anti-tubercular activity of compounds 7a-p and 8a-k was assessed against Mtb H37Rv (ATCC 27294). These compounds demonstrated anti-tubercular activity against H37Rv in the range of 6.25–50 μM. Swiss ADME’s in silico computational studies showed that the ADME parameters were better and had a good pharmacokinetic profile. The compounds 8a, 7a, and 7p showed the most potential as anti-TB activity against Mtb H37Rv in this study, with MIC values of 6.25 μM, 12.5 μM, and 10 μM, respectively.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) is a highly contagious disease that is mainly caused by Mycobacterium tuberculosis (Mtb); it mainly affects the pulmonary respiratory system of the body but is not necessarily restricted to this organ [1]. The World Health Organization considers that, even though TB is curable, the annual cost for its prevention, diagnosis, and treatment would be US$ 13 billion until 2022. India (41%), Indonesia (14%), and the Philippines (12%) are the nations where the number of TB diagnoses increased the highest between 2019 and 2020 [2]. The rise in incidence rates of resistance to commonly used antibiotics, limited health coverage, and insufficient diagnostic procedures are the major reasons for the growing number of deaths due to TB infection. The term multidrug-resistant strain of TB (MDR-TB) is a strain that is resistant to both first-line medications used to treat the infection, such as isoniazid and rifampicin. The extensively drug-resistant strain of TB (XDR-TB) is a subtype of TB that is resistant not only to first-line medications but also to second-line anti-TB agents like fluoroquinolones and to second-line injectable treatments (e.g., kanamycin, amikacin, and capreomycin). The available drugs are not effective against the highly drug-resistant strain of TB (XXDR-TB) [3,4,5,6,7].
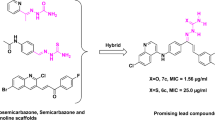
Our interest in creating novel anti-TB agents prompted us to concentrate on structural alterations to the quinoline scaffold, which demonstrated significant antimycobacterial action as previously published by researchers (Fig. 1) [8,9,10,11,12,13,14,15]. According to the previously established structure-activity relationship (SAR), it was found that the nature and position of substituents on the quinoline scaffold were extremely sensitive to maintain the anti-mycobacterial activity against susceptible Mtb strains as well as isoniazid and rifampicin-resistant clinical isolates of Mtb. Bedaquiline is a recently FDA-approved drug that inhibits the mycobacterial ATP synthase. However, several side effects have been associated with the use of bedaquiline. It inhibits the cardiac potassium hERG channel and increases the risk of delayed ventricular repolarization (enhanced QT interval) [16, 17]. So, considering the significant role of quinoline in the research, we have designed and synthesized a series of substituted quinoline analogs. To advance our understanding of previously published SAR, we have reported the antimycobacterial activity of a series of 3-(2-hydroxyquinolin-3-yl)-1-phenylprop-2-en-1-one. Specifically, we varied the substituents at positions 2nd and 6th of the quinoline scaffold, as studied previously to be the most important position for its anti-TB activity [18, 19]. Additionally, we studied the in silico absorption, distribution, metabolism, and excretion (ADME) properties of the quinolines.
Result and discussion
In the present research, we have designated the substituted quinoline scaffold as a core moiety reported to have anti-tubercular activities [11, 20,21,22,23,24]. We have designed quinoline-chalcone hybrid analogs as an anti-tubercular agent by inferring various literature.
Chemistry
The route for synthesizing 3-(2-hydroxyquinolin-3-yl)-1-phenylprop-2-en-1-one (8 a-k) analogs has been described in Scheme 1. The preparation of N-phenylacetamide (3 a-b) was achieved via the reaction of substituted aniline (1) with acetyl chloride (2) in the presence of triethylamine as a base at room temperature. Compound 3 undergoes a Vilsmeier Haack formylation reaction in the presence of a Vilsmeier reagent to yield 2-chloro-3-formylquinoline (4a-b). Further, the compound 2-chloro-3-formylquinoline (4) undergoes nucleophilic addition reaction in the presence of methanol, which acts as a solvent as well as a reagent to give 2-methoxy-3-formylquinoline (5a-c). Compound 5 undergoes Claisen Schmidt condensation with substituted acetophenones (6) gave 3-(2-methoxyquinolin-3-yl)-1-phenylprop-2-en-1-one (7a-p), which further upon demethylation furnished 3-(2-hydroxyquinolin-3-yl)-1-phenylprop-2-en-1-one (8a-k) (Table 1).
Anti-tubercular activity
The minimum inhibitory concentration (MIC) against Mtb H37Rv strain was determined for the synthesized compounds by the previously reported methods [25, 26]. The MIC value is the lowest concentration at which bacterial growth is inhibited. The compounds 7a-p and 8a-k with various substituents at positions 2nd and 6th of the quinoline nucleus were tested for anti-TB activity. Among the series of 7a-p compounds, 7a, 7 g, and 7p showed anti-TB activity against the Mtb H37Rv strain in the 10–50 μM range. 7p, having dimethoxy group, showed a good MIC value of 10 µM while compound 7a showed a MIC value of 12.5 µM with a single substitution of -OMe at position 2nd of the quinoline nucleus (Table 2).
Further, we introduced the -OH group at position 2nd of quinoline via demethylation as series 8a-k compounds. Compounds 8a-k were tested for anti-TB activity against Mtb H37Rv strain in the 6.25–50 μM range. Among them, compound 8a showed the best anti-TB activity, having a MIC value of 6.25 μM (Table 2). While studying the SAR of these molecules, we found that it was advantageous to retain anti-TB activity when substituents such as a methoxy were present at the 2nd position of the quinoline core because a change of methoxy to ethoxy at the 2nd position of the quinoline nucleus led to a loss of the activity. Surprisingly, substitution with a hydroxy group at the 2nd position of the quinoline nucleus showed better activity with MIC of 6.25 μM. Substitution at position 6th of the quinoline nucleus with the electron-withdrawing or electron donor group does not impact the anti-TB activity. The di-methoxy group at the aryl ring positively impacted anti-TB activity (Fig. 2).
Drug likeness and ADME studies
Pre-screening experiments for drug similarity were conducted using Lipinski’s rule of five. Using common pharmacokinetics parameters, such as absorption, distribution, metabolism, and excretion (ADME), these drugs’ various physicochemical characteristics were computed by an in-silico “SWISS ADME predictor” software. This analysis includes the quantitative measurement of drug-like properties such as molecular weight, partition coefficient (Log Po/w), water solubility, number of hydrogen bond acceptors as well as donor, topological polar surface area, gastrointestinal absorption, blood-brain barrier permeability, cytochrome 2D6 (CYP2D6), cytochrome 2C19 (CYP2C19), cytochrome 2C9 (CYP2C9), cytochrome 1A2 (CYP1A2), cytochrome 3A4 (CYP3A4) enzyme inhibition, and skin permeability. According to the outcomes of the in silico study, the most active compounds have an adequate range of heavy atoms (21-24), rotatable bond (3-4), H-bond acceptors (3-5), H-bond donor (1), topological surface area (50.19–96.01 Å2). Additionally, these compounds showed high gastrointestinal absorption and blood-brain barrier (BBB) permeability. The skin permeability was found in the range of −4.71 to −5.58 cm/s, along with Log P values between 2.79 and 4.79, which are strong indicators of oral absorption of listed substances (Table S1, in Supplementary Information). The molecular weight of the synthesized molecule is less than 500 kDa, demonstrating a strong drug likelihood profile and no violations of Lipinski’s criterion. Each compound showed a bioavailability score of 0.55. The Boiled-egg diagram from the SWISS ADME software (Fig. S137, Supplementary Information) is used to identify the lipophilicity and hydrophilicity of the molecules. The Boiled-egg diagram of the predicted molecules (8a) showed good lipophilicity, suggesting preferable cell wall penetration of Mtb bacteria. (See Tables S2 and S3 in Supplementary Information).
