Abstract
Three donor-π-acceptor (D-π-A) dyes (P1–P3) of polymeric metal complexes were synthesized for application in dye-sensitized solar cells (DSSCs). These D-π-A sensitizers use Phenothiazine appended with octyl as donor (D), C=C bond as π-bridge(π), and thiophene-phenanthroline metal complexes as acceptor (A) that can be anchored to the TiO2 surface. They have been characterized and studied by FT-IR, GPC, Elemental analysis, TGA, UV-vis absorption spectroscopy, photoluminescence spectroscopy and cyclic voltammetry. When applied in the DSSCs, the sensitizer P1 exhibits the best energy conversion efficiency of 1.57% (Jsc = 4.12 mA/cm2, Voc = 0.62 V, FF = 61.7%) under simulate AM 1.5G solar irradiation. The TGA data show that these new dyes own good thermal stability. These results will facilitate the understanding of the crucial importance of molecular engineering and pave a new path to design novel conjugated organic polymer dye for highly efficient and stable DSSCs.

Three D-π-A sensitizers that use Phenothiazine appended with octyl as donor, C=C bond as π-bridge, and thiophene-phenanthroline metal complex as acceptor were synthesized. When applied in a dye sensitized solar cell, the sensitizer P1 exhibits the best energy conversion efficiency of 1.57% under simulated AM 1.5G solar irradiation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Dye-sensitized solar cells (DSSCs) as next generation of promising photovoltaic devices have attracted increasing scientific interest since the milestone report by Gratzel’s group.[1] The rise of the energy conversion limit is always dependent on the development of new dyes, as well as new electrolytes,[2–4]which leads to the power conversion efficiency (PCE) increasing from 11 to 12.3% in a recent report.[5] However, more and more queries about the dependence on platinum has been raised, regarding not only its high cost, which limits its wide application and industrialization, but also the thermal treatment for the synthesis of conventional platinum counter electrodes, which limits the application of plastic substrates in DSSCs. Therefore, there has been significant interest in the development of alternative solar cells based on inexpensive materials. Herein, many new types of dyes have emerged, and most of these dyes belong to D-π-A structure. For D-π-A structured organic dyes, triphenenlyamide,[6–8]phenothiazine,[9]coumarin,[10–12]andindoline[13,14]have been successfully used as electron donors. A π-conjugated bridge is used as a linker to connect the electron donor and electron acceptor. Phenyl, thiophene, and its derivatives[15,16] are generally used as π-conjugated bridges in the molecular design of D-π-A structured organic dyes.
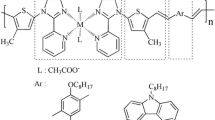
In recent years, polymer dyes have been intensively investigated due to the tailored design of the molecular structure, their high molar extinction coefficients, their environmental friendliness and low cost. However, most unfunctionalized polymers suffer from poor solubility, which limits the ability to characterize the materials in solution and to process them for applications. Besides, polymer materials also have disadvantages that are not shared by metals. Compared to metals, they have lower mechanical strength and relatively low thermal stability. Hence, the idea of combining polymers and metals into metallopolymers that exhibit as many advantages of either parent material as possible and with the number of disadvantages reduced to a minimum has significant appeal. To achieve this goal, significant efforts have focused on the synthesis of polymeric metal complexes and oligomers with pendant organic functional groups. For new type of polymeric metal complex dyes, the energy can be transferred efficiently from conjugated main-chain (as host materials) to metal complexes (as guest) in the main- or side-chain of polymers. In addition, polymers with on-chain metal centres can efficiently avoid the intrinsic defects in the corresponding blend system, such as phase-separation. In this paper, three novel side-chain polymeric complexes, which have a phenothiazine derivative as an electron donor and a thiophene-phenanthroline metal complex as an electron acceptor and an anchoring group, connected with C=C bond as a π-conjugated system, were designed and synthesized for application in dye-sensitized solar cells (DSSCs). It is worth noting that a phenothiazine-based dye contains electron-rich nitrogen and sulfur heteroatoms in the heterocyclic structure, which have a high electron-donating ability, and its non-planar butterfly conformation can sufficiently inhibit molecular aggregation and formation of intermolecular excimers.[17,18]More importantly, the introduction of a thiophene derivative with a rigid conjugated structure to the molecule is beneficial to suppression of rotation in the dye molecule for better delocalization of π-electrons which enhances the light-harvesting capacity of these complexes by increasing their extinction coefficient and shifting their spectral response to the red. Specifically, in order to achieve intense and wide absorption from an organic system, the moiety of metal complex can be employed as ‘light harvesting boosters’, and facilitating greatly the transfer of their captured photon energy to the organic component that is responsible for charge generation in the solar cell. This paper also explored the influence of different coordination metal ions on the energy conversion efficiency. Indeed, several recent reports demonstrate that exchange of a single atom, or a few atoms, in a dye molecule markedly changes the efficiencies of DSSCs comprised of them. Herein, we describe three novel polymeric metal complexes that were designed and synthesized, and the performance parameters of them as dye sensitizers. This research may provide a new insight into the factors which contribute to the high energy conversion efficiencies of D-π-A molecules.
