Abstract
N,N,N′,N′-Tetrachlorobenzene-1,3-disulfonamide and poly(N,N′-dichloro-N-ethyl-benzene-1,3-disulfonamide) are new catalysts promoted by one-pot, facile and four-component synthesis of new substituted 1,4-dihydropyridine derivatives from the reaction of ammonium acetate, aldehydes and various 1,3-dicarbonyl compounds with good to high yields at room temperature under mild conditions. All the synthesized compounds were evaluated for antibacterial and anti-oxidant activities. Antibacterial activity was determined against four Gram-positive and -negative bacteria, and anti-oxidant activity was evaluated by 2,2-diphenyl-1-picrylhydrazyl free radical scavenging. The bioassay results indicated that synthesized 1,4-dihydropyridine derivatives have effective anti-oxidant and antibacterial functions.
Graphical Abstract
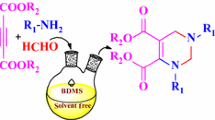
Synthesis and biological evaluation of new series dihydropyridines.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rare structures and diverse functional groups of heterocyclic systems are of great importance in chemical and biological investigations [1]. Dihydropyridine (DHP) nucleus comprises an important class of bioactive molecules in pharmaceutical fields. Also, they are used as reducing agents and versatile synthetic intermediates for the construction of libraries, poly-substituted systems and natural products [2–9]. Variations in structural models of dihydropyridine derivatives may create various biological activities [10–12]. For example, they are analogues of hydrogenase coenzymes [13] and could be used as calcium-channel blockers [14], anti-inflammatory, anti-microbial [15], anti-oxidant, anti-ulcer [16], and anticoagulant [17], and suitable for treatment of multidrug resistance (MDR) (Fig. 1) [18]. Considering the importance of dihydropyridine derivatives, recently a number of methods via multicomponent reaction have been developed [19, 20], by the use of I2 [21], Yb(OTf)3 [22], Sc(OTf)3 [23], HClO4–SiO2 [24, 25], and CuSO4·5H2O [26], including microwave irradiation [27–29], ZnO [30], ZnCl2 [31], ionic liquids [32, 33], magnetic Fe3O4 nanoparticles [34], cobalt nanoparticles [35] and organocatalysts [36–38]. Therefore, the design and synthesis of new 1,4-dihydropyridine derivatives using multicomponent reactions are nowadays structurally desirable.
Experimental
All commercially available chemicals were purchased from Merck and Fluka, and used without further purification unless otherwise stated. Nuclear magnetic resonance 1H and 13C-NMR spectra were recorded on Bruker Avance 500, 400, 300, MHz FT and Varian 90 MHz NMR instrumenta. Chemical shifts were expressed in parts per million (ppm) and J in hertz (Hz). Infrared (IR) spectroscopy was conducted on a Perkin Elmer GX FT-IR spectrometer. Mass spectra was recorded on a Shimadzu QP 1100 BX Mass Spectrometer. Elemental analyses were performed with CHNS-932, Leco, USA.
A sample experimental procedure for the synthesis of ethyl-4-(4-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (1)
A mixture of ammonium acetate (0.077 g, 1 mmol), 4-chlorobenzaldehyde (0.140 g, 1 mmol), dimedone (0.140 g, 1 mmol), ethyl acetoacetate (0.130 g, 1 mmol) and TCBDA (0.06 g, 0.16 mmol) or PCBS (0.05 g), ethanol (2 mL) was stirred at room temperature for 4 h. The progress of the reaction was monitored by TLC (9:3, n-hexane/acetone). After completion of the reaction, ethanol (10 mL) was added. The crude product was filtered, washed with ethanol and dried. The crude product was recrystallized from methanol to give the pure product. Evaporation of the solvent was followed by the addition of CH2Cl2 (10 mL) recycling the catalysts. Yield 0.2 g (70 %); white powder; m.p.: 236–238 °C; IR (KBr, ν max/cm−1): 3290, 3082, 2963, 2930, 1698, 1644, 1611, 1484, 1381, 1280, 1072, 697 cm−1; 1H NMR spectrum (90 MHz, CDCl3): δ H = 0.80 (s, 3H, CH3), 0.97 (s, 3H, CH3), 1.08–1.16 (t, J = 7.2 Hz, 3H, CH3), 2.30–2.07 (m, 7H), 4.04–3.80 (q, J = 6.9 Hz, 2H, OCH2), 4.81 (s, 1H, CH), 7.11 (s, 4H, H Ar), 9.04 (s, 1H, NH) ppm.
