Abstract
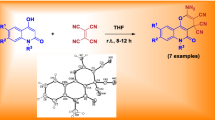
A series of 3,4-disubstituted dihydroquinolin-2(1H)-ones have been synthesized via three-component reaction of 1,3-indandione with isatin derivatives and N-phenacyl pyridinium bromide salts in presence of triethylamine as basic reagent. This procedure affords a highly efficient route for accessing quinolones by ring expansion of isatin via a one-pot pathway. Products were obtained in good to moderate yield without requiring a chromatographic purification step.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
3,4-Dihydroquinolin-2(1H)-ones have attracted attention from chemists and biologists because of their pharmaceutical activities such as phosphodiesterase inhibitory [1], antiplatelet [2], and cardiovascular actions [3]. Several routes have been reported in literature for synthesis of these compounds [4–7]. One of the most important approaches is ring expansion of isatin using different reagents, such as diazomethane [8] or ethyl diazoacetate [9].
On the other hand, the 1,3-indandione motif is widely found in many natural products with useful biological activities [10–12]. Also, its derivatives have been employed in synthesis of potentially important compounds, such as drugs [13] and dyes and pigments [14].
In continuation of our previous work on synthesis of novel heterocyclic compounds [15–19], we report herein an efficient procedure for synthesis of new 3,4-dihydroquinolin-2(1H)-one derivatives bearing a 1,3-indandione moiety via three-component reaction of 1,3-indandione with isatin derivatives and N-phenacyl pyridinium bromide salts (Scheme 1).
Results and discussion
To investigate the reaction conditions, we chose the reaction of isatin, N-(4-chlorophenacyl)pyridinium bromide, and 1,3-indandione as model reaction (Table 1). The results showed that, without basic reagent, the reaction led to no desired product (Table 1, entry 1). So, the reaction was examined in presence of different bases, revealing that triethylamine (NEt3) gave better yields than others (Table 1, entry 5). Then, different amounts of triethylamine were tested; 1 eq. NEt3 was found to be sufficient to promote this reaction. Subsequently, various solvents such as tetrahydrofuran (THF), dichloromethane (DCM), H2O, ethanol, dioxane, and acetonitrile as well as solvent-free condition were examined for the reaction at different temperatures. Using EtOH at 50 °C, the reaction afforded the corresponding product in good yield (88%) after 8 h (Table 1, entry 5).
After optimization of the model reaction, a variety of 3,4-dihydroquinolin-2(1H)-one derivatives were synthesized via three-component reaction of isatin derivatives with 1,3-indandione and phenacyl pyridinium salts according to Scheme 1. The target compounds were obtained in good yield with high purity (Table 2). Their structure was confirmed by infrared (IR), 1H and 13C nuclear magnetic resonance (NMR), heteronuclear single quantum coherence (HSQC), and heteronuclear multiple-bond correlation (HMBC) spectroscopy, and mass spectrometry (MS).
The mass spectrum of 4a displayed the molecular ion (M+· + 2) and (M+·) peaks at m/z 429 and 427, respectively, consistent with the product’s structure. The 1H NMR spectrum of 4a in CDCl3 exhibited one singlet at 6.29 ppm for CH group of quinolone. The 12 aromatic protons appeared as three doublets at 7.03, 7.26, and 7.36 ppm (3 J HH = 8.0, 8.4, and 8.4 Hz, respectively), four multiplets at around 7.25–8.30 ppm, and one triplet at 7.47 ppm (3 J HH = 8.0 Hz). The proton of NH was observed as a broad singlet at 8.38 ppm. The proton-decoupled 13C NMR spectrum of 4a showed 23 distinct resonances, in agreement with the proposed structure. Also, the structure of 4a was confirmed by HSQC and HMBC (Fig. 1). In the HMBC spectrum, the correlations of methine proton (H-32) with nearby carbons helped to identify the final structure of products. This proton at δ 6.29 (s) showed correlation with C-8 (2 J CH), C-10 (2 J CH), C-11 (3 J CH), C-13 (2 J CH), and C-16 (4 J CH). In another proposed product (structure 8 in Scheme 2), the methine proton could not be correlated with the carbonyl of indandione moiety (C-16), within five bonds. On the other hand, the 13C NMR spectrum of this product showed three signals in ketone region (δ = 181.8, 183.0, and 190.7 ppm) and one signal in amide region (δ = 170.1 ppm), in agreement with the structure of 4a.
