Abstract
In this work, four novel D–A polymeric metal complexes (P1–P4) were designed and synthesized as dye sensitizers for high-performance dye-sensitized solar cells (DSSCs), which use cyclopentadithiophene or fluorene derivatives as donor (D) and the 2-(2′-pyridyl)benzimidazole derivative as acceptor (A). All polymers exhibited good thermal stability for their application in DSSCs. DSSCs based on P1 exhibited the best power conversion efficiency of 2.49 % with the cyclopentadithiophene derivative as donor and Cd(II) as coordination ion, which can be attributed to their stronger absorption in the UV–visible region and lower band gap. This study will pave a new path to design novel conjugated organic polymers as dyes for DSSCs.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the consumption of traditional energy (such as coal, oil, and natural gas) which causes serious environmental problems, we have to seek for other clean and renewable new energy (wind, rain, geothermal, and solar energy). Solar energy is recognized as one of the promising sources of energy in the twenty-first century, due to its universality, non-pollution, and security. Dye-sensitized solar cells (DSSCs) [1–5] are considered as one of the most promising next-generation photovoltaic cells, which can perform efficient conversion of solar energy directly into electricity. They have been extensively studied because of their low production cost, and flexible structure since the first report by Grätzel and co-workers in 1991 [6]. The sensitizer is one of the most critical components of DSSCs, which plays an important role by capturing sunlight and transferring electrons. Therefore, to develop new types of dye sensitizers with inexpensive materials for DSSCs is very important, and it has been a challenging topic in the world.
Recently, various types of sensitizers have been studied, and most of them are D–A structures, which consist of the electron-donating (D) group and the electron-accepting (A) group. The sensitizers based on D–A structure exhibit broad and intense spectral absorption, which is considered to be one of the most promising category of dyes. For most D–A sensitizers, using triphenylamine [7–9], indolines [10], carbazole [11–13], phenothiazine [14], a diphenylamine or dialkylamine moiety as electron donor, and a carboxylic acid or cyanoacrylic acid moiety acts as electron acceptor. This structure has many advantages, as follows: firstly, sensitizers based on D–A have better conjugated structure with the introduction of an electron-donating group and an electron-accepting group, which not only can enhance light absorption in the visible region to make the absorption spectrum redshift, but also enhance the transmission performance of the electron. Secondly, the π–π* transitions of electron and intramolecular charge-transformation (ICT) of molecules from an electron-donating group to an electron-accepting group can enhance the light–harvesting ability of sensitizers in the UV–visible region. Meanwhile, it has efficient electron injection from the excited dye to the conduction band (CB) of TiO2 electrode with the acceptor unit. Last but not least, it can suppress the charge recombination between the radical cation dye and the injected electrons.
Sensitizers can be divided into pure organic and metal complexes. In the dye-sensitized solar cell materials, bipyridyl ruthenium [15–17] is most widely used. It has a wider absorption of the ultraviolet to near-infrared light, and an appropriate level to achieve charge separation and regeneration of the dye compared with the titanium dioxide photoanode and I−/I3 − electrolyte. In addition, the higher stability of the molecule structure is in line with the high performance characteristics of the sensitizer and the energy conversion efficiency of DSSCs, which has been reached at 12 % for ruthenium sensitizers [18, 19]. However, the absorption coefficient of ruthenium dye is relatively low (10.000–20.000 M−1 cm−1), coupled with the high cost of ruthenium, so that other new sensitizers have to be studied. Pure organic dyes (metal-free organic dyes) have been thoroughly studied because of their high molar extinction coefficients (50,000–200,000 M−1 cm−1), a variety of synthetic methods, and changeable molecular structures. The energy conversion efficiency of DSSCs based on pure organic dye has been 11 % [20]. However, a relatively narrow absorption band and unstable molecule structure restrict the development of pure organic dye sensitizers; they still need further improvement.
Accordingly, we have made significant effort in the synthesis of polymeric metal complexes. It combines pure organic and metal complexes to form a metal-polymer, which exhibits as many advantages of both parent materials as possible. For example, the d–π feedback bond and the coordinate bond between the metal and the ligand will improve the ability of electronic transmission, meanwhile, with the flexible structure, we can obtain the target molecule by modification, and it has the performance of higher thermal stability as metal complexes, and better dissolution properties as pure organics.
