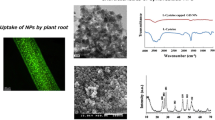
Abstract
The surface characteristics of nanoparticles cause their influx into the environment and lead to their interaction with fungi, algae, and plants. In the present study, the toxic effects of copper oxide nanoparticles were studied on the higher aquatic plant Spirodela polyrrhiza. Copper oxide nanoparticles were synthesized using green sonochemistry and their surface specifications were determined using XRD and SEM. The entrance and uptake of CuO nanoparticles in the roots of S. polyrrhiza was confirmed using fluorescence microscopy. The toxicity of CuO nanoparticles on S. polyrrhiza was investigated by measuring the growth rate (relative frond number), enzymatic activities (peroxidase, superoxide dismutase, and catalase) and content of photosynthetic pigments. In all experiments, the negative effects of CuO nanoparticles on the growth of S. polyrrhiza were confirmed by means of growth and enzymatic and pigment assays. Accordingly, significant changes in antioxidant enzyme activity were achieved. Catalase, peroxidase, and superoxide dismutase activity were increased due to the plant's defence system for scavenging the reactive oxygen species. In addition, relative frond number and chlorophyll content were reduced owing to possible phytotoxicity generated by CuO nanoparticles.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotechnology has been a great field of discovery in recent decades [1]. Nanomaterials are a diverse class of material in tiny scale, and nanoparticles are a subset of nanomaterials with 1–100 nm size in two dimensions [2]. Nanomaterial surface characteristics such as surface area, surface charge, and surface reactivity are noted causes of nanoparticle toxicity [3]. This toxicity has attracted the attention of researchers as they are generated in lofty amounts due to their use in biomedical, pharmaceutical, and industrial applications and their subsequent release into the environment [4]. These nanoparticle surface features are important for their collective behavior that cause their motion in aquatic environment and for their interaction with aquatic organisms [5]. Thus, it is important to know about the risks of nanoparticles in aquatic ecosystems as they effect on health of ecosystems [6].
Among the types of nanoparticles, metal oxide nanoparticles are most commonly used and have many applications in cosmetics and sunscreen [7], gene therapy [8], electrospray disinfectants [9], textiles [10], and many other fields. In recent decades, several studies have reported the potential toxicity of metal oxide nanoparticles [11]; however, little investigation has been done on the toxicity of nanometal oxide in aqueous biosystems [12].
On one hand, copper is an essential element for living organisms, but on the other hand, copper shows a wide range of biolethal activities, particularly on the growth of bacteria, viruses, and fungi [13, 14]. Copper oxide nanoparticles (CuO NPs) have many applications in a range of industries. They are applied in designing high temperature ceramic superconductors, arsenic purge, gas sensors, photovoltaic cells, semiconductors, antifouling paints for boats, antimicrobial coating, and plastics due to their high electrical conductivity, high melting point, and cheap price, as well as other specific applications [15, 16]. Therefore, these many usages cause nanoparticles to be widespread in the environment, covering aqueous ecosystems that are hazardous to live organisms including aquatic plants.
Plants are principal parts of ecosystems and intensely interact with the environment and materials around them; hence, they can be influenced by nanoparticles [17, 18]. With the arrival of nanoparticles into the environment, the toxicity of these nanoparticles was observed in aquatic higher plants [19]. Spirodela polyrrhiza is an aquatic floating plant (Lemnaceae), which has several roots. The plant has fast clonal growth so it tends to form “pads” on lake and pond surfaces [20, 21]. Because of rapid growth and small genome size, S. polyrrhiza is a suitable species for the study of biofuels, carbon cycle and bioremediation [22]. Furthermore, its small size and axenic cultural conditions have made it an ideal plant species for photoperiod, leaf morphogenesis, and toxicological research [23].
Internal regulators that respond to environmental conditions, control plants growth and development. Plants continuously generate reactive oxygen species (ROS) in unsuitable environmental conditions. The aggregation of ROS prevents the growth and development of plants. ROS is removed uninterruptedly by the antioxidant system [24]. This complex defense system contains enzymes such as superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) that helps biological control of ROS [25]. Recently, changes in the activity of some antioxidant enzymes were observed after treatment of plants by different nanoparticles [26]. However, there is a little information about the transport and fate of CuO NPs in plants and to our best knowledge, no research has been conducted on S. polyrrhiza before. Thus, the aims of this study were: (1) the synthesis of CuO NPs and their entrance confirmation into the roots of S. polyrrhiza; (2) investigate the toxic effects of CuO NPs on the activity of antioxidant enzymes and measure photosynthetic pigments content.