The following mechanism can be suggested for the formation of products 4a–g (Scheme 2): In the first step, Knoevenagel condensation between 1,3-indandione and isatin derivatives affords adduct 5 in presence of Et3N. Also, N-phenacyl pyridinium bromide salt is deprotonated under basic condition to give pyridinium ylide 6, which attacks adduct 5 to generate intermediate 7 after ring opening of isatin moiety. Intermediate 7 undergoes intramolecular cyclization along with alkyl shift, and pyridine elimination leads to the corresponding product 4a–g.
Experimental
Materials and techniques
All chemicals and reagents were purchased from Fluka and Merck and used without further purification. Melting points were measured on an Electrothermal 9100 apparatus. NMR spectra were recorded using a Bruker DRX-400 AVANCE instrument (400.1 MHz for 1H, 100.6 MHz for 13C) with CDCl3 as solvent. Chemical shifts (δ) are given in parts per million (ppm) relative to tetramethylsilane (TMS), and coupling constants (J) are reported in hertz (Hz). IR spectra were recorded on a Bruker Vector 22 Fourier-transform (FT)-IR spectrometer. Mass spectra were recorded on a Finnigan MAT-8430 mass spectrometer operating at ionization potential of 70 eV.
General procedure for synthesis of compounds 4a–g
N-Phenacyl pyridinium bromide derivatives were obtained by reaction of phenacyl bromide derivatives and pyridine in acetonitrile medium. Triethylamine (1 mmol) was added to a mixture of isatin derivatives (1 mmol), N-phenacyl pyridinium bromide derivatives (1 mmol), 1,3-indandione (1 mmol), and triethylamine (1 mmol) in 5 ml ethanol charged in a 25-ml round-bottomed flask. The reaction mixture was stirred at 50 °C for appropriate time. After reaction completion as monitored by thin-layer chromatography (TLC) using EtOAc/hexane (1:1) as eluent, solvent was removed under reduced pressure and the product obtained by recrystallization from methanol.
Physical and spectral data for compounds 4a–g
2-(3-(4-Chlorobenzoyl)-2-oxo-2,3-dihydroquinolin-4(1H)-ylidene)-1H-indene-1,3(2H)-dione (4a)
Brown powder; m.p. 275–277 °C; yield 0.38 g, 88%; IR (KBr) (ν max, cm−1): 3443 (NH), 3077 (Csp2–H), 2923 (Csp3–H), 1683 (C=O), 1481 (C=C); 1H NMR (400 MHz, CDCl3), δ H (ppm): 6.29 (s, 1H, CHquinolone), 7.03 (d, 1H, 3 J HH = 8.0, CHAr), 7.26 (d, 2H, 3 J HH = 8.4 Hz, 2CHAr), 7.25–7.28 (m, 1H, CHAr), 7.36 (d, 2H, 3 J HH = 8.4 Hz, 2CHAr), 7.47 (t, 2H, 3 J HH = 8.0 Hz, 2CHAr), 7.82–7.85 (m, 2H, 2CHAr), 8.18–8.20 (m, 1H, CHAr), 8.28–8.30 (m, 1H, CHAr), 8.38 (brs, 1H, NH); 13C NMR (100 MHz, CDCl3), δ C (ppm): 55.1, 121.9, 123.9, 124.2, 127.1, 127.2, 128.9, 129.0, 131.5, 131.6, 132.3, 132.7, 134.1, 134.2, 134.5, 136.9, 138.5, 139.8, 141.1, 170.1, 181.8, 183.0, 190.7; MS, m/z: 429 (M+· + 2), 427 (M+·).