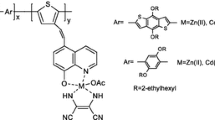
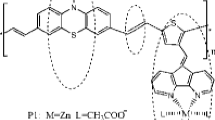
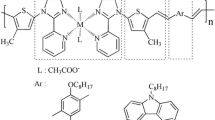
It is worth noting that cyclopentadithiophene or fluorene derivatives are rigid flat structures with larger bandwidth and lower energy gap, which are good electron donors; 2-(1H-benzo[d]imidazol-2-yl)phenol has a large planar rigid structure, which can coordinate with many metal ions, so it is a good acceptor; the carboxyl groups of 3,4-diamino benzoic acid can load on the surface of titanium dioxide steadily, which is a good coreceptor. Based on the above advantages, four novel D–A polymeric metal complexes (P1–P4) have been designed and synthesized using cyclopentadithiophene or a fluorene derivative as donor and a 2-(2′-pyridyl)benzimidazole derivative as acceptor by the Yamamoto coupling reaction for application in DSSCs as a sensitizer. Moreover, the optical properties, thermal properties, and photovoltaic properties of these polymeric metal complexes are also investigated in this paper.
Experimental
Instruments and measurements
Fourier transform infrared (FT-IR) spectra were measured by KBr powder and the range of FT-IR was 450–4000 cm−1. 1H-NMR spectra were measured with a Bruker ARX400 (400 MHz) Germany, using DMSO-d6 or CDCl3 as the solvent, and tetramethylsilane (TMS) as the internal standard (0.00 ppm). Elemental analysis was measured with a Perkin-Elmer 2400 II instrument under nitrogen atmosphere; it was to test C, H, S and N of the molecular components. UV–visible spectra were tested with a PE-Lambda 25 spectrophotometer. Samples were dissolved in N,N-dimethylformamide (DMF), and then diluted it to a concentration of 10−5–10−4 mol L−1. Differential scanning calorimetry (DSC) was measured with a Perkin-Elmer DSC-7 thermal analyzer, and heating it from 25 to 250 °C at a rate of 20 °C min−1. Thermal gravimetric analyses (TGA) were measured with a Shimadzu TGA-7 Instrument at a heating rate of 25 °C min−1 from 25 to 600 °C in a nitrogen atmosphere. Gel permeation chromatography (GPC) analyses were measured with a Waters-1515 model at room temperature, using Waters styragel columns (103, 104, 105 Å) as a separation column, DMF as mobile phase, and polystyrene as calibration. Cyclic voltammetry was measured with a CHI630C Electrochemical Workstation, using a platinum wire electrode as the auxiliary electrode, a glassy carbon electrode as the working electrode, and a saturated calomel electrode (SCE) as reference electrode in a 0.1 mol L−1 [Bu4N]BF4 of DMF solution at a scan rate of 100 mV s−1.
General procedures for fabrication of the DSSCs devices
Titania paste was prepared as follows: immersing the fluorine-doped SnO2 conducting glass (FTO) that was cleaned in 40 mmol L−1 TiCl4 solution for 30 min at 70 °C, and then washing with water and ethanol. The 20-30 nm-particles-sized TiO2 colloid was coated on FTO glass that had been prepared by the sliding glass rod method, and then sinter it for 30 min at 450 °C. This process was done three times to obtain a 15 μm-thickness TiO2 film. After cooling to 100 °C, the TiO2 film was soaked in 0.5 mmol L−1 the dye-sensitized samples in DMF and kept for 24 h in the dark. Then the films were cleaned with anhydrous ethanol. After drying, electrolyte that contains 0.05 mol L−1 I2, 0.5 mol L−1 LiI and 0.5 mol L−1 4-tert-butylpyridine dissolved in a mixed solution of acetonitrile/3-methoxypropionitrile (v/v, 7:3) was dripped on the surface of TiO2 electrodes. A Pt foil used as counter electrode was clipped onto the top of the TiO2 as working electrode. The dye-coated semiconductor film was illuminated through a conducting glass support without mask. Photoelectrochemical performance of the dye-sensitized solar cell was tested with a Keithley 2602 Source meter controlled by a computer. The parameters of the dye-sensitized solar cell were obtained with an intensity of 100 mW cm−2 of the incident light, which was generated by a 500 W Xe lamp with the AM 1.5 G filter with an effective area of 0.2 cm2.