Materials and methods
Plant culture
S. polyrrhiza plants were collected from a pond in Anzali, in northern Iran. Afterwards, they were transferred to the laboratory and then cultured in a glass aquarium using 25 % special nutrient solution. This solution contained 0.205 mmol L−1 CaCl2, 0.0008 mmol L−1 CoCl2, 0.0012 mmol L−1 CuCl2, 0.081 mmol L−1 FeSO4, 0.178 mmol L−1 H3BO3, 0.323 mmol L−1 K2HPO4, 0.202 mmol L−1 K2SO4, 0.739 mmol L−1 KH2PO4, 4.04 mmol L−1 KNO3, 0.826 mmol L−1 MgSO4, 0.047 mmol L−1 MnCl2, 0.026 mmol L−1 Na2-EDTA, 0.00007 mmol L−1 (NH4)6MO7O24. Plants were kept at 20 °C under 16/8 h (light/dark) photoperiod with white fluorescent lamps. Because of the rapid growth of the colonies, the nutrient solution was replaced every week.
CuO NPs synthesis
An ultrasound green chemistry approach was employed to synthesize CuO NPs without using surfactant. Substances used in this work were of analytical reagent grade and purchased from Merck Co. (Germany). Two hundred milliliters of copper acetate II (0.2 mol L−1) was added to 1 mL glacial acetic acid first. Then the achieved solution was poured into a round bottom flask equipped with reflux using sonicator (Sonica, 2200 EP S3, Italy). The frequency was 50–60 kHz. Furthermore, this device includes heating regulation for synthesis of CuO NPs. After boiling, 20 mL sodium hydroxyl (1 mol L−1) was progressively added and the resulting solution was placed into an ultrasonic bath for an hour. The achieved black sediment was rinsed several times with distilled water and pure ethanol to remove residual impurities. The obtained product was dried at room temperature.
CuO NPs specifications
To be assured of the pure synthesis of CuO NPs, X-Ray diffraction pattern of the synthesized nanoparticles was obtained using Siemens X-ray diffractometer apparatus (D5000, Germany) with Cu Kα radiation (λ = 1.54065 Å). To identify the size and morphology of CuO NPs, SEM images of NPs with three different magnifications were taken using a Hitachi S-4200 scanning electron microscopy (Japan).
Uptake of CuO NPs into cells
Epifluorescence microscopy was used to ensure the entry of CuO NPs in the treated plants. Observation of the control and treated plants was performed 5 days after the commencement of treatment. Some roots of these plants were randomly selected and stained by Auramine O (0.1 % in 100 mL water) for 5 min. The samples were observed using an Olympus BX51 fluorescence microscope. The microscope was armed with the catadioptric lenses UMPlanFL-BDP and the BX-RFA (Olympus Optical Co., Ltd. Tokyo, Japan) fluorescence illuminator. The best fluorescent shots were taken when U-MWB3 (480–510 nm) and U-MWG3 (510–550 nm) mirror cube units were used. The depth of field was enhanced by the stack z-projection to exert the final superimposed images [27].
Calculation of S. polyrrhiza growth rate
To consider growth rate affected by CuO NPs, relative frond number (RFN) based on fresh weight was calculated. Thus, solutions with different concentrations (0.5, 1, 1.5, 2, 2.5, 3, and 6 mg L−1) of nanoparticles besides control were prepared. Twenty isometric fronds were placed into each concentration. Prepared samples were maintained at room temperature and incandescent and fluorescent bulbs provided light. The number of healthy fronds was calculated every 4 days for 20 days by stereomicroscope (Olympus, Japan). The value of RFN was counted using Eq. (1) [28, 29].
N 0 and N 1 demonstrate frond numbers at day 0 and day n, respectively.
Enzymatic assays
For enzymatic activity assessment, control samples and different concentrations of CuO NPs including 1, 3, and 6 mg L−1 were prepared. Samples were placed in these solutions, and enzymatic changes were measured on the first, third, and sixth day after treatment. Samples of 0.125 g of fresh plants were weighed for enzyme extraction and were homogenized with extraction buffer (0.01 mol L−1, pH = 7.0) containing 2 % PVP (w/v). The homogenized solution was centrifuged for 20 min (60 × 100 RPM-CAT. NO. 1004) at 4 °C. The supernatant (enzyme extraction) was stored, and sediment was discarded. The following assays were done to evaluate antioxidant enzymes in four replicates.