2 Experimental
2.1 Instruments and measurements
1H NMR spectra were performed in CDCl3 or DMSO and recorded with a Bruker NMR 400 spectrometer, and the internal reference was tetramethylsilane (0.00 ppm). Fourier transform infrared (FTIR) spectra was obtained by using KBr pellets (250 mg of dried KBr and 2 mg of lyophilized sample) with a PerkinElmer Spectrum One. The frequency range of FTIR spectrometer is 450–4000 cm−1. Photoluminescence spectra were obtained with a Perkin Elmer LS55 luminescence spectrometer with a xenon lamp as the light source. UV-visible spectra were obtained with a Lambda 25 spectrophotometer. Samples were dissolved in DMF and diluted to a concentration of 10−5-10−4mol L−1. TGA was performed on Q50 thermal gravimetric analysis instrument in nitrogen atmosphere at a heating rate of 20 K min−1 from 25 to 900°C. DSC was obtained by using a Q10 thermal analyzer in nitrogen at a heating rate of 20°C min−1 from 25 to 250°C. Elemental analysis for C, H and N was carried out by using a Vario EL V5 elemental analyzer instrument made by Elementar Analysensysteme. Gel permeation chromatography (GPC) analyses were performed by a Waters 1515 system equipped with a set of HT3, HT4 and HT5, l-Styragel columns with DMF as eluent (1.0 mL min−1) at 25°C, calibrated using polystyrene standards. Cyclic voltammetry was conducted with a CH Instruments chi630c Electrochemical Workstation, in a 0.1 mol L−1 [Bu4N]BF4 in DMF solution at a scan rate of 100 mV s−1 at room temperature. The working electrode was a glassy carbon electrode, the auxiliary electrode was a platinum wire electrode and a saturated calomel electrode (SCE) was used as reference electrode.
2.2 Fabrication of DSSCs
Titania paste was prepared as follows: Fuorine-doped SnO2 conducting glass (FTO) were cleaned and immersed in isopropanol. The 20–30 nm particles sized TiO2 colloid was coated onto the FTO glass mentioned above by the sliding glass rod method and then sintered at 450°C for 30 min which was done thrice to obtain a TiO2 film of 15 μm thickness. After cooling to 100°C, the TiO2 film was immersed in the dyes dissolved in DMF and kept in dark for 24 h. Then the films were cleaned by anhydrous ethanol. After drying, electrolyte solution containing 0.5 mol L−1 LiI, 0.05 mol L−1 I2 and 0.5 mol L−1 4-tert-Butylpyridine was dripped on the surface of TiO2 electrodes. A Pt foil used as counter electrode was clipped onto the top of the TiO2 acting as the working electrode. The dye-coated semiconductor film was illuminated through a conducting glass support without a mask. Photoelectrochemical performance of the solar cell was measured using a Keithley 2602 Source meter controlled by a computer. The cell parameters were obtained under an incident light with intensity 100 mW cm−2, which was generated by a 500-W Xe lamp passing through an AM 1.5 G filter with an effective area of 0.2 cm2.