Materials
All starting materials were obtained from Shanghai Chemical Reagent Co. Ltd. (Shanghai China) and used without further purification. All solvents used were analytical grade. 2,6-Dibromo-4,4-bis(2-ethylhexyl)-4H-thieno[3′,2′:4,5]cyclopenta[1,2-b]thiophene was purchased from the company of Rui Xun Optoelectronic Materials. 3,5-dibromo-2-hydroxybenzaldehyde, [21] 2-(1H-benzo[d]imidazol-2-yl)-4,6-dibromophenol [22] were synthesized according to the literature methods. N,N-dimethylformamide (DMF) was dried by distillation with CaH2. All other reagents and solvents were commercially purchased and were used as received.
Synthesis of metal complex C1
2-(1H-benzo[d]imidazol-2-yl)-4,6-dibromophenol 0.0923 g (0.25 mmol) and 3,4-diaminobenzoic acid 0.0389 g (0.25 mmol) were dissolved in 15 mL absolute methanol in the 100 mL three-necked round-bottomed flask, then a methanol solution (10 mL) of CdCl2·2.5H2O 0.0688 g (0.3 mmol) was dropped slowly into the three-necked round-bottomed flask under reflux condition. Then the mixture was adjusted carefully to slightly acidic (pH = 5–6) with NaOH (0.1 mol L−1), and reacting for 12 h. Stopping the reaction, and then cooled it to room temperature, a light gray solid was precipitated. It was filtered, and the solid was washed repeatedly with anhydrous methanol until the filtrate was colorless. After dried in vacuum at 60 °C, a pale gray solid (0.1373 g) was obtained, yield 78 %. FT-IR (KBr): 3427 (NH), 3070 (=C–H), 1592 (C=C), 1137 (C=N–Cd), 511 (N–Cd). Anal. Calcd for [C20H16Br2N4O3 CdCl2]: C 34.14, H 2.28, N 7.96. Found: C 34.52, H 2.11, N 7.66.
Synthesis of metal complex C2
The synthetic method was the same as metal complex C1 that has been described above, the difference was that C2 was coordinated with ZnCl2, a yellow solid (0.1248 g) was obtained, yield 76 %. FT-IR (KBr): 3383 (NH), 1566 (C=C), 1104 (C=N–Zn), 508 (N–Zn). Anal. Calcd for [C20H16Br2N4O3 ZnCl2]: C 36.59, H 2.44, N 8.54. Found: C 36.87, H 2.13, N 8.16.
Synthesis of polymeric metal complex P1
The polymer P1 was synthesized according to the Yamamoto reaction. [23] 2,6-Dibromo-4,4-bis(2-ethylhexyl)-4H-thieno[3′,2′:4,5]cyclopenta[1,2-b]thiophene 0.1120 g (0.2 mmol), metal complex C1 0.1406 g (0.2 mmol), zinc 0.0650 g (1 mmol), bis(triphenylphosphine) nickel(II) chloride 0.1312 g (0.2 mmol), triphenylphosphine 0.1048 g (0.4 mmol) and bipyridine 0.0031 g (0.02 mmol) were dissolved in DMF (12 mL) in the single-necked round bottom flask, then the mixture was heated to 90 °C slowly under nitrogen for 48 h. Cooling to room temperature, the mixture was poured into a beaker, then addied a large amount of ethanol, and the solid was precipitated after 5 h, and filtered. The crude product was washed with methanol, distilled water and THF, respectively, and then dried under vacuum for 24 h, a dark gray solid (0.0819 g) was obtained, yield 43 %. FT-IR (KBr): 3420 (NH), 3064 (=C–H), 2961 (vinylic C–H), 1579 (C=C), 1113 (C=N–Cd), 495 (N–Cd). Anal. Calcd for [C45H56N4O3 S2CdCl2] C 57.02, H 5.91, N 5.91, S 6.76. Found: C 56.71, H 5.38, N 6.33, S 6.54. Mn = 8.58 kg mol−1, PDI = 1.68.