The SOD (EC 1.15.1.1) activity was determined by the means of photoreduction prohibition of NBT according to the method of Beyer and Fridovich [30]. The SOD solution mixture contained 2.65 mL potassium phosphate buffer solution (67 mmol L−1, pH = 7.8), 0.1 mL NBT (1.5 mmol L−1), 0.2 mL EDTA (0.1 mmol L−1) including 0.3 mmol L−1 KCN, 50 mL riboflavin (0.12 mmol L−1) and 50 μL enzyme extract. Reaction mixtures were put under 1000 lx light intensity for 15 min, and the absorbance of the solution was recorded at 560 nm. The amount of enzyme, which inhibits 50 % of the NBT reduction under the assay condition, was considered a unit of SOD [31].
POD (EC 1.11.1.7) activity was calculated following the polymerization of guaiacol to tetraguaiacol. The solution mixture contained citrate–phosphate-borate buffer solution (0.1 mol L−1, pH = 7.5), 25 μL enzyme extract, 15 mmol L−1 guaiacol and 3.3 mmol L−1 H2O2. The absorbance increment was recorded at 470 nm for 3 min throughout guaiacol polymerization. One unit of POD activity was considered as the amount of enzyme that can produce one µmol L−1 tetraguaiacol min−1 [ε = 26.6 (mmol L−1)−1 cm−1]. Enzyme activity was represented per mg of protein [32].
CAT (E.C. 1.11.1.6) activity was assessed through the consumption of H2O2 in 3 min by measuring the absorbance decline at 240 nm [ε = 39.4 (mol L−1)−1 cm−1]. The solution mixture included 50 µL enzyme extract, citrate phosphate-borate buffer solution (0.1 mol L−1, pH = 7.5) and 10 mmol L−1 H2O2. One unit of CAT activity was equal to the amount of enzyme needed for reduction of 1 µmol of H2O2 per minute. The enzyme activity was represented per mg of protein [33].
Protein content was appraised with bovine serum albumin as a standard by UV–Vis spectrophotometer (WPA lightwave S2000, England) at 595 nm using the method of Bradford [34].
Photosynthetic pigment content assay
Chlorophyll and carotenoids extraction was obtained from fronds by absolute acetone using the method reported by Lichtenthaler [35]. A 0.1-g sample of fresh fronds from the control and treated plants with different nanoparticle concentrations (1, 3, and 6 mg L−1) were concisely homogenated by 5 mL pure acetone. The mixture was centrifuged at 2000×g for 10 min. The absorbance of the supernatant was measured at 470, 645, and 662 nm. The amount of pigments was quantified using equations defined by Lichtenthaler [35].
Statistical analysis
GraphPad Instat 3 software was exerted using one-way analysis of variance (Anova) with Tukey multiple comparison tests. Assays were done in four replicates. The difference of significance was considered when p was <0.05.
Results and discussion
Features of the synthesized CuO NPs
The XRD analysis demonstrates that all the diffraction peaks of CuO NPs match the pure single-phase of CuO with monoclinic structure (JCPDS 05-0661) [36]. The diffraction patterns were observed to be at 2θ = 35.49, 38.68 to the reflection lines of monoclinic CuO nanoparticles. These results are in fair agreement with the records of Das et al. [37]. Other impurities such as Cu2O or Cu(OH)2 were not spotted, which proves that the synthesized sample was pure. It confirms the successful synthesis of CuO NPs by green sonochemistry (Fig. 1). By using Debye–Scherrer formula (0.9λ/βcosθ), the average crystalline size of synthesized CuO NPs was estimated to be about 50 nm. SEM images show the morphology of synthesized CuO NPs (Fig. 2a–c). As demonstrated, the shape of CuO NPs is globular and aggregated. The diameter of the majority of them is about 50–60 nm (Fig. 3).