Conclusion
In conclusion, 3-(quinolin-3-yl)-1-phenylprop-2-en-1-one analogs were synthesized. The spectroscopic methods, including infrared spectroscopy, 1H- NMR, 13C- NMR, and mass spectrometry, were used to characterize all the synthesized compounds. The anti-TB activities of 3-(2-methoxyquinolin-3-yl)-1-phenylprop-2-en-1-one analogs (7a-p), and 3-(2-hydroxyquinolin-3-yl)-1-phenylprop-2-en-1-one (8a-k), were assessed against the Mtb H37Rv strain. Compounds 7a, 7p, and 8a showed promising MICs of 12.5 μM, 10 μM, and 6.25 μM, respectively. The overall drug-likeness for these compounds seems appropriate, as revealed by an in silico ADME study. Further, 3-(quinolin-3-yl)-1-phenylprop-2-en-1-one analogs containing the quinoline nucleus can be developed with better anti-TB activity by studying target-specific assays and in vivo evaluation in the future.
Experimental
Chemistry
All the commercially available chemicals were received from Sigma-Aldrich and Spectrochem, India, and were used as such without further purification. Glassware that had been air-dried and kept at room temperature was used for all reactions. Thin-layer chromatography was used to monitor chemical reactions (TLC). TLC was carried out using Sigma-Aldrich silica gel 60 F254 TLC plates using a UV-visible chamber for visualization. Compounds were purified by column chromatography with silica gel (100–200 mesh size). A Bruker spectrometer was used to record the IR spectra. Jeol Nuclear Magnetic Resonance ECLDR series spectrometers operating at 500 MHz and 125 MHz were employed to record NMR spectra. Tetramethylsilane (TMS) was used as the internal standard in NMR. The following were the highest multiplicities: s = singlet; d = doublet; dd = double doublet; t = triplet; q = quartet; m = multiplet. J values are expressed in Hz (hertz). The Agilent Mass Spectrometer - Quadrupole Time of Flight (QTOF) was used to record the HRMS spectra.
General procedure for the synthesis of acetanilides (3a-b)
Aniline 1 (1.96 mL, 21.0 mmol, 1 equiv.) was dissolved in dichloromethane (15 mL), then triethylamine (3.19 mL, 23.0 mmol, 1.1 equiv.) was added to the reaction mixture. Further, acetyl chloride 2 (1.49 mL, 21.0 mmol, 1 equiv.) was added dropwise at 0 °C. The reaction mixture was allowed to stir at room temperature for 1 h. A solid precipitate was observed in the reaction mixture. The reaction was monitored with TLC using 10% ethyl acetate in n-hexane. After completion of the reactions, the solvent was evaporated, and the product was extracted by ethyl acetate (50 mL x 3) and water (50 mL) and recrystallized with ethanol to get 77% yield of compound 3a and 79% yield of compound 3b (Table 1).
N-phenylacetamide (3a)
2.23 g; yield: 92%; shiny white crystal; Rf (Hexane: EtOAc 9:1) 0.2; mp: 110–113 °C; FTIR (neat) cm−1: 3287.35, 1948.95, 1666.04, 1592.61, 1529.66, 1422.36, 1309.99, 1166.07, 745.06, 684.28; 1H NMR (500 MHz, CDCl3): δ (ppm) 8.40 (s, 1H), 7.52 (d, J = 7.8 Hz, 2H), 7.26 (t, J = 7.9 Hz, 2H), 7.08 (t, J = 7.4 Hz, 1H), 2.1 (s, 3H); 13C {1H} NMR (125 MHz, CDCl3) δ (ppm): 169.3, 138.2 128.8, 124.4, 124.2, 120.3, 120.3, 24.4; HRMS (ESI) m/z calcd for C8H9NO (M + H)+ = 136.0762, observed 136.0766.
4-Chloro-N-phenyl acetamide (3b)
2.6 g; yield: 89%; white solid powder; Rf (Hexane: EtOAc 9:1) 0.3; mp:160–175 °C; FTIR (neat) cm−1: 3003.11, 1768.84, 1486.96, 1395.61, 1186.10, 1001.15, 814.29; 1H NMR (500 MHz, CDCl3): δ (ppm) 7.44 (d, J = 8.6 Hz, 2H), 7.26 (d, J = 8.6 Hz, 2H), 2.16 (s, 3H); 13C {1H} NMR (125 MHz, CDCl3) δ (ppm): 168.3, 136.5, 129.5, 129.3, 121.1, 24.6; HRMS (ESI) m/z calcd for C8H8ClNO (M + H)+ = 170.0373, observed 170.0339.
General procedure for the synthesis of 2-chloro-3-formyl quinoline (4 a-b)
To N, N-dimethylformamide (4.55 mL, 58.0 mmol, 4 equiv.), phosphorous oxychloride (13 mL 0.000147 mmol, 10 Equiv.) was added dropwise and stirred at 0 °C for 30 min to get Vilsmeier reagent. The substituted acetanilide (2 g, 14.7 mmol, 1 equiv.) was added in the in situ prepared Vilsmeier reagent at 70 °C. After completion of the reaction, the reaction mixture was cooled and poured over crushed ice. Precipitate was filtered, washed with water, and dried to get a yellow crude product. The desired product was isolated with column chromatography (#100–200 mesh size silica) in 10% ethyl acetate in hexane as a mobile phase.
2-Chloro-3-formyl quinoline (4a)
2.5 g; yield 84%; yellow solid powder; Rf (Hexane: EtOAc 9:1) 0.4; mp: 154–156 °C; FTIR (neat) cm−1: 3039.34, 2782.59, 1844.39, 1681.31, 1567.98, 1376.06, 1161.54, 1039.18, 759.34; 1H NMR (500 MHz, CDCl3): δ (ppm) 10.53 (s, 1H), 8.73 (s, 1H), 8.06 (t, J = 8.5 Hz, 1H), 7.97 (d, J = 8.1 Hz, 1H), 7.88 (t, J = 8.2 Hz, 1H) 7.65 (t, J = 7.6 Hz, 1H); 13C {1H} NMR (125 MHz, CDCl3) δ (ppm): 189.2, 150.1, 149.6, 140.3, 133.7, 129.8, 128.6, 128.2, 126.6, 126.4; HRMS (ESI) m/z calcd for C10H6ClNO (M + H)+ = 192.0216, observed 192.0251.
2,6-Dichloro-3-formyl quinoline (4b)
1.5 g; yield 65%; yellow solid powder; Rf (Hexane: EtOAc 9:1) 0.3; mp: 197–199 °C; FTIR (neat) cm−1: 3055.63, 2849.30, 1682.28, 1564.31, 1478.17, 1322.05, 1163.29, 1039.36, 930.47, 824.39, 764.93; 1H NMR (500 MHz, CDCl3) δ (ppm) 10.54 (s, 1H), 8.65 (s, 1H), 8.00 (d, J = 9 Hz, 2H), 7.95 (d, J = 7.8 Hz, 1H), 7.80 (d, J = 6.7 Hz, 1H); 13C {1H} NMR (125 MHz, CDCl3) δ (ppm): 190.9, 160.4, 148.5, 139.2, 134.5, 134.2, 130.2, 128.1, 127.2, 127.1; HRMS (ESI) m/z calcd for C10H5Cl2NO (M + H)+ = 225.9826, observed 225.9826.
General procedure for the synthesis of 2-methoxy-3-formyl quinoline (5 a-c)
2-chloro-3-formylquinoline (0.5 g, 2.6 mmol, 1 equiv.) was dissolved in 10 mL DMF and methanol or ethanol (15 mL) for respective substitution. K2CO3 (1 g, 26.0 mmol, 1 equiv.) was added, and the reaction mixture was refluxed at 70 °C till the completion of the reaction. The solvent was evaporated, and the reaction mixture was poured onto the crushed ice. The precipitate was filtered out using vacuum filtration, dried under vacuum, and recrystallized with ethanol. The desired product 5a was obtained with a good yield of 78%, and all other derivatives were synthesized using the same procedure to get 5b-c (50–77%).