Ethyl-4-(2-methoxyphenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (2)
Yield 0.33 g (91 %); white powder; m.p.: 240–242 °C; IR (KBr, ν max/cm−1): 3285, 3070, 2964, 1689, 1610, 1488, 1381, 1281, 1215, 1028, 752 cm−1; 1H NMR spectrum (300 MHz, CDCl3): δ H = 0.93 (s, 3H, CH3), 1.06 (s, 3H, CH3), 1.16–1.20 (t, J = 7.1 Hz, 3H, CH3), 2.34–2.11 (m, 7H), 3.80 (s, 3H, CH3), 4.05–3.97 (q, J = 9.6 Hz, 2H, OCH2), 5.25 (s, 1H, CH), 5.98 (s, 1H, NH), 6.84–6.77 (m, 2H, H Ar), 7.08–7.05 (m, 3H, H Ar), 7.31–7.29 (m, 1H, H Ar) ppm; 13C NMR (75 MHz, CDCl3): δ C = 14.11 (CH3), 19.31 (CH3), 26.84 (CH3), 29.48 (CH3), 32.55 (CH2), 33.47 (CH2), 41.24 (CH), 50.72 (OCH3), 55.34 (C), 59.56 (OCH2), 105.09, 110.79, 111.02, 119.9 (C Ar), 127.2 (C Ar), 131.2 (C Ar), 134.4, 143.03, 148.37, 157.48, 167.90 (CO2), 195.29 (C=O) ppm; MS m/z = 369 [M]+ (80), 340 (60), 262 (100), 234 (55), 178 (10); Anal. Calcd. for C22H27NO4: C, 71.54; H, 7.31; N, 3.79 %. Found: C, 71.42; H, 7.60; N, 3.81 %.
Ethyl-4-(3-nitrophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate (3)
Yield 0.33 g (88 %); yellow powder. m.p.: 169–171 °C. IR (KBr, ν max/cm−1): 3285, 3076, 2958, 2872, 1705, 1642, 1606, 1534, 1487, 1380, 1352, 1281, 1211, 1072, 682 cm−1; 1H NMR spectrum (300 MHz, CDCl3): δ H = 0.93 (s, 3H, CH3), 1.08 (s, 3H, CH3), 1.22–1.17 (t, J = 7.2 Hz, 3H, CH3), 2.41–2.11 (m, 7H), 4.09–4.02 (q, J = 6.9 Hz, 2H, OCH2), 5.15 (s, 1H, CH), 6.34 (s, 1H, NH), 7.40–7.35 (t, J = 7.8 Hz, 1H, H Ar), 7.74–7.71 (d, J = 7.8 Hz, 1H, H Ar), 7.99–7.96 (d, J = 6.0 Hz, 1H, H Ar), 8.11 (s, 1H, H Ar) ppm; 13C NMR (75 MHz, CDCl3): δ C = 14.16 (CH3), 19.47 (CH3), 27.07 (CH3), 29.35 (CH3), 32.75 (CH2), 36.94 (CH2), 40.95 (CH), 50.54 (C), 60.05 (OCH2), 105.1, 111.2, 121.2 (C Ar), 122.8 (C Ar), 128.5, 134.8, 144.3, 148.2, 148.7, 149.1 (C Ar), 166.8 (CO2), 195.4 (C=O) ppm; MS m/z = 384 [M]+ (12), 367 (24), 262 (100), 234 (74), 178 (24). Anal. Calcd. for C21H24N2O5: C, 65.62; H, 6.25; N, 7.29 %. Found: C, 64.58; H, 6.48; N, 7.18 %.