2.3 Synthesis
2.3.1 Materials
All starting materials were obtained from Shanghai Chemical Reagent Co. Ltd. (Shanghai China) and used without further purification. All solvents used were analytical grade. 2,5-Dibromo-3-triphenylphosphoniomethylbromo-thiophene (1), 4,5-Diazafluoren-9-one (2),[19]2,5-dibromo-3-(4,5-diaza- fluorene-9-vinyl)thiophene(3)[20],10-octyl-3,7-divinyl-10H-phenothiazine (4)[21,22]were synthesized according to the literature methods. Triethylamine was purified by distillation over KOH. DMF and THF were dried by distillation over CaH2. All other reagents and solvents were purchased and used as received.
2.3.2 Synthesis of 2,5-dibromo-3-(4,5 -diaza-fluorene-9-vinyl) thiophene (3)
A solution of 2,5-Dibromo-3-triphenylphosphoniomethylbromo-thiophene (1.194 g, 2 mmol) and 4,5-Diazafluoren-9-one (0.361 g, 2 mmol) in 40 mL absolute alcohol was stirred at 0°C under N2 atmosphere. Then the solution was added EtONa slowly, the mixture reacted for 24 h at 60°C. Next, the mixture was cooled at room temperature and extracted with CH2Cl2. The organic phase was washed with water, dried with MgSO4 and evaporated. The solvent was removed under reduced pressure. A pale white solid was gained, washed with methanol repeatedly. (0.208 g, yield 25%)1H NMR(CDCl3, δ, ppm): 8.65 ∼8.75 (d, 2H), 8.17 ∼8.23 (d, 2H), 7.82 ∼7.88 (d, 2H) 7.23 (s, 1H), 7.35 (s, 1H) Anal. Calcd for[C16H8Br2N2S]: C, 45.74; H, 1.92; Br, 38.04; N, 6.67; S, 7.63 Found: C, 45.62; H, 1.78; N, 6.57.
2.3.3 Synthesis of Metal Complex C1
A methanol solution (10 mL) of Zn(CH3COO)2⋅2H2O (0.26 g, 1.2 mmol) was slowly dropped into absolute alcohol which dissolved with 2,5-dibromo-3-(4,5 -diaza-fluorene-9-vinyl) thiophene (0.5 g, 1.2 mmol) and diaminomaleonitrile (0.13 g, 1.2 mmol). The reaction system was neutralized carefully with NaOH (1 m) to slightly acidic pH and refluxed for 12 h. The reaction system was then cooled to room temperature, filtered, washed twice with methanol and with water repeatedly, and then dried under vacuum at room temperature overnight. A white lead solid was gained (0.69 g, yield 81%). FT-IR (KBr, cm−1): 2227(-CN), 1647 (C=N), 1523(C=C), 1233(=C-N), 1115(C=N-M), 545(N-M). Anal. Calcd. for [C24H18Br2N6O4SZn]: C, 40.50; H, 2.55; Br, 22.45; N, 11.81; O, 8.99; S, 4.51; Zn, 9.19 Found: C, 40.18; H, 2.42; N, 11.46.
2.3.4 Synthesis of metal complex C2
With the same method as described for metal complex C1, synthesis yielded a pink–yellow solid (0.61 g, yield 78%). FT-IR (KBr, cm−1): 2233(-CN), 1659 (C=N), 1543(C=C), 1247 (=C-N), 1123 (C=N-M), 540 (N-M). Anal. Calcd for[C20H12Br2Cl2N6SNi]: C, 36.52; H, 1.84; Br, 24.29; Cl, 10.78; N, 12.78; Ni, 8.92; S, 4.87 Found: C, 36.40; H, 1.71; N, 12.63.