Synthesis of polymeric metal complex P2
The synthetic method was the same as metal complex P1 that has been described above, the difference was that P2 reacted with C2, a dark yellow solid (0.1005 g) was obtained, yield 56 %. FT-IR (KBr): 3407 (NH), 2969 (vinylic C–H),1527 (C=C), 1085 (C=N–Zn), 497 (N–Zn). Anal. Calcd for [C45H56N4O3 S2ZnCl2] C 60.00, H 6.22, N 6.22, S 7.11. Found: C 60.57, H 6.08, N 6.43, S 7.25. Mn = 10.83 kg mol−1, PDI = 1.59.
Synthesis of polymeric metal complex P3
The synthetic method was the same as metal complex P1 that has been described above, the difference was that P3 reacted with alkyl fluorene, a gray solid (0.0914 g) was obtained, yield 49 %. FT-IR (KBr): 3425 (NH), 3066 (=C–H), 2937 (vinylic C–H), 1585 (C=C), 1122 (C=N–Cd), 502 (N–Cd). Anal. Calcd for [C49H60N4O3 Cd Cl2] C 62.89, H 6.42, N 5.99. Found: C 62.53, H 6.21, N 6.38. Mn = 7.54 kg mol−1, PDI = 1.71.
Synthesis of polymeric metal complex P4
The synthetic method was the same as metal complex P1 that has been described above, the difference was that P4 reacted with C2 and alkyl fluorene, a yellow solid (0.0909 g) was obtained, yield 51 %. FT-IR (KBr): 3398 (NH), 2966 (vinylic C–H), 1533 (C=C), 1092 (C=N–Zn), 504 (N–Zn). Anal. Calcd for [C49H60N4O3 ZnCl2] C 66.22, H 6.76, N 6.31. Found: C 66.68, H 6.42, N 6.14. Mn = 8.97 kg mol−1, PDI = 1.64.
Result and discussion
Synthesis and characterization
The detailed synthetic routes of the monomers and the four polymeric metal complexes are depicted in Scheme 1. The target four polymers were obtained by the Yamamoto coupling reaction with metal complexes C1, C2 as acceptor, and alkyl cyclopentadithiophene or alkyl fluorene derivatives as donor.
The 1H NMR spectrum of the intermediate compound 2-(1H-benzo[d]imidazol-2-yl)-4,6-dibromophenol is shown in Fig. 1, which was tested in DMSO-d6. The signal of 13.86 ppm is due to the hydrogen protons of NH in the benzimidazole, and the signals of 7.70 ppm, 7.33 ppm are attributed to the hydrogen protons of benzene in the benzimidazole. The hydrogen proton of benzene is observed at 8.32 and 7.91 ppm.
Figure 2a, b show the FT-IR spectra of the polymers P1–P4 and their corresponding metal complexes C1, C2. The figures show that, there is a broad absorption band at 3427 and 3383 cm−1, which can be attributed to the stretching vibration of N–H in the molecule, so it is not easy to see the O–H stretching vibrations of the water molecules and −COOH. The absorption peaks at 1592 and 1566 cm−1 are due to the stretching vibration of C=C, and the absorption peaks at 1137, 1104 cm−1 can be attributed to the stretching vibration of C=N–M in the C1, C2. Meanwhile, there are absorption peaks at 511 and 508 cm−1, respectively, which are due to the stretching vibration of N–M in the C1 and C2. All these prove that C1 and C2 have been successfully obtained. Compared with C1 and C2, the corresponding absorption peaks (C=C, C=N–M, N–M) of polymers P1–P4 appeared red shifted to some extent, which is due to the extension of the conjugation chain after polymerization. Polymers P1–P4 have similar absorption at about 2966 cm−1, due to the stretching vibration of alkyl chain, which illuminates that alkyl cyclopentadithiophene or alkyl fluorene have been embedded in the molecular chain of metal complexes successfully.
The data of gel permeation chromatography (GPC) is shown in Table 1. The results show that the degree of polymerization of the polymer P1–P4 is not too high, it is due to the fact that there is no soluble group in the metal complexes, which result in their weak solubility in the solvent. Coupled with the larger molecular structure of acceptor and donor, this results in a larger steric hindrance, which may decrease the collision in the reaction, and finally they are not efficient for polymerization.
All above results demonstrated that the target product P1–P4 has been synthesized successfully.