3-Acetyl-4-(2-methoxyphenyl)-2,7,7-trimethyl-1,4,5,6,7,8-hexahydroquinoline-5-one (4)
Yield 0.29 g (88 %); yellow powder. m.p.: 210–212 °C. IR (KBr, ν max/cm−1): 3289, 3300, 2960, 2930, 1666, 1611, 1473, 1379, 1220, 1142, 762; 1H NMR spectrum (300 MHz, CDCl3): δ H = 0.93 (s, 3H, CH3), 1.04 (s, 3H, CH3), 2.33–2.09 (m, 10 H), 3.84 (s, 3H, OCH3), 5.36 (s, 1H, CH), 6.64 (s, 1H, NH), 6.83-6.78 (t, J = 6.6 Hz, 2H, H Ar), 7.18–7.06 (m, 2H, H Ar) ppm; 13C NMR (75 MHz, CDCl3): δ C = 19.60 (CH3), 27.04 (CH3), 29.19 (CH3), 29.46 (CH3), 31.95 (CH2), 32.56 (CH2), 40.96 (CH), 50.78 (OCH3), 55.46 (C), 111.1, 111.5, 114.5, 120.6, 127.6 (C Ar), 129.8, 134.6, 141.0, 148.8, 155.9 (C Ar), 195.4 (C=O), 200.9 (C=O) ppm; MS m/z = 339 [M]+ (10), 309 (56), 232 (100), 190 (10), 77 (18); Anal. Calcd. for C21H25NO3: C, 74.33; H, 7.37; N, 4.12 %. Found: C, 74.36; H, 7.83; N, 4.15 %.
A sample experimental procedure for the synthesis of ethyl-2-methyl-4-(3-methylphenyl)-5-oxo-4,6,7,8-tetrahydro-1H-quinoline-3-carboxylate (5)
A mixture of ammonium acetate (0.077 g, 1 mmol), 3-methylbenzaldehyde (0.120 g, 1 mmol), 1,3-cyclohexanedione (0.112 g, 1 mmol), ethyl acetoacetate (0.130 g, 1 mmol) and TCBDA (0.06 g, 0.16 mmol) or PCBS (0.05 g), ethanol (2 mL) was stirred at room temperature for the appropriate time. The progress of the reaction was monitored by TLC (9:2, n-hexane/acetone). After completion of the reaction, ethanol (10 mL) was added. The crude product was filtered, washed with ethanol and dried. The crude product was recrystallized from methanol to give the pure product. Evaporation of the solvent was followed by the addition of CH2Cl2 (10 mL) recycling the catalysts. Yield 0.22 g (70 %); white powder; m.p.: 195–197 °C; IR (KBr, ν max/cm−1): 3373, 3295, 3075, 2960, 2940, 1722, 1693, 1608, 1485, 1377, 1219, 1181, 1073 cm−1; 1H NMR spectrum (400 MHz, CDCl3): δ H = 1.20–1.23 (t, J = 6.0 Hz, 3H, CH3), 1.89–2.05 (m, 2H, CH2), 2.29 (s, 3H, CH3), 2.31–2.37 (m, 2H, CH2), 2.38 (s, 3H, CH3), 2.46–2.42 (m, 2H, CH2) 4.10–4.04 (q, J = 8.1 Hz, 2H, CH2), 5.07 (s, 1H, CH), 6.14 (s, 1H, NH), 6.93–6.94 (t, J = 2.7 Hz, 1H, H Ar), 7.12–7.09 (m, 3H, H Ar) ppm; 13C NMR (100 MHz, CDCl3): δ C = 14.20 (CH3), 19.41 (CH3), 21.02 (CH3), 21.57 (CH2), 27.48 (CH2), 36.28 (CH2), 37.05 (CH), 59.80 (OCH2), 106.1, 113.4, 125.0, 126.8, 127.8, 128.8, 137.2 (C Ar), 143.2 (C Ar), 147.0, 149.6, 167.5 (CO2), 195.7 (C=O) ppm; MS m/z = 325 [M]+ (33), 295 (16), 234 (100), 205 (77), 160 (16); Anal. Calcd. for C19H21NO2: C, 73.84; H, 7.07; N, 4.30 %. Found: C, 73.67; H, 7.32; N, 3.71 %.