2.3.5 Synthesis of metal complex C3
With the same method as described for metal complex C1, synthesis yielded a yellow solid (0.56 g, yield 72%). FT-IR: 2231 (-CN), 1651 (C=N), 1536 (C=C), 1262 (=C-N), 1110 (C=N-M), 555 (N-M). Anal. Calcd for [C20H12Br2Cl2N6SCo]: C, 36.50; H, 1.84; Br, 24.28; Cl, 10.77; Co, 8.96; N, 12.77; S, 4.87. Found: C, 36.35; H, 1.69; N, 12.61;
2.3.6 Synthesis of polymeric metal complex P1
The polymeric metal complex P1 was synthesized according to the literature.[23] A mixture of metal complex C1 (0.22 g, 0.315 mmol), 10-octyl-3,7-divinyl-10H-phenothiazine (0.11 g, 0.315 mmol), Pd(OAc)2 (0.0029 g, 0.013 mmol), triethylamine (3 mL), tri-o-tolylphosphine (0.022 g, 0.072 mmol) and DMF (8 mL) heated at 90°C in the presence of nitrogen for 36 h. After the reaction mixture was cooled to room temperature and filtered, the filtrate was poured into methanol. The resulting precipitate was filtered and washed with cold methanol. The crude product was purified by dissolving it in THF and precipitating into methanol to afford a dust-colour solid (0.145 g, yield 53%). FT-IR (KBr, cm−1): 3043 (=C-H), 2918, 2843 (vinylic C–H), 2217(-CN), 1632 (C=N), 1531 (C=C), 1094 (C=N-M), 533 (N-M). Anal. Calcd for [C49H48O4N7S2Zn]:C, 63.39; H, 5.21; N, 10.56; O, 6.89; S, 6.91; Zn, 7.04. Found: C, 63.26, H, 5.07; N, 10.43. Mn =10.0 Kg/mol, PDI =1.33.
2.3.7 Synthesis of polymeric metal complex P2
A similar synthetic method as for P1 was applied. Mix metal complex C2 (0.21 g, mmol), 10-octyl-3,7-divinyl-10H-phenothiazine (0.11 g, mmol), Pd(OAc)2 (0.0029 g, 0.013 mmol), triethylamine (3 mL), tri-o-tolylphosphine (0.022 g, 0.072 mmol) and DMF (8 mL), and finally afforded a yellow solid (0.12 g, yield 47%) FT-IR (KBr, cm−1) : 3045 (=C-H), 2920, 2850 (vinylic C–H), 2227(-CN), 1641(C=N), 1535 (C=C), 1089 (C=N-M), 531 (N-M). Anal. Calcd for [C45H42N7S2 Ni] C, 61.80; H, 4.84; Cl, 8.11; N, 11.21; S, 7.33; Ni, 6.71; S, 7.33. Found: C, 61.67, H, 4.71; N, 11.08; Mn =7.7 Kg/mol, PDI =1.38.
2.3.8 Synthesis of polymeric metal complex P3
A similar synthetic method as for P1 was applied. Mix metal complex C3 (0.21 g, 0.315 mmol), 10-octyl-3,7-divinyl-10H-phenothiazine(0.11 g, 0.315 mmol), Pd(OAc)2 (0.0029 g, 0.013 mmol), triethylamine (3 mL), tri-o-tolylphosphine (0.022 g, 0.072 mmol) and DMF (8 mL), and finally afforded a brown solid (0.104 g, yield 41%) FT-IR (KBr, cm−1) : 3038 (=C-H), 2914, 2847 (vinylic C–H), 2231(-CN), 1635(C=N), 1541 (C=C), 1087 (C=N-M), 537 (N-M). Anal. Calcd for [C45H42Cl2N7S2Co]: C, 61.78; H, 4.84; Cl, 8.11; N, 11.21; S, 7.33; Co, 6.74. Found: C, 61.64; H, 4.73; N, 11.09. Mn =6.8 Kg/mol, PDI =1.35.