Microscopic observations and CuO NPs effect on cells
Fluorescence microscopy
To confirm the entrance and localization of CuO NPs to the root tissues, treated plants with different nanoparticle concentrations were investigated by fluorescence microscopy. Fluorescence microscopic images of treated plants with 3 and 6 mg L−1 CuO NPs stained with Auramine O verified black spots (nanoparticle aggregation) in root tissues (Fig. 4c, d). In contrast, fluorescence microscopic images did not show any black spots in the control specimens (Fig. 4a). In the roots treated with 1 mg L−1 CuO NPs, the spots were also not observed (Fig. 4b). Probably because of the low concentration of nanoparticles, no aggregation occurred in the cells. The agglomeration and size of the optical signals were directly related to nanoparticle concentration, i.e. in high concentrations they were dense and bigger compared to low concentrations, thus they tend to aggregate in higher concentrations. CuO NPs can enter the cell through cell wall pores and intercellular plasmodesmata by the means of osmotic pressure, capillary forces, or via the regulated symplastic route [38]. Some features of nanoparticles such as size, shape, and surface charge can influence their localization to the plant [39]. Similar results in Glycin max affected by magnetite nanoparticles were shown by fluorescence microscopy imaging [40].
Effect of CuO NPs on growth
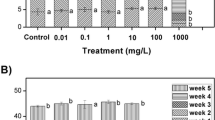
One of the growth indicators is the assessment of RFN affected by nanoparticles. Hence, S. polyrrhiza was exposed to increasing concentrations of CuO NPs (0.5, 1, 1.5, 2, 2.5, 3, and 6 mg L−1) during 20 days with 4-day intervals (Fig. 5). As a result, RFN correlated with concentrations of CuO NPs and exposure time. Therefore, concentration augmentation reduced RFN significantly compared to control samples as time progressed. S. polyrrhiza was sensitive to the concentrations over 0.5 mg L−1 of CuO NPs and showed the toxic symptoms. This reduction in growth is attributed to the inhibitory effect of nanoparticles, which was shown in some previous studies on S. polyrrhiza influenced by L-Cys-capped CdS and ZnO NPs [19, 41], Triticum aestivum and Landoltia punctata by CuO NPs, Lemna jibba and Lemna minor by Ag NPs [42, 43]. Thus, this inhibition that shows toxicity can be explained as protein synthesis and photosynthesis restriction [44, 45].
Effect of CuO NPs on enzymatic activities
ROS production and oxidative stress induction are the major toxicological mechanisms of NPs uptake. Abundant ROS could be produced even when only small amounts of CuO NPs are entered into cells [46]. NPs are able to interact with biomolecules due to their large specific surface area with highly reactive and electronic density characterization [47]. During this process, chemical reactions take place and increase superoxide radical (O ·−2 ) formation, which leads to ROS accumulation and oxidative stress [48]. ROS induce the biological defense system to remove reactive intermediates or repair the damage [49]. ROS production leads to a range of biological responses including the antioxidant responses engaged in oxidative stress [50]. Upon entering the cell, particles may induce intracellular oxidative stress by unbalancing the oxidant and anti-oxidant processes. Excessive oxidative stress may also modify proteins, lipids, and nucleic acids, which further stimulates the anti-oxidant defense system or even leads to cell death [49].
The activities of antioxidant enzymes including SOD, POD, and CAT are usually altered due to oxidative stress [51, 52]. SOD is usually the first defense step against reactive oxygen species and catalyzes the dismutation of superoxide radical into hydrogen peroxide [53].
The activities of SOD, POD, and CAT were assayed under different concentrations of CuO NPs on the first, third, and sixth days after treatment. SOD activity was increased in the treated plants with CuO NPs at 1, 3, and 6 mg L−1 concentrations on the first and third days compared to control (Fig. 6). The 3 mg L−1 treatment caused the most raise in all reaction times. These differences in SOD activity were significant except for 1 mg L−1 on the first and sixth days and 6 mg L−1 on the sixth day (p < 0.001). For the first day, it can be explained that the defense system has not started working properly yet, ascribed to low nanoparticle concentration. For the last day, it might be because of the stress from the high concentration of CuO nanoparticles or possibly the abundant formation of reactive oxygen species that causes tissue destruction, and subsequently a decrease in SOD activity. Accordingly, the SOD activity increased significantly in S. polyrrhiza exposed to the increasing concentrations of ZnO NPs (p < 0.05) compared to the control samples [41]. In the other study, exposure to increasing concentrations of silver nanoparticles has elevated the SOD activity in S. polyrrhiza [54].