3-Acetyl-4-(2-hydroxy-5-bromophenyl)-2-methyl-4,6,7,8-tetrahydro-1H-quinoline-5-one (6)
Yield 0.32 g (85 %); white powder; m.p.: 209–211 °C; IR (KBr, ν max/cm−1): 3420, 3281, 3088, 2951, 2900, 1714, 1580, 1511, 1472, 1378, 1237, 1195, 1027 cm−1; 1H NMR spectrum (300 MHz, DMSO-d6): δ H = 1.62–1.58 (m, 1H, CH2), 1.98–1.93 (d, J = 12.9 Hz, 1H, CH2), 2.03 (s, 3H, CH3), 2.23 (s, 3H, CH3), 2.40–2.30 (m, 2H, CH2), 3.02 (s, 1H, NH), 4.42 (s, 1H, CH), 6.75-6.72 (d, J = 8.7 Hz, 1H, H Ar), 7.17–7.14 (d, J = 7.8 Hz, 1H, H Ar), 7.36 (s, 1H, H Ar), 7.68 (s, 1H, OH) ppm; 13C NMR (75 MHz, DMSO-d6): δ C = 20.07 (CH3), 20.74 (CH3), 28.74 (CH2), 30.54 (CH2), 32.99 (CH2), 47.34 (CH), 84.45, 109.1, 111.4, 118.0, 129.4, 130.4 (C Ar), 131.0, 150.0, 150.7, 192.7 (C=O), 204.4 (C=O) ppm; MS m/z = 378 [M]+ (50), 360 (66), 332 (31), 204 (100), 162 (53); Anal. Calcd. for C20H22BrNO: C, 57.6; H, 4.80; N, 3.73 %. Found: C, 56.98; H, 4.83; N, 3.69 %.
A sample experimental procedure for the synthesis of ethyl-4-(3-methylphenyl)-2-methyl-5-oxo-4,5-dihydro-1H-indeno-[1,2-b]pyridine-3-carboxylate (7)
A mixture of ammonium acetate (0.077 g, 1 mmol), 3-methylbenzaldehyde (0.120 g, 1 mmol), 1,3-indandione (0.146 g, 1 mmol), ethyl acetoacetate (0.130 g, 1 mmol) and TCBDA (0.06 g, 0.16 mmol) or PCBS (0.05 g), ethanol (2 mL) was stirred at room temperature for the appropriate time. The progress of the reaction was monitored by TLC (9:3, n-hexane/acetone). After completion of the reaction, ethanol (10 mL) was added. The crude product was filtered, washed with ethanol and dried. The crude product was recrystallized from methanol to give the pure product. Evaporation of the solvent was followed by the addition of CH2Cl2 (10 mL) recycling the catalysts. Yield 0.33 g (92 %); red powder; m.p.: 256–258 °C; IR (KBr, ν max/cm−1): 3436, 3274, 3192, 3083, 2984, 1670, 1637, 1512, 1189, 1085 cm−1; 1H NMR spectrum (500 MHz, CDCl3): δ H = 1.17 (t, J = 7.0 Hz, 3H, CH3), 2.3 (s, 3H, CH3), 2.51 (s, 3H, CH3), 4.08 (q, 2H, CH2), 6.86 (s, 1H, NH), 6.96 (d, J = 3.5 Hz, 1H, H Ar), 7.15–7.11 (m, 4H, H Ar), 7.29 (s, 1H, H Ar), 7.38–7.37 (d, J = 6.0 Hz, 2H, H Ar) ppm; 13C NMR (100 MHz, CDCl3): δ C = 14.01 (CH3), 19.66 (CH3), 21.47 (CH3), 37.24 (CH), 60.01 (OCH2), 108.2, 110.6, 117.1, 121.3, 125.0, 127.2, 128.0, 128.6, 130.0 (C Ar), 131.2, 134.0, 136.1, 137.5, 143.2 (C Ar), 145.6, 153.2, 167.4 (CO2), 192.4 (C=O) ppm; MS m/z = 359 [M]+ (6.2), 298 (100), 268 (31), 206 (31), 178 (35); Anal. Calcd. for C23H21NO3: C, 76.86; H, 5.89; N, 3.90 %. Found: C, 76.53; H, 5.87; N, 3.69 %.