3 Result and Discussion
3.1 Synthesis and characterization
Our synthetic routes toward the low-band gap model complexes, the tethered intra-molecular charge transfer (ICT) monomer, and the corresponding metal-containing polymer are presented in scheme 1. The general approach of introducing transition metals into conjugated polymers has received considerable attention, in particular due to the potential to manipulate the electronic properties of these materials. These three novel branched-chain conjugated polymers were synthesized by the Heck coupling reaction.[24]
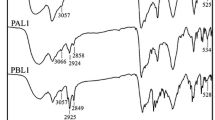
Figures 1 and 2 are the1H NMR spectrum of the intermediate compound 2,5-dibromo-3-(4,5-diaza-fluorene-9-vinyl)thiophene (3) and 10-octyl-3,7-divinyl-10H-phenothiazine (4). The synthesis of polymeric metal complexes could be determined by FT-IR spectroscopy and Elemental analysis. Figure 3a and figure 3b present the FT-IR spectra of ligand–metal complexes C1, C2, C3 and their corresponding conjugated polymers P1, P2, P3. The strong signal peak at about 2230 cm−1 of the metal complexes is attributed to the N-coordinated thiocyanate group ((C ≡N)), and it becomes weaker after polymerization. Absorption peaks appeared at 1115, 1123 and 1110 cm−1 can be ascribed to the C=N-metal stretching vibration of C1, C2 and C3. Some peaks of three corresponding polymeric metal complexes appears red shift to a certain extent because of the extension of conjugation chain after polymerization. For example, the stretching frequency of C=N-metal of P1, P2 and P3 appeared at 1094, 1089 and 1087 cm−1, respectively. The peaks at 545, 540 and 551 cm−1 are due to the N–metal vibrations of metal compounds C1, C2 and C3. Accordingly, the analogous peaks of polymeric metal complexes also have bathochromic-shift and appear at 533, 531 and 537cm−1, respectively. The appearance of similar bands at 2922 and 2847 cm−1 of the target polymers are associated with the CH2 asymmetric and symmetric stretching vibration, which indicates that phenothiazine derivatives have been successfully embedded in the molecular chain.
Gel permeation chromatography (GPC) studies shows that P1, P2 and P3 have number average molecular weight at 10.0, 7.7 and 6.8 kg/mol (11, 9 and 8 repeating units on average for P1, P2 and P3, respectively) with a relatively broad polydispersity index (PDI) between 1.33–1.38 for P1–P3 (table 1). The GPC results combined with elemental analysis indicate that the co-polymerization has been taken place between monomers and the target dyes have been obtained.
3.2 Thermal properties
As an important property of organic materials used in solar cell, thermal properties of the polymeric metal complexes were investigated by TGA and differential scanning calorimetric (DSC) analysis. The TGA traces are shown in figure 4, and the corresponding data are listed in table 1. The results of TGA show the excellent stability of polymers P1–P3 with loss 5% weight temperatures (Td) of 329, 303 and 289°C under nitrogen, respectively. The glass transition temperatures (Tg) of P1, P2 and P3 are 157, 142 and 149°C. Good thermal stability and high values of Tg are important parameters for polymers incorporated in DSSCs devices, because they provide resistance against the deformation or degradation of the active layers.
3.3 Optical properties of the polymers
The UV-vis absorption spectra of the metal complexes and their target polymeric metal complexes in DMF solution are shown in figures 5a and 5b. The corresponding optical data of the polymeric metal complexes are summarized in table 2. All the maximum absorption peaks of the co-polymers are shifted toward the longer-wavelength region relative to their metal complexes that appeared at about 360 nm. In comparison to the acceptor moiety (metal complexes), these three polymeric metal complexes (P1–P3) give two distinct absorption bands, one weak absorption band in the near-UV region (320–375 nm) corresponding to the π-π* transition of the conjugated molecule, and the other band in the visible region (390–455 nm) that can be attributed to an intra-molecular charge transfer (ICT) between the electron acceptor metal-phenanthroline unit and the electron donating phenothiazine moiety.[25] This significant expansion of its absorption band is favourable to the enhancement of energy conversion efficiency when these dyes applied in DSSCs devices.