SOD activity in S. polyrrhiza treated with CuO NPs that shows significant differences at different concentrations on various experimental days. Different letters indicate significant differences and treatments sharing the same letter(s) do not show significant differences at p ≤ 0.05 according to Tukey’s HSD Test. The error bars represent standard deviation of the mean (n = 3 replicates)
Enhancement of POD activity was significant at 3 and 6 mg L−1 CuO NPs in all the experiment days (first, third, and sixth) (p < 0.05). POD activity was not promoted significantly during the treatment at 1 mg L−1 (Fig. 7). This POD activity increment could be the result of high ROS production by CuO NPs in cells, which leads to H2O2 breakup. Willekens et al. [55] suggested that bulk H2O2 scavenging occurs by CAT, whereas POD can break up H2O2 residue, therefore, removing hydroxyl radicals. Some studies correlate with our finding such as the elevation of POD activity exposed to Ag NPs with concentration dependency in S. polyrrhiza [54], and POD activity enhancement in Phaseolus vulgaris exposed to CeO2 nanoparticles [56].
POD activity in S. polyrrhiza treated with CuO NPs that shows significant differences at different concentrations on various experimental days. Different letters indicate significant differences and treatments sharing the same letter(s) do not show significant differences at p ≤ 0.05 according to Tukey’s HSD Test. The error bars represent standard deviation of the mean (n = 3 replicates)
CAT activity was enhanced significantly (p < 0.05) by the increasing concentrations of CuO NPs compared to the control but 1 and 3 mg L−1 treatment did not show a significant increase on the sixth day (Fig. 8). It means CuO NPs induce ROS production. Similar results were achieved in other studies: S. polyrrhiza was exposed to ZnO nanoparticles and showed enhancing CAT activity [41] and different concentrations of Ag NPs that showed increase in CAT activity [54]. In P. vulgaris treated with CeO2, CAT activity was raised [56]. Also, increased activity of CAT was observed in Medicago sativa exposed to CdSe/ZnS NPs [57].
CAT activity in S. polyrrhiza treated with CuO NPs that shows significant differences at different concentrations on various experimental days. Different letters indicate significant differences and treatments sharing the same letter(s) do not show significant differences at p ≤ 0.05 according to Tukey’s HSD Test. The error bars represent standard deviation of the mean (n = 3 replicates)
Effect of CuO NPs on chlorophyll and carotenoids content
Chlorophyll a, b and total chlorophyll content in S. polyrrhiza were examined 6 days after exposing to CuO NPs and showed a significant decrease (p < 0.05). This decrease in the content of chlorophyll a, b and total chlorophyll was evident at 3 and 6 mg L−1 (p < 0.05) concentrations. However, carotenoids did not show any significant differences in various concentrations of CuO NPs (Fig. 9). In another study on S. polyrrhiza, the decrease in chlorophyll a content was reported to be influenced by Ag NPs [58]. Furthermore, a decrease was observed in chlorophyll a, b and total chlorophyll content in seedling of Arabidopsis thaliana and chlorophyll b and carotenoids content in P. vulgaris exposed to Ag NPs as well [59, 60].
Effect of CuO NPs on photosynthetic pigments content. The difference is significant in chlorophyll a, b and total chlorophyll but insignificant in carotenoids. Different letters indicate significant differences and treatments sharing the same letter(s) do not show significant differences at p ≤ 0.05 according to Tukey’s HSD Test. The error bars represent standard deviation of the mean (n = 3 replicates)
Total chlorophyll content decrease affected by nanoparticles, might be attributed to either plant biomass reduction or oxidative stress [58, 61, 62]. Reduction in chlorophyll content can be explained by the inhibition of chlorophyll biosynthesis [63]. These pigments reductions imply direct decreases in photosynthetic activity that exerts reduced carbon fixation and possible effects at the whole plant level [64]. Therefore, the decreasing content of photosynthetic pigments can be used to evaluate the potential phytotoxicity [65].
Conclusion
Our results conclusively confirmed the ability of S. polyrrhiza in uptaking the CuO NPs. Fluorescence microscopic studies approved the entrance of CuO NPs into root cells of the plant. S. polyrrhiza showed significant toxicological symptoms by treating with increasing concentrations of CuO NPs. This toxicity can be confirmed by RFN and photosynthetic pigments content decrease versus the increase in antioxidant enzymes activity including SOD, POD, and CAT. Increasing activity of antioxidant enzymes suggests ROS production under nanoparticles stress and the effort of the plant system to protect cells against ROS. Therefore, these enzymes play possibly an essential role in preventing oxidative damage.