Ethyl-4-(2-hydroxy-5-bromophenyl)-2-methyl-5-oxo-4,5-dihydro-1H-indeno-[1,2-b]pyridine-3-carboxylate (8)
Yield 0.38 g (88 %); orange powder; m.p.: 235–237 °C; IR (KBr, ν max/cm−1): 3430, 3210, 3080, 2985, 1738, 1659, 1548, 1460, 1093 cm−1; 1H NMR spectrum (400 MHz, DMSO-d6): δ H = 1.11–1.08 (t, J = 7.2 Hz, 3H, CH3), 1.94 (s, 3H, CH3), 3.23–3.24 (d, J = 2.8 Hz, 1H, CH), 4.06–4.01 (q, J = 7.2 Hz, 2H, CH2), 4.13–4.12 (d, 1H, NH), 6.75–6.73 (d, J = 8.4 Hz, 1H, H Ar), 7.45–7.16 (m, 6H, H Ar), 9.60 (br, 1H, OH) ppm; 13C NMR (100 MHz, DMSO-d6): δ C = 14.33 (CH3), 24.63 (CH3), 30.36 (CH), 44.38 (OCH2), 60.96, 83.34, 107.65, 112.0, 118.6, 119.6, 120.6 (C Ar), 127.8 (C Ar), 130.1, 130.1, 131.0, 131.4, 135.9, 136.2, 151.5, 160.9 169.4 (CO2), 186.5 (C=O) ppm; MS m/z = 441 [M]+ (31), 325 (17), 268 (100), 234 (89), 206 (19); Anal. Calcd. for C22H18BrNO4: C, 60.01; H, 4012; N, 3.18 %. Found: C, 59.25; H, 3.94; N, 2.87 %.
A sample experimental procedure for the synthesis of ethyl-5-phenyl-2,2,7-trimethyl-4-oxo-5,8-dihydro-4H-1,3-dioxa-8-aza-naphthalene-6-carboxylate (9)
A mixture of ammonium acetate (0.077 g, 1 mmol), benzaldehyde (0.106 g, 1 mmol), 2,2-dimethyl-1,3-dioxane-4,6-dione (0.144 g, 1 mmol), ethyl acetoacetate (0.130 g, 1 mmol) and TCBDA (0.06 g, 0.16 mmol) or PCBS (0.05 g), ethanol (2 mL) was stirred at room temperature for the appropriate time. The progress of the reaction was monitored by TLC (8:2, n-hexane/acetone) until completion of the reaction. Evaporation of the solvent under reduced pressure was followed by the addition of methanol (2 mL) to give the pure product. Evaporation of methanol and then the addition of CH2Cl2 (10 mL) recycling the catalysts. Yield 0.29 g (86 %); white powder; m.p.: 158–160 °C. IR (KBr, ν max/cm−1): 3426, 2985, 2800, 1736, 1704, 1555, 1501, 1443, 1387, 1266, 1061 cm−1; 1H NMR spectrum (90 MHz, DMSO-d6): δ H = 1.18 (t, J = 4.8 Hz, 3H, CH3), 1.63 (s, 3H, CH3), 2.06 (s, 6H, 2 CH3), 3.46 (q, 2H, CH2), 5.96 (s, 1H, CH), 7.25–7.40 (m, 6H, H Ar, NH) ppm; 13C NMR (100 MHz, DMSO-d6): δ C = 13.82 (CH3), 19.78 (CH3), 27.89 (CH3), 28.48 (CH3), 49.06 (CH), 58.10 (OCH2), 59.66 (C), 95.69, 105.6, 127.4, 128.6, 128.9, 129.2, 129.8 (C Ar), 130.0 (C Ar), 153.4, 160.8, 166.8 (CO2), 167.6 (CO2) ppm; MS m/z = 343 [M]+ (9), 341 (28), 208 (50), 104 (86), 77 (100); Anal. Calcd. for C19H21NO5: C, 66.47; H, 6.12; N, 4.08 %. Found: C, 65.40; H, 6.10; N, 3.81 %.