3.4 Electrochemical properties
To estimate the energy levels of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of the polymers, which are important properties for organic materials used in solar cells, cyclic voltammetry method was employed in DMF solution containing [Bu4N]BF4 as supporting electrolyte and SCE as reference electrode at a scan rate of 100 mV/s,. All the electrochemical properties of the polymers are listed in table 3. When a saturated calomel electrode (SCE) is used as the reference electrode, HOMO and LUMO energy levels as well as the energy gaps of the polymers were calculated accordingto the equations.[26,27]:HOMO = −e(Eox+4.40) (eV); LUMO = −e(Ered+4.40) (eV); Eg =e(Eox −Ered) (eV). Figure 6 shows cyclic voltammetry curves of P1–P3. The HOMO energy value of P1, P2 and P3 were calculated to be −5.68, −5.72 and −5.74 eV. It is not difficult to find that their HOMO is below the top of the valence band of I\(_{\mathrm {3}}^{\mathrm {-}}\)/I− redox couple (−4.83 eV vs NHE), which ensures electrons could transmit between the redox couple and dyes successfully. Accordingly, the LUMO energy value of P1, P2 and P3 were calculated to be −3.37, −3.33 and −3.30 eV, and this meets the requirement that their energy level is above the redox mediator TiO2(−4.40 eV vs NHE). Consequently, the energy gap of the polymers follows the order as P1 <P2 <P3, which implies that the size of their corresponding voltage will exhibit opposite trend compared to the energy gap when these polymers are used as sensitizers, and this result may relate to the electronic configuration of metal ions.
3.5 Photovoltaic properties
The current–voltage curves (C-V curves) of the three polymeric metal complexes (P1–P3) are shown in figure 7, and the corresponding open-circuit voltage (Voc), fill factor (ff), short-circuit current (Jsc) and other relevant data are listed in table 4. It shows that the Voc values of P1, P2 and P3 dyes are 0.62, 0.57 and 0.54 V, respectively. We can clearly see that the Voc shows a downward trend, which is consistent with the speculation we aforementioned. This clearly shows that the control of the molecular orbital energies of the metal and conjugated oligomer or polymer is also important in manipulating the electronic and photophysical properties of these systems. The Jsc data of the three polymer is very low (P1, Jsc =4.12 mA cm−2; P2, Jsc =3.64 mA cm−2; P3, Jsc =3.77 mA cm−2). This limitation is related to the fact that the charge transport in conjugated polymers is a function of intra-chain charge diffusion and inter-chain interactions. We have attempted to hydrolyze one of cyano groups into carboxyl group, but failed. Adsorption effects of cyano group that can be adsorbed on the titanium dioxide by chelating on the surface of titanium dioxide nanoparticles is not as well as carboxyl group, for another, the molecular weight of polymer is very big, these lead to a poor interface between TiO2 surface and the polymeric dyes, which decreases the electron injection efficiency, then enforcing the charge recombination and making charge transfer blocked, and finally leading to a low Jsc for the polymeric dyes. The charge carrier mobility in these
materials is usually limited by disorder effects, which prevents efficient inter-chain coupling and leads to materials with one-dimensional electronic properties. From table 4, we can clearly see that the power conversion efficiency of P1 is higher than that of P2 and P3, which attributes to its relatively high Jsc and Voc. Figure 8 shows the input photon to converted current efficiency (IPCE) curves of these polymeric metal complexes. The maximum external quantum efficiency (EQE) of P1, P2 and P3 are very low and no more than 40%, which is an important aspect and we need to improve and obtain high current density in our future work.
4 Conclusions
In summary, three novel π-conjugated polymers tethered with metal-containing low-band gap units were synthesized by Heck coupling reaction and systemically studied their performance as a dye sensitizer. These dyes bearing functionalized metal complex exhibited high molecular weight, good film-forming properties, and excellent solution processability. The results for these three dyes indicate that minor changes in the molecular design of the polymeric metal complex dye may bring profound impact in its energy conversion efficiency when they are applied in DSSCs.
The energy conversion efficiency of P1, P2 and P3 are 1.57%, 1.31% and 1.22%, respectively, suggesting further optimization is essential before application in DSSCs.