Thermal properties
Thermal stability of the dye molecules determines the working life of the photovoltaic devices. The thermal stability of four polymeric metal complexes (P1–P4) were studied by thermal gravimetric analysis (TGA) and the differential scanning calorimetry test (DSC). The results of TGA and DSC are shown in Table 1, and the TGA curve of polymer P1–P4 is shown in Fig. 3. The T d (the temperature at which they lose 5 % of their own weight) of the polymer P1–P4 are 293, 282, 332, and 356 °C, respectively. These data are all higher than 280 °C, which is in line with the thermal performance requirements of a dye-sensitizer. Their glass transition temperatures (T g) are 147, 139, 158, and 167 °C, respectively. These data suggest that the thermal performance of P3, P4 are better than that of P1 and P2, which illustrates that the rigidity structure of alkyl benzene is better than alkyl thiophene. In conclusion, these four polymeric metal complexes (P1–P4) show a better thermal stability, which is very favorable to extend the working life of the device.
Optical properties
UV–Vis absorption spectra reflect the sunlight capturing capability of dye molecules in the UV–visible region, which is an important aspect to assess the dye sensitizer.
The UV–Vis absorption spectra of polymers P1–P4 and their corresponding metal complexes C1, C2 are shown in Fig. 4, which is measured in DMF solution, and the corresponding optical data are shown in Table 2. The maximum absorption wavelengths of the metal complexes C1, C2 were at 386, and 377 nm, which is due to the π–π* electronic transitions of molecule [24]. After polymerization, the conjugate structure of the molecules is extended to some extent, due to the combination of donor and acceptor. The maximum absorption wavelengths of P1–P4 are red-shifted to 484, 475, 435, and 404 nm, respectively, since the charge transfer from donor to acceptor is in the entire molecule. Meanwhile, we can also see that polymeric metal complexes P1, P2 have relatively weak absorption peaks at 525 and 519 nm, although the absorption intensity is not too high, the photon flux of this area is very large, which is very advantageous to improve the efficiency of solar cells. Obviously, the polymer with a Cd(II) and cyclopentadithiophene derivative has better absorption than that with a Zn(II) and fluorene derivative in the UV–Vis area, which can be attributed to the bigger radius of Cd(II) than of Zn(II), and a stronger electron-donating ability of cyclopentadithiophene derivative than fluorene derivative. In summary, the introduction of cyclopentadithiophene derivative in the donor and the introduction of Cd(II) in the acceptor can contribute to the ultraviolet–visible absorption of dye molecules.
Electrochemical properties
The electrochemical properties are an important aspect to evaluate the performance of a sensitizer, which can be tested by cyclic voltammetry (CV) to estimate the lowest unoccupied molecular orbital (LUMO) energy levels and the highest occupied molecular orbital (HOMO) energy levels of the polymer. It is an important basis for determining whether the dye can be applied in the dye-sensitized solar cells. On the one hand, the LUMO energy level of the dye should be higher than the conduction band energy level (E CB) of TiO2, to achieve the charge separation; on the other hand, the HOMO energy level of dye must be lower than the redox potential of the electrolyte (e.g., I −/I −3 ), to achieve recycling of the dye. LUMO and HOMO energy levels can be calculated from the onset reduction potentials (E red) and the onset oxidation potentials (E ox) of the polymers according to equations: [25, 26]
The cyclic voltammograms of polymer P1–P4 are shown in Fig. 5, the corresponding energy levels are shown in Fig. 6, and the corresponding data are shown in Table 2. The E ox values of P1, P2, P3 and P4 are 1.10, 1.12, 1.18, and 1.20 V, respectively; The E red values of P1–P4 are −0.92, −0.97, −1.07 and −1.13 V, respectively. According to equations above, the corresponding HOMO level are −5.50, −5.52, −5.58 and −5.60 eV, respectively; LUMO energy levels are −3.48, −3.43, −3.37 and −3.27 eV, respectively. These results show that the HOMO levels of the four polymers (P1–P4) are lower than the redox potential of the electrolyte (−4.83 eV vs NHE), and LUMO energy levels of the four polymers (P1–P4) are higher than the conduction band energy level of titanium dioxide (−4.26 eV vs NHE). In other words, electrons can be transferred successfully among the dye molecules, TiO2 conduction band, and the electrolyte, to achieve charge separation and recycling of the dye molecules. The energy gap (E g) of polymer P1–P4 are 2.02, 2.09, 2.21, and 2.33 eV, respectively. Compared with P3 and P4, the E g of P1 and P2 is smaller, this is due to the stronger electron-donating of cyclopentadithiophene derivative, which also implies that polymers with cyclopentadithiophene derivative are more suitable to be applied as sensitizer in the DSSCs.