2-(3-Benzoyl-2-oxo-2,3-dihydroquinolin-4(1H)-ylidene)-1H-indene-1,3(2H)-dione (4b)
Yellow powder; m.p. 266–268 °C; yield 0.26 g, 65%; IR (KBr) (ν max, cm−1): 3449 (NH), 3020 (Csp2–H), 2930 (Csp3–H), 1698 (C=O), 1475 (C=C); 1H NMR (400 MHz, CDCl3), δ H (ppm): 6.32 (s, 1H, CHquinolone), 7.02 (dd, 1H, J 1 = 8.4 Hz, J 2 = 1.2 Hz, CHAr), 7.24–7.30 (m, 3H, 3CHAr), 7.39 (dd, 2H, J 1 = 8.0 Hz, J 2 = 1.2 Hz, 2CHAr), 7.43–7.49 (m, 3H, 3CHAr), 7.81–7.84 (m, 2H, 2CHAr), 8.16–8.19 (m, 1H, CHAr), 8.28–8.30 (m, 1H, CHAr), 8.35 (brs, 1H, NH); 13C NMR (100 MHz, CDCl3), δ C (ppm): 55.2, 121.8, 123.9, 124.1, 127.1, 127.2, 127.6, 128.5, 131.5, 131.6, 132.4, 132.7, 133.3, 134.2, 134.4, 135.9, 137.0, 138.8, 141.0, 170.4, 181.9, 183.1, 192; MS, m/z: 393 (M+·).
2-(6-Chloro-3-(4-chlorobenzoyl)-2-oxo-2,3-dihydroquinolin-4(1H)-ylidene)-1H-indene-1,3(2H)-dione (4c)
Yellow powder; m.p. 261–263 °C; yield 0.38 g, 83%; IR (KBr) (ν max, cm−1): 3442 (NH), 3086 (Csp2–H), 2940 (Csp3–H), 1697 (C=O), 1479 (C=C); 1H NMR (400 MHz, CDCl3), δ H (ppm): 6.30 (s, 1H, CHquinolone), 7.01 (d, 1H, 3 J HH = 8.4 Hz, CHAr), 7.29 (d, 2H, 3 J HH = 8.8 Hz, 2CHAr), 7.41 (d, 2H, 3 J HH = 8.8 Hz, 2CHAr), 7.42 (d, 1H, 3 J HH = 8.4 Hz, CHAr), 7.50 (d, 4 J HH = 2.4 Hz, 1H, CHAr), 7.82–7.86 (m, 2H, 2CHAr), 8.17–8.19 (m, 1H, CHAr), 7.26–8.28 (m, 1H, CHAr), 9.0 (brs, 1H, NH); 13C NMR (100 MHz, CDCl3), δ C (ppm): 55.1, 123.3, 125.0, 127.1, 127.3, 129.0; 129.1, 129.8, 131.4, 131.6; 132.1, 132.2, 133.9, 134.4, 134.7, 135.5, 138.8, 140.0; 140.1, 170.0, 181.7, 182.5; 190; MS, m/z: 465 (M+· +4), 463 (M+· +2), 461.2 (M+·).
2-(3-Benzoyl-6-chloro-2-oxo-2,3-dihydroquinolin-4(1H)-ylidene)-1H-indene-1,3(2H)-dione (4d)
Yellow powder; m.p. 266–268 °C; yield 0.33 g, 76%; IR (KBr) (ν max, cm−1): 3429 (NH), 2923 (Csp3–H), 2853 (Csp3–H), 1666 (C=O), 1456 (C=C); 1H NMR (400 MHz, CDCl3), δ H (ppm): 6.31 (s, 1H, CHquinolone), 7.03 (d, 1H, 3 J HH = 8.8 Hz, CHAr), 7.28 (d, 1H, 4 J HH = 2.8 Hz, CHAr), 7.30 (d, 1H, 3 J HH = 7.2 Hz, CHAr), 7.40–7.48 (m, 5H, 5CHAr), 7.80–7.85 (m, 2H, 2CHAr), 8.15–8.17 (m, 1H, CHAr), 8.26–8.28 (m, 1H, CHAr), 9.35 (brs, 1H, NH); 13C NMR (100 MHz, CDCl3), δ C (ppm): 55.2, 123.3, 125.0, 127.1, 127.2, 127.7, 128.6, 129.6, 131.4, 131.5, 132.0, 132.2, 133.5, 134.3, 134.6, 135.7, 135.8, 139.1, 139.8, 170.5, 181.7, 182.6; 191.5; MS, m/z: 429 (M+· + 2), 427 (M+·).