References
J.R. Peralta-Videa, L. Zhao, M.L. Lopez-Moreno, G. de la Rosa, J. Hong, J.L. Gardea-Torresdey, J. Hazard. Mater. 186, 1 (2011)
M.C. Roco, J. Nanopart. Res. 5, 3–4 (2003)
X. Zhu, J. Wang, X. Zhang, Y. Chang, Y. Chen, Chemosphere 79, 9 (2010)
P. Manchikanti, T.K. Bandopadhyay, NanoEthics 4, 1 (2010)
E. Navarro, A. Baun, R. Behra, N.B. Hartmann, J. Filser, A.-J. Miao, A. Quigg, P.H. Santschi, L. Sigg, Ecotoxicology 17, 5 (2008)
M. Moore, Environ. Int. 32, 8 (2006)
A. Nel, T. Xia, L. Mädler, N. Li, Science 311, 5761 (2006)
S. Jin, K. Ye, Biotechnol. Prog. 23, 1 (2007)
X. Zhu, J. Wang, X. Zhang, Y. Chang, Y. Chen, Chemosphere 79, 9 (2010)
A. Becheri, M. Dürr, P.L. Nostro, P. Baglioni, J. Nanopart. Res. 10, 4 (2008)
L. Reijnders, J. Clean. Prod. 14, 2 (2006)
C. Blaise, F. Gagné, J. Férard, P. Eullaffroy, Environ. Toxicol. 23, 5 (2008)
G. Grass, C. Rensing, M. Solioz, Appl. Environ. Microbiol. 77, 5 (2011)
C.E. Santo, D. Quaranta, G. Grass, Microbiologyopen 1, 1 (2012)
J. Zhu, D. Li, H. Chen, X. Yang, L. Lu, X. Wang, Mater. Lett. 58, 26 (2004)
C. Saison, F. Perreault, J.-C. Daigle, C. Fortin, J. Claverie, M. Morin, R. Popovic, Aquat. Toxicol. 96, 2 (2010)
N. Durán, P.D. Marcato, Nano-Antimicrobials (Springer, Berlin, 2012), pp. 337–374
N. Asmathunisha, K. Kathiresan, Colloids Surf. B Biointerfaces 103, 5 (2013)
A. Khataee, A. Movafeghi, F. Nazari, F. Vafaei, M.R. Dadpour, Y. Hanifehpour, S.W. Joo, J. Nanopart. Res. 16, 12 (2014)
W.S. Hillman, Am. J. Bot. 46, 8 (1959)
R.L. Thompson, J. Ky. Acad. Sci. 66, 2 (2005)
W. Wang, R.A. Kerstetter, T.P. Michael J. Bot. 2011, 9 (2011)
E. Wangermann, E. Ashby, New Phytol. 50, 2 (1951)
A. Polle, Plant Physiol. 126, 1 (2001)
J.E. Weckx, H.M. Clijsters, Physiol. Plant. 96, 3 (1996)
M. Hatami, M. Ghorbanpour, Turk. J. Biol. 38, 1 (2014)
A. Movafeghi, M.R. Dadpour, S. Naghiloo, S. Farabi, Y. Omidi, Flora-Morphol. Distribut. Funct. Ecol. Plants 205, 4 (2010)
K. Mitsou, A. Koulianou, D. Lambropoulou, P. Pappas, T. Albanis, M. Lekka, Chemosphere 62, 2 (2006)
A. Khataee, M. Fathinia, S. Joo, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 112, 13 (2013)
W.F. Beyer, I. Fridovich, Anal. Biochem. 161, 2 (1987)
C.C. Winterbourn, B.M. McGrath, R.W. Carrell, Biochem. J. 155, 10 (1976)
B. Chance, A. Maehly, Methods Enzymol. 2, 12 (1955)
R.F. Beers, I.W. Sizer, J. Biol. Chem. 195, 1 (1952)
M.M. Bradford, Anal. Biochem. 72, 1 (1976)
H.K. Lichtenthaler, Methods Enzymol. 148C, 33 (1987)
V.V.T. Padil, M. Černík, Int. J. Nanomed. 8, 1 (2013)
D. Das, B.C. Nath, P. Phukon, S.K. Dolui, Colloids Surf. B Biointerfaces 101, 4 (2013)
B. Nowack, T.D. Bucheli, Environ. Pollut. 150, 1 (2007)
H. Zhu, X. Wang, Y. Li, Z. Wang, F. Yang, X. Yang, Chem. Commun. 34, 3 (2009)
M.H. Ghafariyan, M.J. Malakouti, M.R. Dadpour, P. Stroeve, M. Mahmoudi, Environ. Sci. Technol. 47, 18 (2013)