2-Methoxy-3-formylquinoline (5a)
1.9 g; yield 89%; yellow solid powder; Rf (Hexane: EtOAc 9:1) 0.7; mp: 158–160 °C; FTIR (neat) cm−1: 3035.50, 2945.03. 2788.66, 2347.42, 1684.23, 1598.70, 1487.91, 1386.55, 1336.58, 1153.54, 1106.44, 1004.94, 869.58, 755.08; 1H NMR (500 MHz, CDCl3) δ (ppm): 10.45 (s, 1H), 8.56 (s, 1H), 7.84 (t, J = 8.7 Hz, 2H), 7.72 (t, J = 6.9 Hz, 1H), 7.41 (t, J = 7.9 Hz, 1 Hz), 4.17 (s, 3H); 13C {1H} NMR (125 MHz, CDCl3) δ (ppm): 189.4, 161.3, 149.0, 140.1, 132.6, 129.3, 127.3, 125.1, 124.4, 120.1, 53.92; HRMS (ESI) m/z calcd for C11H9NO (M + H)+ = 188.0712, observed 188.0646.
6-Chloro-2-methoxy quinolone-3-carbaldehyde (5b)
1.8 g; yield 81%; yellow solid powder; Rf (Hexane: EtOAc 9:1) 0.6; mp: 156–172 °C; FTIR (neat) cm−1: 2998.00, 2869.59, 1688.69, 1599.06, 1467.41, 1390.03, 1341.05, 1259.64, 1126.33, 1003.56, 937.15, 825.18; 1H NMR (500 MHz, CDCl3) δ (ppm): 10.44 (s, 1H) 8.46 (s, 1H) 7.79 (d, J = 10 Hz, 2H), 7.65 (d, J = 10 Hz, 1H), 4.16 (s, 3H)); 13C {1H} NMR 125 MHz, CDCl3) δ (ppm): 189.1, 161.4, 147.4, 138.98, 133.2, 130.5, 128.9, 128.2, 125.0, 120.7, 54.1; HRMS (ESI) m/z calcd for C11H8ClNO2 (M + H)+ =222.0322, observed 222.0331.
2-Ethoxy quinoline-3-carbaldehyde (5c)
0.25 g; yield 69%; pale yellow solid powder; Rf (Hexane: EtOAc 2:8) 0.7; mp: 168–180 °C; FTIR cm−1: 3054.13, 2979.28, 2865.06, 1685.40,1598.13, 1462.79; 1H NMR (500 MHz, CDCl3) δ (ppm): 10.48 (s, 1H), 8.56 (s, 1H), 7.82 (d, J = 9.0 Hz, 2H), 7.72–7.69 (m, 1H), 7.40 (t, J = 7.8 Hz, 1H), 4.64 (q, J = 7.1 Hz, 2H), 1.49 (t, J = 7.1 Hz, 3H); 13C {1H} NMR(125 MHz, CDCl3) δ (ppm): 189.6, 161.1, 149.1,139.7, 132.5, 129.8, 127.3, 124.9, 124.3, 120.0, 62.4, 14.5 ESI-HRMS (m/z) calcd C12H11NO2 [M + H+] = 202.0868; found 202.0866.
General procedure for the synthesis of 3-(2-methoxyquinolin-3-yl)-1-phenyl prop-2-en-1-one (7 a-p)
To the solution of KOH (0.085 g, 1.59 mmol, 3 equiv.) in ethanol, substituted acetophenone (0.53 mmol,1 equiv.) was added and stirred over 10 min at 0 °C temperature. To this reaction mixture 2-methoxy-3-formylquinoline (0.1 g, 0.53 mmol, 1 equiv.) was added, and the reaction was kept at room temperature. The reaction mixture was stirred magnetically until both starting materials were consumed. After completion of the reaction, the solvent was evaporated, and crushed ice was added to the round-bottomed flask. The product got precipitated. The precipitate was filtered, collected, dried, and then characterized. The desired product (7 a-p) was obtained with a good yield (Table 1).
3-(2-Methoxyquinolin-3-yl)-1-phenylprop-2-en-1-one (7a)
1.9 g; yield 74%; slightly yellow powder; Rf (Hexane: EtOAc 9:1) 0.5; mp: 162–189 °C; FTIR (neat) cm−1: 2925.94, 1663.18, 1592.72, 1440.45, 1363.14, 1265.96, 1000.61, 849.71, 744.92, 682.66, 643.30: 1H NMR (500 MHz, DMSO-d6) δ (ppm): 8.94 (s, 1H), 8.13 (t, J = 12 Hz 3H), 7.96 (d, J = 8.6 Hz, 2H), 7.90 (d, J = 7.9 Hz, 1H), 7.7(d, J = 8.25 Hz, 2H), 7.7-7.6 (m, 2H), 7.58(t, J = 7.5 Hz, 1H), 4.07 (s, 3H); 13C {1H} NMR (125 MHz DMSO-d6) δ (ppm): 189.5, 160.1, 146.8, 138.8, 137.9, 137.7, 134.4, 133.9, 131.6, 129.4, 129.0, 128.9, 128.4, 127.1, 125.4, 125.3, 125.0, 119.9, 54.6; HRMS (ESI) m/z calcd for C19H15NO2 (M + H)+ = 290.1181, observed 290.1138.
3-(2-Methoxyquinolin-3-yl)-1-(p-tolyl)prop-2-en-1-one (7b)
1.8 g; yield 87%; slightly yellow powder; Rf (Hexane: EtOAc 9:1) 0.4; mp: 173–189 °C; FTIR (neat) cm−1 2918.96, 1661.91, 1448.82, 1266.17, 1171.30, 979.99, 815.93, 743.20; 1H NMR (500 MHz, CDCl3), δ (ppm): 8.23 (s, 1H) 7.99 (d, J = 13.7 Hz, 3H), 7.85–7.82 (m, 2H), 7.75 (d, J = 8.0 Hz, 1H), 7.64 (t, J = 7.7 Hz, 1H), 7.39 (t, J = 7.5 Hz, 1H), 7.29 (d, J = 8.0 Hz, 2H), 4.16 (s, 3H), 2.43 (s, 3H); 13C {1H} NMR (125 MHz, CDCl3) δ (ppm): 190.2, 160.3, 146.8, 143.7, 138.8, 135.6, 130.7, 129.4, 128.8, 128.0, 127.1, 125.2, 125.1, 124.6, 120.4, 53.9, 21.7; HRMS (ESI) m/z calcd for C20H17NO2 (M + H)+ = 304.1293, observed 304.1434.
1-(4-Methoxyphenyl)-3-(2-methoxyquinolin-3-yl)prop-2-en-1-one (7c)
1.8 g; yield 92%; slightly yellow powder; Rf (Hexane: EtOAc 9:1) 0.5; mp: 175–189°C; FTIR (neat) cm−1: 2921.98, 1647.96, 1580.67, 1393.16, 1339.74, 1255.02, 1162.62, 995.40, 825.56, 761.14, 670.16; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 8.91 (s, 1H) 8.09 (d, J = 15 Hz, 3H) 8.03 (d, J = 8.2 Hz, 2H) 7.92 (d, J = 15.7 Hz, 1H), 7.75 (d, J = 8.2 Hz, 1H), 7.68–7.44 (m, 1H), 7.09 (d, J = 8.0 Hz, 2H), 4.06 (s, 3H), 3.84 (s, 3H); 13C {1H} NMR (125 MHz DMSO-d6) δ (ppm): 187.7, 164.1, 163.9, 160.3, 146.7, 138.4, 136.8, 131.5, 130.8, 128.9, 127.1, 125.4, 125.0, 120.0, 114.6, 56.1, 54.3; HRMS (ESI) m/z calcd for C20H17NO2 (M + H)+ = 320.1287, observed 320.1423.