2-(3-Benzoyl-6-bromo-2-oxo-2,3-dihydroquinolin-4(1H)-ylidene)-1H-indene-1,3(2H)-dione (4e)
Yellow powder; m.p. 259–261 °C; yield 0.33 g, 70%; IR (KBr) (ν max, cm−1): 3424 (NH), 3066 (Csp2–H), 2937 (Csp3–H), 1665 (C=O), 1477 (C=C); 1H NMR (400 MHz, CDCl3), δ H (ppm): 6.26 (s, 1H, CHquinolone), 7.08 (d, 1H, 3 J HH = 8.4 Hz, CHAr), 7.29 (t, 2H, 3 J HH = 8.4 Hz, CHAr), 7.39 (d, 2H, 3 J HH = 7.2 Hz, CHAr), 7.46 (t, 1H, 3 J HH = 7.2 Hz, CHAr), 7.53 (dd, 1H, J 1 = 8.8 Hz, J 2 = 2 Hz, CHAr), 7.56 (d, 1H, 4 J HH = 2 Hz, CHAr), 7.81–7.83 (m, 2H, 2CHAr), 8.14–8.16 (m, 1H, CHAr), 8.25–8.27 (m, 1H, CHAr), 9.02 (brs, 1H, NH); 13C NMR (100 MHz, CDCl3), δ C (ppm): 55.3, 116.9, 123.7, 125.3, 127.1, 127.2, 127.6, 128.6, 131.4, 132.2, 133.5, 134.3, 134.6, 134.8, 135.8, 136.4, 139.1, 139.6, 139.8, 169.7, 181.7, 182.6, 191.8; MS, m/z: 475 (M+· + 2), 473 (M+·).
2-(6-Bromo-3-(4-chlorobenzoyl)-2-oxo-2,3-dihydroquinolin-4(1H)-ylidene)-1H-indene-1,3(2H)-dione (4f)
Yellow powder; m.p. 228–230 °C; yield 0.41 g, 80%; IR (KBr) (ν max, cm−1): 3431 (NH), 2925 (Csp3–H), 2855 (Csp3–H), 1680 (C=O), 1480 (C=C); 1H NMR [400 MHz, dimethyl sulfoxide (DMSO)], δ H (ppm): 6.13 (s, 1H, CHquinolone), 7.01 (d, 1H, 3 J HH = 8.8 Hz, CHAr), 7.29 (d, 2H, 3 J HH = 8.4 Hz, 2CHAr), 7.46 (d, 2H, 3 J HH = 8.4 Hz, 2CHAr), 7.58–7.65 (m, 2H, 2CHAr), 7.95 (t, 2H, 3 J HH = 5.8 Hz, 2CHAr), 8.07 (t, 1H, 3 J HH = 6 Hz, CHAr), 8.19 (d, 1H, 3 J HH = 5.6 Hz, CHAr), 10.86 (brs, 1H, NH); 13C NMR (100 MHz, CDCl3), δ C (ppm): 57.2, 106.4, 110.8, 122.8, 123.2, 127.2, 127.3, 129.1, 129.2, 130.2, 132.4, 134.4, 134.5, 134.7, 135.1, 135.7, 139.4, 141.6, 143.5, 169.2, 180.8, 181.7, 187.8; MS, m/z: 507 (M+· + 2), 505 (M+·).
2-(3-Benzoyl-6-nitro-2-oxo-2,3-dihydroquinolin-4(1H)-ylidene)-1H-indene-1,3(2H)-dione (4g)
Yellow powder; m.p. 233–235 °C; yield 0.36 g, 83%; IR (KBr) (ν max, cm−1): 3429 (NH), 3085 (Csp2–H), 2925 (Csp3–H), 1698 (C=O), 1482 (C=C); 1H NMR (400 MHz, DMSO), δ H (ppm): 6.16 (s, 1H, CHquinolone), 6.92 (d, 1H, 3 J HH = 8.4 Hz, CHAr), 7.00 (dd, 1H, J 1 = 5.0, J 2 = 3.2 Hz, CHAr), 7.22 (dd, 1H, J 1 = 5.0, J 2 = 3.2 Hz, CHAr), 7.68 (t, 1H, 3 J HH = 8.0, CHAr), 7.79–7.82 (m, 1H, CHAr), 8.06–8.12 (m, 3H, 3CHAr), 8.28 (t, 1H, 3 J HH = 7.6, CHAr), 8.74 (t, 1H, 3 J HH = 8.0, CHAr), 8.93 (s, 1H, CHAr), 9.00 (d, 1H, 3 J HH = 5.6, CHAr), 10.67 (s, 1H, NH); 13C NMR (100 MHz, DMSO), δ C (ppm): 66.7, 109.6, 117.4, 119.5, 126.0, 128.3, 128.7, 129.1, 129.6, 130.0, 133.9, 135.2, 136.5, 140.1, 142.4, 146.7, 146.8, 149.1, 170.9, 181.1, 182.2, 191.1; MS, m/z: 438 (M+·).