Ethyl-5-(2,4-dichlorophenyl)-2,2,7-trimethyl-4-oxo-5,8-dihydro-4H-1,3-dioxa-8-aza-naphthalene-6-carboxylate (10)
Yield 0.28 g (70 %); white powder; m.p.: 183–184 °C; IR (KBr, ν max/cm−1): 3369, 3077, 3001, 2928, 1769, 1740, 1587, 1470, 1384, 1277, 1102, 1024 cm−1; 1H NMR spectrum (90 MHz, DMSO-d6): δ H = 1.52 (t, J = 6.8 Hz, 3H, CH3), 2.08 (s, 9H, 3CH3), 3.52 (q, 2H, CH2), 4.02 (s, 1H, CH), 6.54 (s, 1H, NH), 7.11–7.68 (m, 3H, H Ar) ppm; 13C NMR 100 MHz, DMSO-d6): δ C = 14.4 (CH3), 18.6 (CH3), 35.0 (2CH3), 36.5 (CH) 59.9 (OCH2), 103 (C), 128.1 (C Ar), 129.1, 129.7, 132.5, 133.6, 138.8, 150.2, 166.4, 169.3 (2CO2) ppm; MS m/z = 412 [M]+ (45), 376 (9), 295 (16), 262 (100), 179 (33), 149 (66); Anal. Calcd. for C19H19Cl2NO5: C, 55.33; H, 4.65; N, 3.40 %. Found: C, 54.32; H, 4.43; N, 2.91 %.
6-Acetyl-5-(2,4-dichlorophenyl)-2,2,7-trimethyl-5,8-dihydro-4H-1,3-dioxa-8-aza-naphthalene-4-one (11)
Yield 0.30 g (80 %); white powder.; m.p.: 189–191 °C; IR (KBr, ν max/cm−1): 3175, 3001, 3001, 2928, 1660, 1565, 1454, 1347, 1260, 1203, 1058, 756 cm−1; 1H NMR spectrum (300 MHz, DMSO-d6): δ H = 1.80 (s, 3H, CH3), 1.88 (s, 3H, CH3), 2.07 (s, 6H, CH3), 4.86 (s, 1H, CH), 7.12-7.09 (d, J = 8.4 Hz, 1H, H Ar), 7.47-7.43 (d, J = 8.4 Hz, 1H, H Ar), 7.73 (s, 1H, H Ar) ppm; 13C NMR (75 MHz, DMSO-d6): δ C = 26.58 (CH3), 27.44 (CH3), 30.69 (CH), 41.88 (C), 106.9, 127.3 (C Ar), 127.5, 128.6, 129.2, 133.5, 136.1, 158.7, 160.2 (CO2), 206.4 (C=O) ppm; MS m/z = 381 [M]+ (2.7), 296 (61), 282 (16), 241 (16), 199 (100) ppm; Anal. Calcd. for C18H17Cl2NO4: C, 56.56; H, 4.48; N, 3.66 %. Found: C, 55.56; H, 4.13; N, 3.59 %.
A sample experimental procedure for the synthesis of 9-(phenyl)-3,3,6,6-tetra-methyl-1,2,3,4,5,6,7,8-octahydro-acridine-1,8-dione (16)
A mixture of ammonium acetate (0.077 g, 1 mmol), benzaldehyde (0.106 g, 1 mmol), dimedone (0.280 g, 2.1 mmol) and TCBDA (0.06 g, 0.16 mmol) or PCBS (0.05 g), ethanol (2 mL) was stirred at room temperature for the appropriate time. The progress of the reaction was monitored by TLC (9:3, n-hexane/acetone). After completion of the reaction, ethanol (10 mL) was added. The crude product was filtered, washed with ethanol and dried. Evaporation of the solvent was followed by the addition of CH2Cl2 (10 mL) recycling the catalysts.
9-(2,4-dichlorophenyl)-3,3,6,6-tetra-methyl-2,4,5,7-tetraoxa-1,3,6,8-tetrahydro-acridine-1,8-dione (17)
Yield 0.34 g (80 %); white powder; m.p.: 168–170 °C; IR (KBr, ν max/cm−1): 3129, 3077, 3001, 2882, 1779, 1743, 1589, 1477, 1395, 1320, 1273, 1201, 1020 cm−1; 1H NMR spectrum (400 MHz, DMSO-d6): δ H = 1.81 (s, 6H, 6H, 2CH3), 1.89 (s, 6H, 2CH3), 4.88 (s, 1H, CH), 7.10–7.13 (t, J = 8.4 Hz, 1H, H Ar), 7.45–7.48 (q, J = 8.4 Hz, 1H, H Ar), 7.74–7.75 (d, J = 2.0 Hz, 1H, H Ar) ppm; 13C NMR (100 MHz, DMSO-d6): δ C = 27.0 (CH3), 27.9 (CH3), 42.3 (CH), 107.4, 127.8, 128.0 (C Ar), 129.0, 129.6, 133.9, 136.5, 159.1, 160.6 ppm.