1-(4-Fluorophenyl)-3-(2-methoxyquinolin-3-yl)prop-2-en-1-one (7d)
1.6 g; yield 89%; yellow solid powder; Rf (Hexane:EtOAc 9:1) 0.5; mp: 165–182 °C; FTIR (neat) cm−1: 3743.43, 2360.18, 1658.01, 1590.59, 1397.35, 1334.92, 1274.34, 1216.20, 974.03, 828.77, 746.19, 671.49; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 8.80 (s,1H), 8.22–8.18 (m, 2H), 8.07 (d, J = 7.8 Hz, 1H) 7.91 (d, J = 7.0 Hz, 1H), 7.86 (d, J = 8.1 Hz, 1H), 7.74 (d, J = 10 Hz, 1H), 7.66 (d, J = 7.6 Hz, 1H), 7.45 (t, J = 7.5 Hz, 1H), 7.38 (t, J = 7.5 Hz, 2H), 4.04 (s, 3H); 13C {1H} NMR (125 MHz DMSO-d6) δ (ppm): 187.9, 166.6, 160.6 (d, J = 250.6 Hz), 156.6, 154.6, 150.0, 146.8, 138.7, 137.8, 134.5, 132.0, 132.0 (dd, J = 9.3 Hz), 131.6, 128.9, 127.1, 125.3, 125.3, 124.6, 119.8, 116.5, 116.3 (d, J = 21.6 Hz), 54.3; HRMS (ESI) m/z calcd for C19H14FNO (M + H)+ = 308.1087, observed 308.1587.
1-(3-Bromophenyl)-3-(2-methoxyquinolin-3-yl)prop-2-en-1-one (7e)
1.8 g; yield 80%; slightly yellow powder; Rf (Hexane:EtOAc 9:1) 0.5; mp: 169–175 °C; FTIR (neat) cm−1 2980.48, 2356.77, 1570.06, 1406.20, 1267.80, 1176.54, 985.04, 848.78, 758.05, 673.4; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 8.92 (s, 1H), 8.25 (t, J = 7.6 Hz, 1H), 8.10 (d, J = 7.8 Hz, 1H), 8.03 (t, J = 7.8 Hz, 1H), 7.95 (d, J = 8.0 Hz, 1H), 7.88 (d, J = 8.0 Hz, 1H), 7.86 (m, 1H), 7.74 (d, J = 8.2 Hz, 1H), 7.71 (m, 1H), 7.51 (t, J = 7.9 Hz, 1H), 7.47 (m, 1H), 4.04 (s, 3H); 13C {1H} NMR (125 MHz DMSO-d6) δ (ppm): 188.1, 160.0, 146.9, 139.9, 138.6, 138.3, 136.4, 131.7, 131.6, 131.4, 129.0, 128.0, 127.1, 125.4, 125.3, 124.3, 122.9, 119.7, 54.3; HRMS (ESI) m/z calcd for C19H14BrNO (M + H)+ = 368.0286, observed 368.0281.
1-(4-Bromophenyl)-3-(2-methoxyquinolin-3-yl)prop-2-en-1-one (7f)
1.5 g; yield 79%; white solid powder; Rf (Hexane: EtOAc 9:1) 0.5; mp: 159–175 °C; FTIR (neat) cm−1 2309.77, 1659.89, 1594.61, 1397.34, 1339.43, 1275.50, 998.53, 825.59, 749.37, 663.38; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 8.92 (s, 1H), 8.11–8.03 (m, 3H) 7.95 (d, J = 15.7 Hz, 2H), 7.89 (d, J = 8 Hz, 1H), 7.81–7.74 (m, 3H), 7.69 (t, J = 7.3 Hz, 1H), 4.06 (s, 3H); 13C {1H} NMR (125 MHz DMSO-d6) δ (ppm): 188.6, 160.1, 146.9, 138.8, 138.1, 136.8, 132.4, 131.7, 131.0, 129.0, 128.0, 127.1, 125.4, 125.3, 124.6, 119.8, 54.4; HRMS (ESI) m/z calcd for C19H14BrNO (M + H)+ = 368.0286 observed 368.0280.
1-(4-Chlorophenyl)-3-(2-methoxyquinolin-3-yl)prop-2-en-1-one (7g)
1.4 g; yield 83%; slightly yellow powder; Rf (Hexane: EtOAc 9:1) 0.3; mp: 157–164 °C; FTIR (neat) cm−1: 3854.97, 3747.71, 2989.78, 2382.83, 2309.67, 1663.56, 1600.10, 1400.77, 1342.26, 1280.71, 1007.11, 827.93, 751.89; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 8.93 (s, 1H), 8.15 (d, J = 10 Hz, 2H), 8.10 (s, 1H), 7.98–7.94 (m, 1H), 7.90–7.88 (m, 1H) 7.78–7.75 (m, 1H), 7.72–7.68 (m, 1H), 7.64 (d, J = 8.5 Hz, 2H), 7.47–7.44 (m, 1H), 4.06 (s, 3H); 13C {1H} NMR (125 MHz DMSO-d6) δ (ppm): 188.4, 160.1, 146.9, 138.8, 138.1, 136.5, 131.7, 130.9, 130.4, 129.5, 129.0, 127.1, 125.4, 125.3, 124.6, 119.8, 54.4; HRMS (ESI) m/z calcd for C19H14ClNO2 (M + H)+ = 324.0791, observed 324.0737.
3-(2-Methoxyquinolin-3-yl)-1-(3-nitrophenyl) prop-2-en-1-one (7h)
2.5 g; yield 84%; orange solid powder; Rf (Hexane: EtOAc 9:1) 0.5; mp: 162–179 °C; FTIR (neat) cm−1: 3027.52, 2858.65, 1683.24, 1593.69, 1498.46, 1470.07, 1435.98, 1381.43, 1333.22, 1253.74, 1200.01, 1147.35, 1105.20, 1002.01, 868.92, 750.70, 719.07, 688.47; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 8.86 (s, 1H), 8.73 (s, 1H), 8.50 (d, J = 7.4 Hz, 1H), 8.44 (d, J = 8.1 Hz, 1H), 8.07–7.96 (m, 2H), 7.88 (d, J = 7.9 Hz, 1H), 7.84 (t, J = 8.0 Hz, 1H), 7.75 (d, J = 8.3 Hz, 1H), 7.68 (t, J = 7.1 Hz, 1H), 7.44 (t, J = 7.6 Hz, 1H), 4.08 (s, 3H); 13C {1H} NMR (125 MHz DMSO-d6) δ (ppm): 188.1, 160.1, 148.8, 147.0, 139.2, 139.0, 135.1, 131.7, 131.1, 129.0, 128.9, 127.1, 125.3, 125.2, 123.3, 123.2, 119.8, 119.6, 54.2; HRMS (ESI) m/z calcd for C19H14N2O4 (M + H)+ = 335.1012, observed 335.1144.
1-(2,6-Dichlorophenyl)-3-(2-methoxyquinolin-3-yl)prop-2-en-1-one (7i)
1.6 g; yield 85%; slightly brown powder; Rf (Hexane:EtOAc 9:1) 0.4; mp: 154–173 °C; FTIR (neat) cm−1 3059.52, 2363.07, 1662.91,1582.37, 1460.88, 1340.15, 1271.56, 1072.02, 997.77, 839.03, 739.85; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 8.79 (s,1H), 7.87 (d, J = 8.0 Hz, 1H), 7.78 (d, J = 7.0 Hz, 1H), 7.75 (d, J = 8.3 Hz, 1H), 7.70 (t, J = 7.5 Hz, 2H), 7.63 (t, J = 7.5 Hz, 2H), 7.50 (d, J = 1.8 Hz, 1H), 7.46 (d, J = 15 Hz, 1H), 4.02 (s, 3H); 13C {1H} NMR (125 MHz DMSO-d6) δ (ppm): 192.7, 159.9, 146.9, 140.6, 140.3, 137.7, 136.4, 132.0, 131.8, 131.3, 130.3, 129.1, 129.0, 128.2, 127.0, 125.4, 125.2, 119.2, 54.4; HRMS (ESI) m/z calcd for C19H13Cl2NO2 [M + H]+ = 358.0402, observed 358.0406.