Conclusions
We demonstrate a new and efficient procedure for synthesis of novel 3,4-dihydroquinolin-2(1H)-one derivatives through a new ring expansion reaction of isatin derivatives using N-phenacyl pyridinium bromide salts. Some advantages of this synthetic development are utilization of nonhazardous solvent, good product yield, simple experimental procedure, and easy workup.
References
G.R. Martinez, K.A. Walker, D.R. Hirschfeld, J.J. Bruno, D.S. Yang, P.J. Maloney, J. Med. Chem. 35, 620 (1992)
C.C. Tzeng, I. Chen, Y.L. Chen, T.C. Wang, Y.L. Chang, C.M. Teng, Helv. Chim. Acta 83, 349 (2000)
C.T. Alabaster, A.S. Bell, S.F. Campbell, P. Ellis, C.G. Henderson, D.S. Morris, D.A. Roberts, K.S. Ruddock, G.M.R. Samuels, M.H. Stefaniak, J. Med. Chem. 32, 575 (1989)
K. Li, L.N. Foresee, J.A. Tunge, J. Org. Chem. 70, 2881 (2005)
D.V. Kadnikov, R.C. Larock, J. Org. Chem. 69, 6772 (2004)
W. Zhou, L.R. Zhang, N. Jiao, Tetrahedron 65, 1982 (2009)
A.Z. Irini, W. Alan, E.W. Jan, F.H. Rodger, W.D. Stevan, Tetrahedron Lett. 48, 3549 (2007)
B.A. Johnsen, K. Undheim, Acta Chem. Scand. Sect. B 38, 109 (1984)
S.Y. Sit, F.J. Ehrgott, J. Gao, N.A. Meanwell, Bioorg. Med. Chem. Lett. 6, 499 (1996)
P.A. Evans, T.A. Brandt, Tetrahedron Lett. 37, 1367 (1996)
D. Pizzirani, M. Roberti, M. Recanatini, Tetrahedron Lett. 48, 7120 (2007)
D. Pizzirani, M. Roberti, S. Grimaudo, A.D. Cristina, R.M. Pipitone, M. Tolomeo, M. Recanatini, J. Med. Chem. 52, 6936 (2009)
D. Leblois, S. Piessard, G. Le Baut, P. Kumar, J.D. Brion, L. Sparfel, R.Y. Sanchez, M. Juge, J.Y. Petit, L. Welin, Eur. J. Med. Chem. 22, 229 (1987)
K. Bello, L. Cheng, J. Griffiths, J. Chem. Soc. Perkin Trans. 2, 815 (1987)
R. Baharfar, N. Shariati, C. R. Chim. 17, 413 (2014)
R. Baharfar, S. Mohajer, Catal. Lett. 146, 1729 (2016)
R. Baharfar, R. Azimi, J. Chem. Sci. 127, 1389 (2015)
R. Baharfar, S. Asghari, S. Rassi, M. Mohseni, Res. Chem. Intermed. 41, 6975 (2015)
R. Baharfar, N. Shariati, Aust. J. Chem. 67, 1646 (2014)
Acknowledgements
This research was supported by the Research Council of the University of Mazandaran, Iran.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baharfar, R., Asghari, S., Tahiyyati, F. et al. Three-component synthesis of quinolone derivatives bearing 1,3-indandione moiety using pyridinium salts. Res Chem Intermed 43, 3007–3014 (2017). https://doi.org/10.1007/s11164-016-2808-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2808-0