Screening of DPPH radical scavenging activity
Free radical scavenging activity of the new synthesized compounds was evaluated by the stable radical DPPH. Thrn, 0.3 mmol DMSO solution of DPPH (1 mL) was added to samples (2.5 mL) containing different synthesized 1,4-DHPs. The samples were first kept in a dark place at room temperature and their absorbance was read at 517 nm after 30 min. The anti-oxidant activity (AA) was determined using the following formula:
Blank samples contained (1 mL) DMSO + (2.5 mL) from various concentrations of synthesized compounds; the control sample contained (1 mL) of (0.3 mmol) DPPH + (2.5 mL) DMSO. The optic density of the samples, the control and the empty samples were measured in comparison with DMSO. Ascorbic acid was used as a positive control and experiments were carried out in triplicate. The discoloration was plotted against the sample concentration in order to calculate the IC50 value, which is the amount of sample necessary to decrease the absorbance of DPPH by 50 % (Table 3).
Screening of antimicrobial activity
Synthesized 1,4-dihydropyridine derivatives were tested by the disc diffusion method against a panel of microorganisms including two Gram-positive bacteria, namely Bacillus cereus (PTCC 1247) and Staphylococcus aureus (Wild) and two Gram-negative bacteria, namely Escherichia coli (Wild) and Serratia marcescens (PTCC 1111). The complexes were dissolved in hexane to a final concentration of 1 mg/mL then sterilized by filtration (0.45 μm; Millipore). All tests were carried out with 10 mL of suspension containing 1.5 × 108 bacteria/mL and spread on nutrient agar medium. A negative control was prepared by DMSO. Gentamicin, Penicillin, Streptomycin and Cephalexin were used as positive standard discs for the comparison of antibacterial activity. Positive and negative controls are shown in Table 5 (below).
Statistical analysis
The evaluation of antibacterial and anti-oxidant assays was carried out in triplicate and recorded as mean ± standard deviation. Analysis of variance was performed by Excel and SPSS procedures. p value <0.05 was regarded as significant.
Results and discussion
As a part of our research into the application of N-halosulfunamides in organic synthesis [39–43], we report one-pot, four-component synthesis of 1,4-dihydropyridine derivatives from ammonium acetate, cyclic and acyclic 1,3-dicarbonyl compounds and various aldehydes in the presence of N,N,N′,N′-tetrachlorobenzene-1,3-disulfonamid (TCBDA) and poly(N,N′-dichloro-N-ethyl-benzene-1,3-disulfonamide) (PCBS) [44, 45] as catalyst at room temperature and under mild conditions (Scheme 1).
Providing the TCBDA and PCBS are easy and stable under atmospheric conditions for 2 months, after the completion of the reaction, the catalyst can be recovered and can be reused several times without significant decrease of the yield (Scheme 2).
In order to optimize the reaction conditions, we first examined the effect of TCBDA and/or PCBS for the synthesis of 1,4-DHPs under Hantzsch condensation from 4-chlorobenzaldehyde (1.0 mmol), ethyl acetoacetate (1.0 mmol), ammonium acetate (1.0 mmol) and dimedone (1.0 mmol) as a model reaction. It was observed that, in the absence of the catalyst, only a trace of ethyl-4-(4-chlorophenyl)-2,7,7-trimethyl-5-oxo-1,4,5,6,7,8-hexahydroquinoline-3-carboxylate was formed, even after prolonged reaction times (Table 1, entry 8). So, the effect of the catalyst was also investigated in various conditions and the results are summarized in Table 1. It was determined that TCBDA (0.06 g, 0.16 mmol) or PCBS (0.05 g) and ethanol were optimal to this reaction (Table 1, entries 1, 2).