3-(6-Chloro-2-methoxyquinolin-3-yl)-1-(p-tolyl)prop-2-en-1-one (7j)
1.7 g; yield 87%; slightly white powder; Rf (Hexane: EtOAc 9:1) 0.4; mp: 157–168 °C; FTIR (neat) cm−1 2974.54, 1586.83, 1386.72, 1262.86, 1161.80, 959.70, 814.62; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 8.82 (s, 1H), 8.01 (d, J = 7.5 Hz, 3H),7.91 (d, J = 8.2 Hz, 1H), 7.85 (d, J = 15.7 Hz, 1H), 7.74 (d, J = 8.9 Hz, 1H), 7.66 (d, J = 8.9 Hz, 1H), 7.36 (d, J = 8.0 Hz, 2H), 4.05 (s, 3H), 2.37 (s, 3H); 13C {1H} NMR (125 MHz DMSO-d6) δ (ppm): 188.8, 160.4, 145.2, 144.4, 137.7, 136.8, 135.2, 131.6, 129.9, 129.3, 129.1, 129.1, 127.3, 126.1, 125.7, 121.0 54.5, 21.7; HRMS (ESI) m/z calcd for C20H16ClNO2 (M + H)+ = 338.0948, observed 338.0960.
1-(2-Bromophenyl)-3-(2-methoxyquinolin-3-yl) prop-2-en-1-one (7k)
2.0 g; yield 90%; pale yellow solid powder; Rf (Hexane: EtOAc 9:1) 0.5; mp: 154–172 °C; FTIR cm−1: 3060.26, 2925.24, 1662.43, 1598.52, 1471.52, 1441.70, 1399.47, 1341.25, 1281.82, 1089.87, 1007.33, 828.89, 752.37, 670.93; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 8.70 (s, 1H), 7.86 (d, J = 8 Hz, 1H), 7.73 (t, J = 7.9 Hz, 2H), 7.67 (t, J = 8.2 Hz, 1H), 7.59–7.56 (m, 1H), 7.52 (d, J = 5.8 Hz, 2H), 7.44–7.41 (m, 3H), 4.03 (s, 3H); 13C {1H} NMR (125 MHz DMSO-d6) δ (ppm): 194.5, 159.8, 146.9, 141.0, 140.2, 139.9, 133.7, 132.5, 131.9, 129.7, 129.1, 129.0, 128.4, 127.0, 125.4, 125.2, 119.2, 119.1, 54.3; HRMS (ESI) m/z calcd for C19H14BrNO (M + H)+ = 368.0286 observed 368.0280.
1-(4-Aminophenyl)-3-(2-methoxyquinolin-3-yl)prop-2-en-1-one (7l)
1.15 g; yield 84%; yellow powder, Rf (Hexane: EtOAc 9:1) 0.2; mp: 162–181 °C; FTIR cm−1:3695.00, 3670.66, 3277.41, 2712.89, 1639.23, 1610.11, 1570.17, 1541.72, 1473.43, 1438.61, 1397.61, 1343.96, 1247.19, 1155.26, 1004.42, 854.86, 782.02, 747.52, 649.40; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 8.88 (s, 1H), 8.03 (d, J = 15.6 Hz, 1H), 7.91–7.82 (m, 4H), 7.76 (d, J = 8.3 Hz, 1H), 7.69–7.66 (m, 1H), 7.45 (t, J = 7.4 Hz, 1H), 6.61 (d, J = 8.6 Hz, 2H), 6.19 (s, 2H), 4.07 (s, 3H); 13C {1H} NMR (125 MHz, DMSO-d6)δ (ppm): 186.0, 160.1, 154.6, 146.5, 137.8, 135.0, 131.7, 131.2, 128.8, 127.0, 125.6, 125.5, 125.3, 120.4, 113.3, 54.3. ESI-HRMS (m/z) calcd C19H16N2O2 [M + H+] 305.1290; found 305.1288.
1-(2-Aminophenyl)-3-(2-ethoxyquinolin-3-yl)propanone (7m)
1.34 g; yield 82%; yellow solid powder; Rf (Hexane:EtOAc 9:1) 0.28, mp: 170–180 °C; FTIR cm−1 3382.29, 3015.18, 2884.92, 1640.24, 1574.60, 1155.26; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 8.89 (s, 1H), 8.15 (d, J = 15.6 Hz, 1H), 8.03 (d, J = 8.1 Hz, 1H), 7.89–7.84 (m, 2H), 7.73 (d, J = 8.3 Hz, 1H), 7.67 (t, J = 7.5 Hz, 1H), 7.46–7.42 (m, 3H), 7.27 (t, J = 7.5 Hz, 1H), 6.78 (d, J = 8.3 Hz, 1H), 6.58 (t, J = 7.5 Hz, 1H), 4.53 (q, J = 7.0 Hz, 2H), 1.43 (t, J = 7.1 Hz, 3H); 13C {1H} NMR (125 MHz, DMSO-d6)δ (ppm): 190.6, 159.7, 152.7, 146.6, 138.3, 135.7, 135.0, 131.6, 131.3, 128.8, 127.0, 126.3, 125.3, 125.2, 120.3, 117.8, 117.5, 115.0, 62.5, 14.9 ESI-HRMS (m/z) calcd C20H18N2O [M + H+] 319.1447; found 319.1419.
1-(4-Aminophenyl)-3-(2-ethoxyquinolin-3-yl)propanone (7n)
1.15 g; yield 86%; yellow solid powder; Rf (Hexane: EtOAc 9:1) 0.28; mp: 170–180 °C; FTIR cm−1: 3334.03, 3216.21, 3047.84, 2960.40, 1632.24, 1589.00; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 8.86 (s, 1H), 8.06 (d, J = 15.6 Hz, 1H), 7.90–7.82 (m, 4H), 7.73 (d, J = 8.4 Hz, 1H), 7.66 (t, J = 7.5 Hz, 1H), 7.44 (t, J = 7.4 Hz, 1H), 6.61 (d, J = 8.4 Hz, 2H), 6.19 (s, 2H), 4.53 (q, J = 10 Hz, 2H), 1.43 (t, J = 5 Hz, 3H); 13C {1H} NMR (125 MHz, DMSO-d6) δ (ppm): 186.1, 159.8, 154.6, 146.6, 138.0, 135.2, 131.6, 131.2, 128.7, 127.0, 125.7, 125.4, 125.3, 125.2, 120.4, 113.3, 62.5, 14.9; ESI-HRMS (m/z) calcd C20H18N2O [M + H+] 319.1447; found 319.1428.
1-(2-Aminophenyl)-3-(2-methoxyquinolim-3-yl)propanone (7o)
1.14 g; yield 89%; yellow solid powder, Rf (Hexane: EtOAc 9:1) 0.24, mp: 180–190 °C; FTIR cm−1: 3695.00, 3670.66, 3277.41, 2712.89, 1639.23, 157.17, 1155.26; 1H NMR (500 MHz, DMSO-d6): δ (ppm): 8.89 (s, 1H), 8.11 (d, J = 15.6 Hz, 1H), 8.04 (d, J = 7.2 Hz, 1H), 7.88–8.84 (m, 2H), 7.75 (d, J = 8.3 Hz, 1H), 7.67–7.66 (m, 1H), 7.45–7.42 (m, 3H), 7.28–7.25 (m, 1H), 6.79–6.78 (m, 1H), 6.60–6.57 (m, 1H), 4.06 (s, 3H); 13C {1H} NMR (125 MHz, DMSO-d6) δ (ppm): 190.6, 160.1, 152.7, 146.6, 138.0, 135.5, 135.0, 131.8, 131.3, 128.8, 127.0, 126.4, 125.5, 125.3, 120.3,117.5, 115.0, 54.3, ESI-HRMS (m/z) calcd C19H16N2O2 [M + H+] 305.1290; found 305.1288.