Photovoltaic properties
The photocurrent–voltage (J–V) curves of polymer P1–P4 as the sensitizers of dye-sensitized solar cell are shown in Fig. 7, and corresponding data of short-circuit current density (J sc), open-circuit voltage (V oc), fill factor (FF) and power conversion efficiency (η) are shown in Table 3. Seen from the data in the table, V oc and J sc of P1 are at maximum, which corresponds to the highest power conversion efficiency (2.49 %). The J sc follow the order of P1 (4.98 mA cm−2) > P2 (4.82 mA cm−2) > P3 (4.51 mA cm−2) > P4 (4.36 mA cm−2). Obviously, the J sc of dyes do not exceed 5 mA cm−2, which is probably due to the narrow and short absorption spectra that limited the use of long wavelengths energy. Compared with P3 and P4, the J sc of P1 and P2 are a little larger, which may be due to the stronger electron-donating ability of cyclopentadithiophene derivative than the fluorene derivative, which can produce relatively more excited state electrons. Meanwhile, the J sc of polymers with Cd(II) are higher than the corresponding polymers with Zn(II), this may be due to the larger radius of Cd(II), which makes it have a relatively strong complexing ability, and the transmission ability of electrons in the molecule is also relatively strong. It is not difficult to find that the Voc is gradually decreased along with the order of P1 (0.73 V), P2 (0.69 V), P3 (0.68 V) and P4 (0.64 V), which is attributed to the fact that their HUMO–LUMO band gaps were broaden gradually, and the excitation of sensitizers is relatively difficult [27, 28].
The IPCE curves of polymer P1–P4 as the sensitizers of a dye-sensitized solar cell are shown in Fig. 8. From the graph, it is evident that the four polymer molecules all have a spectral response at 400–600 nm. Wherein, P1 has the highest external quantum efficiency value. Although the maximum value of IPCE of dye molecules has a certain degree of improvement, it is still relatively low compared with the ruthenium dye, and it also shows the reason for the lower J sc of dye molecules. Therefore, in our future work of designing molecule structure for the dye, we must improve the molecular structure, and try to make it redshift to expand the absorption range of the dye, and ultimately improving the efficiency of DSSCs.
Conclusions
Four novel D–A polymeric metal complexes dyes (P1–P4) containing alkyl cyclopentadithiophene or an alkyl fluorene derivative as electron donor (D), benzimidazole metal complex as the electron acceptor (A) and carboxyl group (−COOH) as an anchor group were designed, synthesized, and applied for DSSCs. All polymers exhibited good thermal stability, which is in line with the thermal performance requirements of the photovoltaic device. Moreover, the optical properties and photovoltaic properties of these polymeric metal complexes are also investigated. The results show that the polymer with cyclopentadithiophene derivatives and Cd(II) ion complexes exhibits better efficiency because of the stronger electron-donating ability of the cyclopentadithiophene derivative and the stronger complexing ability of the Cd(II) ion. Although the conversion efficiency of DSSCs has a certain degree of improvement, compared with the traditional ruthenium dyes, there is still a long way to go. Therefore, in our future work of designing molecule structure for the dye, we must improve the molecular structure, and try to make it redshift to expand the absorption range of the dye, and ultimately improving the efficiency of DSSCs.