References
O’Regan B and Gratzel M 1991 Nature 353 737
Chen T, Hu W H, Song J L, Guai G H and Li C M 2012 Adv. Funct. Mater. 22 5245
Chang S, Li Q, Xiao X D, Wong K Y and Chen T 2012 Energy Environ. Sci. 5 9444
Chen T, Guai G H, Gong C W, Hu H, Zhu J X, Yang H B, Yan Q Y and Li C M 2012 Energy Environ. Sci. 5 6294
Yella A, Lee H W, Tsao H N, Yi C Y, Chandiran A, Nazeeruddin M K, Diau E W G, Yeh C Y, Zakeeruddin S M and Gratzel M 2011 Science 334 629
Hagberg D P, Edvinsson T, Marinado T, Boschloo G, Hagfeldt A and Sun L A 2006 Chem. Commun. 21 2245
Tian H, Yang X, Chen R, Zhang R, Hagfeldt A and Sun L 2008 J. Phys. Chem. C. 112 11023
Kitamura T, Ikeda M, Shigaki K, Inoue T, Anderson N A, Lian T and Yanagida S 2004 Chem. Mater. 16 1806
Tian H, Yang X, Chen R, Li L, Hagfeldt A and Sun L 2007 Chem. Commun. 36 3741
Hara K, Sayama K, Ohga Y, Shinpo A, Suga S and Arakawa H A 2001 Chem. Commun. 6 569
Wang Z S, Cui Y, Hara K, Dan-Oh Y, Kasada C and Shinpo A A 2007 Adv. Mater. 19 1138
Hara K, Wang Z, Sato T, Furube A, Katoh R, Sugihara H, Dan-oh Y, Kasada C, Shinpo A and Suga S 2005 J. Phys. Chem. B. 109 15476
Zhu W, Wu Y, Wang S, Li W, Li X, Chen J, Wang Z S and Tian H 2011 Adv. Funct. Mater. 21 756
Liu B, Zhu W, Zhang Q, Wu W, Xu M, Ning Z, Xie Y and Tian H 2009 Chem. Commun. 13 1766
He J, Guo F, Li X, Wu W, Yang J and Hua J 2012 Chem- Eur. J. 18 7903
Liu B, Wang R, Mi W, Li X and Yu H 2012 J. Mater. Chem. 22 15379
Tian H N, Yang X C, Chen R K, Pan Y Z, Li L, Hagfeldt A and Sun L C 2007 Chem. Commun. 43 3741
Wu W J, Yang J B, Hua J L, Tang J, Zhang L, Long Y T and Tian H 2010 J. Mater. Chem. 20 1772
Henderson L J Jr., Fronczek F R and Cherry W R 1984 J. Am. Chem. SOC. 106 5876
Hubert C, Tran K, Hauquier F, Cougnon C, Pilard J-F, Gosselin P, Rault-B J and Raoult E 2007 New J. Chem. 31 1730
Morin J F, Drolet N, Tao Y and Leelerc M 2004 Chem. Mater 16 4619
Kim S W, Shim S C and Jung B et a1. 2002 Polymer 43 4297
Ziegler C B and Heck R F 1978 J. Org. Chem. 43 2941
Robert G, Charles H, Freiser R, Friedel L, Hilliard E and Johnston W D 1956 Spectochimica. Acta. 8 1
Ng S C, Xu J M and Chan H S O 2000 Synth. Met. 110 311
Cho N S, Hwang D H and Jung B J 2004 Macromolecules. 37 5265
Bessho T, Constable E C, Graetzel M, Hernandez Redondo A, Housecroft C E, Kylberg W, Nazeeruddin Md K, Neuburger M and Schaffner S 2008 Chem. Commun. 32 3717
Acknowledgments
This work was financially supported by the Open Project Program of Key Laboratory of Environmentally Friendly Chemistry and Applications of Ministry of Education, China (No. 09HJYH10).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
XIE, Q., ZHOU, J., HU, J. et al. Synthesis and Photovoltaic properties of branched chain polymeric metal complexes containing Phenothiazine and Thiophene derivative for dye-sensitized solar cells. J Chem Sci 127, 395–403 (2015). https://doi.org/10.1007/s12039-015-0790-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0790-5