C. Hu, Y. Liu, X. Li, M. Li, Arch. Environ. Contamin. Toxicol. 64, 4 (2013)
E.J. Gubbins, L.C. Batty, J.R. Lead, Environ. Pollut. 159, 6 (2011)
J. Shi, C. Peng, Y. Yang, J. Yang, H. Zhang, X. Yuan, Y. Chen, T. Hu, Nanotoxicology 8, 2 (2014)
R. El-Shahate, M. El-Araby, E. Eweda, M. El-Berashi, J. Am. Sci. 7, 1 (2011)
A. Sood, S. Pabbi, P. Uniyal, Russ. J. Plant. Physiol. 58, 4 (2011)
Y. Toduka, T. Toyooka, Y. Ibuki, Environ. Sci. Technol. 46, 14 (2012)
T. Pisanic, S. Jin, V. Shubayev, From in vivo and In vitro models to health risks (Wiley, London, UK, 2009)
B. De Berardis, G. Civitelli, M. Condello, P. Lista, R. Pozzi, G. Arancia, S. Meschini, Toxicol. Appl. Pharmacol. 246, 3 (2010)
H. Yang, C. Liu, D. Yang, H. Zhang, Z. Xi, J. Appl. Toxicol. 29, 1 (2009)
T. Xia, M. Kovochich, M. Liong, L. Mädler, B. Gilbert, H. Shi, J.I. Yeh, J.I. Zink, A.E. Nel, ACS Nano 2, 10 (2008)
S. Fenik, T. Trofimyak, Y.B. Blyum, Usp. Sovrem. Biol. 115, 3 (1995)
S. Devi, M. Prasad, Russ. J. Plant. Physiol. 52, 2 (2005)
R.G. Alscher, N. Erturk, L.S. Heath, J. Exp. Bot. 53, 372 (2002)
H.S. Jiang, X.N. Qiu, G.B. Li, W. Li, L.Y. Yin, Environ. Toxicol. Chem. 33, 6 (2014)
H. Willekens, S. Chamnongpol, M. Davey, M. Schraudner, C. Langebartels, M. Van Montagu, D. Inzé, W. Van Camp, The EMBO J. 16, 16 (1997)
S. Majumdar, J.R. Peralta-Videa, S. Bandyopadhyay, H. Castillo-Michel, J.-A. Hernandez-Viezcas, S. Sahi, J.L. Gardea-Torresdey, J. Hazard. Mater. 278, 9 (2014)
A.R. Santos, A.S. Miguel, A. Macovei, C. Maycock, A. Balestrazzi, A. Oliva, P. Fevereiro, BMC Biotechnol. 13, 1 (2013)
H.S. Jiang, M. Li, F.Y. Chang, W. Li, L.Y. Yin, Environ. Toxicol. Chem. 31, 8 (2012)
S. Najafi, R. Heidari, R. Jamei, Bull. Environ. Pharmacol. Life Sci. 3, 2 (2014)
H. Qian, X. Peng, X. Han, J. Ren, L. Sun, Z. Fu, J. Environ. Sci. 25, 9 (2013)
C. Ma, S. Chhikara, B. Xing, C. Musante, J.C. White, O.P. Dhankher, ACS Sustain. Chem. Eng. 1, 7 (2013)
A. Oukarroum, S. Polchtchikov, F. Perreault, R. Popovic, Environ. Sci. Pollut. Res. 19, 5 (2012)
K.-J. Dietz, M. Baier, U. Krämer, Heavy Metal Stress in Plants (Springer, Berlin, 1999), pp. 73–97
A.J. Baker, P.L. Walker, Heavy Metal Toler. Plant Evolut. Asp. 2, 11 (1990)
P. Harbison, Mar. Pollut. Bull. 17, 6 (1986)
Acknowledgments
The authors thank the University of Tabriz (Iran) for all support and guidance. We also acknowledge the support of Iran Science Elites Federation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khataee, A., Movafeghi, A., Mojaver, N. et al. Toxicity of copper oxide nanoparticles on Spirodela polyrrhiza: assessing physiological parameters. Res Chem Intermed 43, 927–941 (2017). https://doi.org/10.1007/s11164-016-2674-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2674-9