1-(3,4-Dimethoxyphenyl)-3-(2-methoxyquinolin-3-yl)prop-2-en-1-one (7p)
1.5 g; yield 78%; slightly yellow powder; Rf (Hexane:EtOAc 9:1) 0.3; mp: 168–179 °C; FTIR cm−1: 2921.98, 1647.96, 1580.67, 1393.74, 1339.74, 1255.02, 1162.62, 995.40, 825.56, 761.14, 670.16; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 8.89 (s, 1H) 8.10 (d, J = 15 Hz, 1H) 7.93 (m, 3H), 7.90 (s, 1H),7.76 (d, J = 7.58 Hz, 1H), 7.69 (d, J = 6.8 Hz, 1H), 7.45 (d, J = 6.7 Hz, 1H), 7.09 (d, J = 8.3 Hz, 1H), 4.07(s, 3H), 3.84 (s, 6H); 13C {1H} NMR (125 MHz DMSO-d6) δ (ppm): 187.6, 160.3, 153.9, 149.3, 146.8, 138.8, 135.6, 131.4, 130.8, 128.9, 128.8, 127.1, 125.3, 124.9, 124.0, 120.0, 111.3, 111.1, 56.3, 56.0, 53.9; HRMS (ESI) m/z calcd for C21H19NO4 (M + H)+ = 350.1348, observed 350.1412.
General procedure for the synthesis of 3-(2-hydroxyquinoline-3-yl)-1-phenyl prop-2-en-1-one (8 a-k)
To a solution of 3-(2-methoxyquinolin-3-yl)-1-phenylprop-2-en-1-one (0.5 g, 1.72 mmol, 1 equiv.) in DMF, concentrated HCl (4.32 mmol, 2.5 equiv.) was added dropwise and refluxed at 80 °C for 4 h. After completion of the reaction, the product was added into crushed ice and precipitates were filtered, washed, dried and characterized. The desired product 3-(2-hydroxyquinolin-3-yl)-1-phenylprop-2-en-1-one (8 a-k) was obtained in a good yield (Table 1).
(E)-3-(2-Hydroxyquinolin-3-yl)-1-phenylprop-2-en-1-one (8a)
2.2 g; yield 90%, yellow solid powder; Rf (Hexane: EtOAc 4:6) 0.4; mp: 171–189 °C; FTIR cm−1: 3484.92, 2975.40, 2866.14, 1056.21; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 12.06 (s, 1H), 8.58 (s, 1H), 8.28 (d, J = 7.8 Hz, 1H), 8.03 (d, J = 7.4 Hz, 2H), 7.77 (d, J = 7.8 Hz, 1H), 7.68 (d, J = 7.8 Hz, 1H), 7.64 (t, J = 7.4 Hz, 1H), 7.57- 7.51 (m, 3H), 7.32 (d, J = 8.2 Hz, 1H), 7.20 (t, J = 7.4 Hz, 1H); 13C {1H} NMR (125 MHz, DMSO-d6) δ (ppm): 190.0, 161.4, 141.9, 139.6, 139.5, 138.1, 133.7, 132.3, 129.4, 129.2, 128.8, 126.3, 124.4, 122.9, 119.6, 115.6; ESI-HRMS (m/z) calcd C18H13NO2 [M + H+] = 276.1012; found 276.1064.
(E)-3-(2-Hydroxyquinolin-3-yl)-1-(p-tolyl)prop-2-en-1-one (8b)
2.1 g; yield 87%; yellow solid powder; Rf (Hexane: EtOAc 4:6) 0.4; mp: 163–181 °C; FTIR cm−1: 3665.47, 2973.12, 1658.67, 1055.99; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 12.05 (s, 1H), 8.54 (s, 1H), 8.26 (d, J = 7.7 Hz, 1H), 7.93 (d, J = 7.2 Hz, 2H), 7.75 (d, J = 7.7 Hz, 1H), 7.67 (d, J = 7.4 Hz, 1H), 7.51 (t, J = 6.8 Hz, 1H), 7.33 (d, J = 7.9 Hz, 3H), 7.18 (t, J = 6.9 Hz, 1H), 2.34 (s, 3H); 13C {1H} NMR (125 MHz, DMSO-d6) δ (ppm): 189.4, 161.4, 144.1, 141.7, 139.5, 139.2, 135.6, 132.3, 129.9, 129.2, 129.0, 126.4, 124.4, 122.9, 119.6, 115.6, 21.7; ESI-HRMS (m/z) C19H15NO2calcd [M + H+] = 290.1212; found 290.1261.
(E)-3-(2-Hydroxyquinolin-3-yl)-1-(4-methoxyphenyl) prop-2-en-1-one (8c)
2.6 g; yield 92%; yellow solid powder; Rf (Hexane: EtOAc 4:6) 0.4; mp: 164–173 °C; FTIR cm−1: 3664.03, 2976.02, 1596.88, 1055.96; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 11.98 (s, 1H), 8.47 (s, 1H), 8.29 (d, J = 7.7 Hz, 1H), 8.03 (d, J = 7.9 Hz, 1H), 7.92 (d, J = 8.1 Hz, 1H), 7.73 (d, J = 7.7 Hz, 1H), 7.45 (s, 1H), 7.36 (d, J = 7.0 Hz, 1H), 7.22 (d, J = 8.1 Hz, 1H), 7.07 (d, J = 8.1 Hz, 2H), 6.97 (d, J = 8.3 Hz, 1H), 3.82 (s, 3H); 13C {1H} NMR (125 MHz, DMSO-d6) δ (ppm): 188.5, 163.7, 161.3, 141.2, 138.8, 137.6, 133.4, 131.8, 131.2, 131.1, 128.4, 126.6, 124.7, 119.6, 115.5, 114.6, 56.1; ESI-HRMS (m/z) calcd C19H15NO3 [M + H+] = 306.1112; found 306.0526.
(E)-1-(4-Fluorophenyl)-3-(2-hydroxyquinolin-3-yl) prop-2-en-1-one (8d)
2.4 g; yield 89%; yellow solid powder; Rf (Hexane: EtOAc 4:6) 0.4; mp: 168–181 °C; FTIR cm−1: 3708.18, 2973.89, 1669.97, 1592.04, 1056.07, 753.42; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 12.08 (s, 1H), 8.58 (s, 1H), 8.27 (d, J = 7.8 Hz, 1H), 8.12 (t, J = 8.2 Hz, 2H), 7.78 (d, J = 7.7 Hz, 1H), 7.68 (d, J = 7.8 Hz, 1H), 7.53 (t, J = 7.8 Hz, 1H), 7.38 (t, J = 8.7 Hz, 2H), 7.31 (d, J = 8.2 Hz, 1H), 7.20 (t, J = 7.4 Hz, 1H); 13C {1H} NMR (125 MHz, DMSO-d6) δ (ppm): 188.8, 161.4, 141.8, 139.7, 139.6, 132.2, 131.7, 131.7 (d, J = 9.3 Hz), 129.2, 129.2, 124.6, 122.8, 119.6, 116.4, 116.2 (d, J = 21.7 Hz), 115.6; ESI-HRMS (m/z) calcd C18H12FNO2 [M + H+] = 294.0912; found 294.0890.