References
J.N. Schrauben, Y.X. Zhao, C. Mercado, P.I. Dron, J.L. Ryerson, J. Michl, K. Zhu, J.C. Johnson, ACS Appl. Mater. Interfaces 7, 2286 (2015)
S. Karthikeyan, J.Y. Lee, J. Phys. Chem. A 117, 10973 (2013)
A.R. Pascoe, F. Huang, N.W. Duffy, Y.B. Cheng, J. Phys. Chem. C 118, 15154 (2014)
J. Warnan, J. Gardner, L.L. Pleux, J. Petersson, Y. Pellegrin, E. Blart, L. Hammarström, F. Odobel, J. Phys. Chem. C 118, 103 (2014)
M. Ince, J.H. Yum, Y. Kim, S. Mathew, M. Grätzel, T. Torres, M.K. Nazeeruddin, J. Phys. Chem. C 118, 17166 (2014)
B. O’Regan, M. Gratzel, Nature 353, 737 (1991)
Y.L. Tan, M. Liang, Z.Y. Lu, Y.Q. Zheng, X.N. Tong, Z. Sun, S. Xue, Org. Lett. 16, 3978 (2014)
G.H. Wu, F.T. Kong, Y.H. Zhang, X.X. Zhang, J.Z. Li, W.C. Chen, W.Q. Liu, Y. Ding, C.N. Zhang, B. Zhang, J.X. Yao, S.Y. Dai, J. Phys. Chem. C 118, 8756 (2014)
S.G. Esteban, P. Cruz, A. Aljarilla, L.M. Arellano, F. Langa, Org. Lett. 13, 5362 (2011)
F. Alberto, M.P. Laurence, W. Hongxia, M. Hidetoshi, M. Frank, J. Phys. Chem. C 114, 11822 (2010)
Y. Uemura, T.N. Murakami, N. Koumura, J. Phys. Chem. C 118, 16749 (2014)
P. Thongkasee, A. Thangthong, N. Janthasing, T. Sudyoadsuk, S. Namuangruk, T. Keawin, S. Jungsuttiwong, V. Promarak, Appl. Mater. Interfaces 6, 8212 (2014)
T. Sudyoadsuk, S. Pansay, S. Morada, R. Rattanawan, S. Namuangruk, T. Kaewin, S. Jungsuttiwong, V. Promarak, Eur. J. Org. Chem. 23, 5051 (2013)
Z. Iqbal, W.Q. Wu, D.B. Kuang, L.Y. Wang, H. Meier, D.R. Cao, Dyes Pigm. 96, 722 (2013)
C.Y. Chen, N. Pootrakulchote, T.H. Hung, C.J. Tan, H.H. Tsai, S.M. Zakeeruddin, C.G. Wu, M. Gratzel, J. Phys. Chem. C 115, 20043 (2011)
R.M. O’Donnell, P.G. Johansson, M. Abrahamsson, G.J. Meyer, Inorg. Chem. 52, 6839 (2013)
W.B. Heuer, H.L. Xia, W. Ward, Z. Zhou, W.H. Pearson, M.A. Siegler, A.A.N. Sarjeant, M. Abrahamsson, G.J. Meyer, Inorg. Chem. 51, 3981 (2012)
Y. Cao, Y. Bai, Q. Yu, Y. Cheng, S. Liu, D. Shi, F. Gao, P. Wang, J. Phys. Chem. C 113, 6290 (2009)
M. Gratzel, Acc. Chem. Res. 42, 1788 (2009)
M. Green, K. Emery, Y. Hishikawa, W. Warta, E. Dunlop, Prog. Photovoltaics Res. Appl. 22, 1 (2014)
R. Cordoba, N.S. Tormo, A.F. Medarde, J. Plumet, Bioorg. Med. Chem. 15, 5300 (2007)
D. Secci, A. Bolasco, M. D’Ascenzio, F.D. Sala, M. Yáñez, S. Carradoria, J. Heterocycl. Chem. 49, 1187 (2012)
B.K. An, Y.H. Kim, D.C. Shin, Macromolecules 34, 3993 (2001)
J.H. Jia, Y. Zhang, P.C. Xue, P. Zhang, X. Zhao, B.J. Liu, R. Lu, Dyes Pigm. 96, 407 (2013)
Y. Li, Y. Cao, J. Gao, D. Wang, G. Yu, A.J. Heeger, Synth. Met. 99, 243 (1999)
N.S. Cho, D.H. Wang, B.J. Jung, E. Lim, J. Lee, H.K. Shim, Macromolecules 37, 5265 (2004)
T. Marinado, K. Nonomura, J. Nissfolk et al., Langmuir 26, 2592 (2009)
C. Teng, X. Yang, S. Li et al., Chem. Eur. J. 16, 13127 (2010)
Acknowledgments
This work was financially supported by the Open Project Program of Key Laboratory of Environmentally Friendly Chemistry and Applications of Ministry of Education, China (No. 09HJYH10).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Xie, Q., Liao, Y. et al. Novel dyes of branched chain polymeric metal complexes based on cyclopentadithiophene derivatives: synthesis, characterization and photovoltaic performance for dye-sensitized solar cells. Res Chem Intermed 42, 6163–6179 (2016). https://doi.org/10.1007/s11164-016-2452-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2452-8