(E)-1-(3-Bromophenyl)-3-(2-hydroxyquinolin-3-yl)prop-2-en-1-one (8e)
2.0 g; yield 86%; yellow solid powder; Rf (Hexane: EtOAc 4:6) 0.4; mp: 160–176 °C; FTIR cm−1: 3708.98, 2974.41, 1674.89, 1056.75, 748.64; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 12.10 (s, 1H), 8.50 (s, 1H), 7.71 (d, J = 7.8 Hz, 1H), 7.65 (d, J = 6.4 Hz, 1H), 7.61 (s, 1H), 7.54- 7.42 (m, 4H), 7.35 (d, J = 8.0 Hz, 1H), 7.28 (d, J = 8.1 Hz, 1H), 7.18 (t, J = 7.4 Hz, 1H); 13C {1H} NMR (125 MHz, DMSO-d6) δ (ppm): 195.0, 161.1, 142.5, 142.0, 141.4, 139.7, 133.3, 132.5, 132.2, 129.4, 129.4, 128.6, 128.3, 125.9, 122.8, 119.5, 119.0, 115.6; ESI-HRMS (m/z) calcd C18H12BrNO2 [M + H+] = 354.0112; found 354.0507.
(E)-1-(4-Bromophenyl)-3-(2-hydroxyquinolin-3-yl)prop-2-en-1-one (8f)
1.6 g; yield 83%; yellow solid powder; Rf (Hexane: EtOAc 4:6) 0.4; mp: 169–180 °C; FTIR cm−1: 3671.15, 2974.33, 1665.15, 1056.17; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 12.08 (s, 1H), 8.58 (s, 1H), 8.25 (d, J = 8.0 Hz, 1H), 7.96 (d, J = 8.4 Hz, 2H), 7.79–7.75 (m, 3H), 7.67 (d, J = 7.8 Hz, 1H), 7.53 (t, J = 8.0 Hz, 1H), 7.31 (d, J = 8.2 Hz, 1H), 7.20 (t, J = 7.5 Hz, 1H); 13C {1H} NMR (125 MHz, DMSO-d6)δ (ppm): 189.2, 161.4, 142.2, 140.1, 139.5, 137.1, 132.5, 130.8, 129.3, 128.0, 127.8, 127,8, 126.3, 124.0, 122.9, 119.5, 115.6; ESI-HRMS (m/z) calcd C18H12BrNO2 [M + H+] = 354.0112; found 354.0072.
(E)-1-(4-Chlorophenyl)-3-(2-hydroxyquinolin-3-yl)prop-2-en-1-one (8g)
1.6 g; yield 83%; yellow solid powder; Rf (Hexane: EtOAc 4:6) 0.4; mp: 170–185 °C; FTIR cm−1: 3667.54, 2974.16, 1663.17, 1055.98; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 12.08 (s, 1H), 8.58 (s, 1H), 8.26 (d, J = 7.7 Hz, 1H), 8.0 (d, J = 7.6 Hz, 2H), 7.78 (d, J = 7.7 Hz, 1H), 7.68 (d, J = 7.7 Hz, 1H), 7.62 (d, J = 7.5 Hz, 2H), 7.53 (t, J = 7.7 Hz, 1H), 7.31 (d, J = 8.1 Hz, 1H), 7.20 (t, J = 7.3 Hz, 1H); 13C {1H} NMR (125 MHz, DMSO-d6) δ (ppm): 188.9, 161.4, 142.1, 140.0, 139.5, 138.6, 136.7, 132.4, 130.7, 129.5, 129.3, 126.3, 124.0, 122.9, 119.5, 115.6; ESI-HRMS (m/z) calcd C18H12ClNO2 [M + H+] = 310.0612; found 310.0608.
(E)-3-(2-Hydroxyquinolin-3-yl)-1-(3-nitrophenyl)prop-2-en-1-one (8h)
1.4 g; yield 80%; yellow solid powder; Rf (Hexane: EtOAc 4:6) 0.4; mp: 174–183 °C; FTIR cm−1: 3398.10, 2974.85, 1652.08, 1258.41, 1056.41, 856.74, 700.46; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 11.93 (s, 1H), 8.69 (s, 1H), 8.57 (s, 1H), 8.44 (t, J = 8.3 Hz, 2H), 8.29 (d, J = 7.5 Hz, 1H), 7.85 (t, J = 8.2 Hz, 2H), 7.70 (d, J = 6.3 Hz, 1H), 7.55 (t, J = 7.5 Hz, 1H), 7.34 (d, J = 7.9 Hz, 1H), 7.34 (d, J = 7.9 Hz, 1H), 7.21 (t, J = 7.0 Hz, 1H); 13C {1H} NMR (125 MHz, DMSO-d6)δ (ppm): 195.4, 161.1, 142.8, 142.3, 141.2, 139.6, 133.9, 133.6, 132.7, 132.2, 129.4, 128.5, 128.3, 125.7, 122.9, 119.0, 115.6; ESI-HRMS (m/z) calcd C18H12N2O4 [M + H+] = 321.0912; found 321.1187.
(E)-1-(2,6-Dichlorophenyl)-3-(2-hydroxyquinolin-3-yl)prop-2-en-1-one (8i)
1.9 g; yield 90%; yellow solid powder; Rf (Hexane: EtOAc 4:6) 0.4; mp: 168–175 °C; FTIR cm−1: 3664.28, 2974.09, 1671.34, 1056.52; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 11.93 (s, 1H), 8.42 (s, 1H), 7.69 (d, J = 7.8 Hz, 1H), 7.66 (t, J = 6.4 Hz, 1H), 7.64 (d, J = 6.7 Hz, 1H), 7.57- 7.50 (m, 3H), 7.40 (d, J = 8.0 Hz, 1H), 7.32 (t, J = 6.1 Hz, 1H), 7.17 (q, J = 6.6 Hz, 1H); 13C {1H} NMR (125 MHz, DMSO-d6) δ (ppm): 193.5, 161.1, 143.3, 142.7, 139.7, 137.9, 136.0, 132.7, 131.6, 131.0, 130.2, 129.5, 128.6, 128.2, 125.6, 123.0, 119.4, 115.5; ESI-HRMS (m/z) calcd C18H11Cl2NO2 [M + H+] = 344.0212; found 344.0212.
(E)-3-(6-Chloro-2-hydroxyquinolin-3-yl)-1-(p-tolyl) prop-2-en-1-one (8j)
1.7 g; yield 87%, yellow solid powder; Rf (Hexane: EtOAc 4:6) 0.4; mp: 171–187 °C; FTIR cm−1: 3711.29, 2974.94, 2359.83, 1667.82, 1056.01, 746.11; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 8.64 (s, 1H), 8.22 (d, J = 7.8 Hz, 1H), 8.14 (s, 1H), 8.03 (d, J = 7.7 Hz, 1H), 7.83 (d, J = 8.4 Hz, 1H), 7.69 (d, J = 7.9 Hz, 1H), 7.53 (q, J = 6.9 Hz, 2H), 7.35 (d, J = 5.5 Hz, 1H), 7.20 (t, J = 7.5 Hz, 1H), 2.44 (s, 3H); 13C {1H} NMR (125 MHz, DMSO-d6) δ (ppm): 189.0, 161.3, 141.9, 140.4, 140.2, 139.7, 136.1, 132.3, 131.6, 131.2, 129.2, 127.8, 126.4, 124.3, 122.8, 122.8, 119.6, 115.7, 31.1; ESI-HRMS (m/z) calcd C19H14ClNO2 [M + H+] = 324.0812; found 324.2176.
(E)-1-(2-Bromophenyl)-3-(2-hydroxyquinolin-3-yl)prop-2-en-1-one (8k)
1.4 g; yield 86%; yellow solid powder; Rf (Hexane: EtOAc 4:6) 0.4; mp: 167–178 °C; FTIR cm−1: 3661.09, 2973.10, 1661.23, 1056.36; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 11.92 (s, 1H), 8.42 (s, 1H), 7.70 (d, J = 7.9 Hz, 1H), 7.66–7.62 (m, 2H), 7.51-7.47 (m, 3H), 7.40-7.37 (m, 3H), 7.17 (t, J = 7.5 Hz, 1H); 13C {1H} NMR (125 MHz, DMSO-d6) δ (ppm): 195.3, 161.1, 142.8, 142.6, 142.3, 142.2, 139.6, 133.6, 132.6, 132.2, 129.4, 128.5, 128.3, 125.7, 122.7, 119.5, 119.0, 115.6; ESI-HRMS (m/z) calcd C18H12BrNO2 [M + H+] = 354.0112; found 354.0072.
Anti-tubercular assays
Microbiological cultures and MIC determination were done by previously reported methods [25, 26] In brief, Mtb H37Rv strain was cultured in Middlebrook (MB) 7H9 broth supplemented with 10% albumin dextrose saline, 0.2% glycerol, and 0.05% Tween 80. The compounds were dissolved in DMSO to prepare 50 mM stocks. To determine MIC, the compounds were serially diluted two-fold in 96-well plates. Further, early-log phase Mtb culture (OD600nm ~ 0.2) was diluted 1000 times, added to the compounds and incubated at 37 °C for 14 days. MIC was calculated as the lowest concentration at which no visible bacterial pellet was observed.
Data availability
Data will be made available on request.
References
Allué-Guardia A, García JI, Torrelles JB. Evolution of drug-resistant mycobacterium tuberculosis strains and their adaptation to the human lung environment. Front Microbiol. 2021;12:1–21.
WHO. Global tuberculosis report 2023 [Internet]. WHO. 2023. Available from: https://www.who.int/publications/i/item/9789240083851.
Quenard F, Fournier PE, Drancourt M, Brouqui P. Role of second-line injectable antituberculosis drugs in the treatment of MDR/XDR tuberculosis. Int J Antimicrob Agents. 2017;50:252–4.
Bendre AD, Peters PJ, Kumar J. Tuberculosis: past, present and future of the treatment and drug discovery research. Curr Res Pharmacol Drug Discov. 2021;2:100037.
Wang MG, Wu SQ, He JQ. Efficacy of bedaquiline in the treatment of drug-resistant tuberculosis: a systematic review and meta-analysis. BMC Infect Dis BioMed Central. 2021;21:1–10.
Nguyen TVA, Anthony RM, Bañuls AL, Vu DH, Alffenaar JWC. Bedaquiline resistance: its emergence, mechanism, and prevention. Clin Infect Dis. 2018;66:1625–30.
Fujiwara M, Kawasaki M, Hariguchi N, Liu Y, Matsumoto M. Mechanisms of resistance to delamanid, a drug for Mycobacterium tuberculosis. Tuberculosis. 2018;108:186–94.
Vangapandu S, Jain M, Jain R, Kaur S, Singh PP. Ring-substituted quinolines as potential anti-tuberculosis agents. Bioorganic Med Chem. 2004;12:2501–8.
Mandewale MC, Patil UC, Shedge SV, Dappadwad UR, Yamgar RS. A review on quinoline hydrazone derivatives as a new class of potent antitubercular and anticancer agents. Beni-Suef Univ J Basic Appl Sci. 2017;6:354–61.
Upadhayaya RS, Vandavasi JK, Kardile RA, Lahore SV, Dixit SS, Deokar HS, et al. Novel quinoline and naphthalene derivatives as potent antimycobacterial agents. Eur J Med Chem. 2010;45:1854–67.
Muscia GC, Buldain GY, Asís SE. Design, synthesis and evaluation of acridine and fused-quinoline derivatives as potential anti-tuberculosis agents. Eur J Med Chem. 2014;73:243–9.
Jain PP, Degani MS, Raju A, Anantram A, Seervi M, Sathaye S, et al. Identification of a novel class of quinoline-oxadiazole hybrids as anti-tuberculosis agents. Bioorganic Med Chem Lett. Elsevier Ltd. 2016;26:645–9.
Singh S, Kaur G, Mangla V, Gupta MK. Quinoline and quinolones: promising scaffolds for future antimycobacterial agents. J Enzyme Inhib Med Chem. 2015;30:492–504.
Liu B, Li F, Zhou T, Tang XQ, Hu GW. Quinoline derivatives with potential activity against multidrug-resistant tuberculosis. J Heterocycl Chem. 2018;55:1863–73.
Keri RS, Patil SA. Quinoline: a promising antitubercular target. Biomed Pharmacother. 2014;68:1161–75.
van Heeswijk RPG, Dannemann B, Hoetelmans RMW. Bedaquiline: a review of human pharmacokinetics and drug-drug interactions. J Antimicrob Chemother. 2014;69:2310–8.
Lakshmanan M, Xavier AS. Bedaquiline—the first ATP synthase inhibitor against multi drug resistant tuberculosis. J Young Pharm. 2013;5:112–5.
Huitric E, Verhasselt P, Andries K, Hoffner SE. In vitro antimycobacterial spectrum of a diarylquinoline ATP synthase inhibitor. Antimicrob Agents Chemother. 2007;51:4202–4.
Andries K, Verhasselt P, Guillemont J, Göhlmann HWH, Neefs JM, Winkler H, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307:223–7.
Abdelrahman MA, Salama I, Gomaa MS, Elaasser MM, Abdel-Aziz MM, Soliman DH. Design, synthesis and 2D QSAR study of novel pyridine and quinolone hydrazone derivatives as potential antimicrobial and antitubercular agents. Eur J Med Chem. 2017;138:698–714.
Menteşe E, Bektaş H, Sokmen BB, Emirik M, Çakır D, Kahveci B. Synthesis and molecular docking study of some 5,6-dichloro-2-cyclopropyl-1H-benzimidazole derivatives bearing triazole, oxadiazole, and imine functionalities as potent inhibitors of urease. Bioorganic Med Chem Lett. 2017;27:3014–8.
Jj C, Se A. Natural and synthetic quinoline derivatives as anti- tuberculosis agents. Austin Tuberc Res Treat. 2017;2:2–4.
Akula M, Sridevi JP, Yogeeswari P, Sriram D, Bhattacharya A. New class of antitubercular compounds: synthesis and anti-tubercular activity of 4-substituted pyrrolo[2,3-c]quinolines. Monatshefte fur Chemie. 2014;145:811–9.
Nayyar A, Malde A, Jain R, Coutinho E. 3D-QSAR study of ring-substituted quinoline class of anti-tuberculosis agents. Bioorganic Med Chem. 2006;14:847–56.
Kidwai S, Park C, Mawatwal S, Tiwari P, Jung MG, Gosain TP, et al. Dual mechanism of action of 5-Nitro-1,10- phenanthroline against mycobacterium tuberculosis. Antimicrob Agents Chemother. 2017;61:e00969–17.
Singh P, Rawat S, Agrahari AK, Singh M, Chugh S, Gurcha S, et al. NSC19723, a thiacetazone-like benzaldehyde thiosemicarbazone improves the efficacy of TB drugs in vitro and in vivo. Microbiol Spectr. 2022;10:e0259222.
Acknowledgements
NB, SG, and AS acknowledge the Department of Pharmaceuticals, Ministry of Chemicals, and Fertilizers for the fellowship. RS is a senior fellow of Welcome Trust-DBT India Alliance. RS is a recipient of the Ramalingaswami fellowship and the National Bioscience Award from the Department of Biotechnology. NSS acknowledges MK Bhan Young Research Fellowship, Department of Biotechnology. The authors also acknowledge Mr. Saqib Kidwai for his assistance in MIC studies.
Funding
RS acknowledges the funding received from the Translational Research program from the Department of Biotechnology (BT/PR30159/MED/15/188/2018).
Author information
Authors and Affiliations
Contributions
NB performed the synthesis, which was analysed by SG. The anti-tubercular evaluation of synthesized compounds was done by NSS. SG and AS analysed the computational data. GLK and RS designed the concept for synthesis and evaluation, respectively. All the authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhanwala, N., Sundaramoorthy, N.S., Gollapudi, S. et al. Design, synthesis, anti-tubercular activity, and computational studies of novel 3-(quinolin-3-yl)-1-phenylprop-2-en-1-one derivatives. Med Chem Res (2024). https://doi.org/10.1007/s00044-024-03295-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00044-024-03295-z