Therefore, in order to determine the scope and generality of this reaction, cyclocondensation of a number of various cyclic and acyclic 1,3-dicarbonyl compounds, aromatic, heteroaromatic and aliphatic aldehydes and ammonium acetate as a source of nitrogen was applied and the corresponding 1,4-dihydropyridine derivatives were synthesized in good to high yields. The results are summarized in Table 2.
Furthermore, under optimized reaction conditions, we selected dimedone and 2,2-dimethyl-1,3-dioxane-4,6-dione for the synthesis of structurally symmetrical 1,4-DHPs, and, as shown in Table 2, the desired products were achieved in good to high yields (Table 2, entries 16, 17).
Also, our experiments determined that TCBDA is a reusable catalyst. Therefore, it was applied for the synthesis of 3-acetyl-4-(2-hydroxy-5-bromophenyl)-2-methyl-4,6,7,8-tetrahydro-1H-quinoline-5-one so that after three runs of reusing the catalyst, its catalytic activity was almost the same as that of a fresh catalyst (Table 2, entry 6). The results of catalytic activity of TCBDA for all three consecutive runs are shown in Table 2.
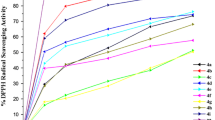
All synthesized 1,4-dihydropyridine derivatives were screened for in vitro anti-oxidant and antibacterial activities. Anti-oxidant properties, especially radical scavenging activities, are very important due to the deleterious role of free radicals in foods and biological systems [46]. The free radical scavenging activity of the synthesized compounds (2–16) was assayed by 2,2-diphenyl-1-picrylhydrazyl (DPPH) and comparison with ascorbic acid as a standard. Assessment of anti-oxidant activity showed that all compounds have good free radical scavenging activity but, in 1 mg/mL concentration, compounds 4, 6, 9, 11, 12, 4, 9, 11 and 17 show a remarkable amount of scavenging activity (>50 %) compared with standard ascorbic acid (Table 3). The higher anti-oxidant activity is reflected in a lower IC50 (mg/mL). The effectiveness of anti-oxidants as DPPH radical scavengers ranged in the following descending order: ascorbic acid > 11 > 4 > 6 ≥ 12 ≥ 17 ≥ 9 ≥ 7 ≥ 8 > 16 ≥ 15 ≥ C 3 ≥ 10 > 13 ≥ C 2 > 14 ≥ 5 (Table 3). The above results indicate that the synthesized compounds may be used in the treatment of diseases caused by free radicals.
The in vitro antibacterial activity of the synthesized compounds (2–16) were assayed against four various bacterial strains like S. aureus (Gram-positive), B.cereus (Gram-positive), E. coli (Gram-negative) and S. marcescens (Gram-negative). The synthesized compounds were dissolved in dimethyl sulfoxide (DMSO) at concentrations of 1, 0.1 and 0.01 mg/mL. Since dimethyl sulfoxide was used as solvent control against all tested organisms, no activity was detected (Table 5). Most of the studied compounds in this research represented antibacterial activity especially on Gram-negative bacteria. It was observed that products of 3, 8, 10 and 15 have most antibacterial activity against E. coli (−) and S. marcescens (−) and products of 2, 4, 5, 6, 11 and 12 have low activity against most of the tested organisms. In the tested organisms, S. aureus (+) is the most resistant bacterium. Generally, antibacterial activity of compounds is attributed mainly to its major components. However, today, it is known that the synergistic or antagonistic effect of one compound in a minor percentage of mixtures has to be considered [47]. In the synthesis of 1,4-DHPs 2–4, the introduction of −NO2 at the meta position of phenyl (3) increased the antibacterial activity against the tested bacteria compared with the 2-OCH3 group on the benzene ring analogues (2, 4). Also, between analogues 9 and 10 of 1,4-DHPs, 10 enhanced activity against many of tested bacteria. The introduction of −OH and −Br substituent on the benzene ring (8) showed a decrease the antibacterial activity except against E. coli (−). 1,4-DHP (15) shows significant activity against E. coli (−) and S. marcescens (−) bacteria compared with unsubstituted phenyl analogues (13). 1,4-DHP (8) has a good antibacterial activity against bacterial strains. Also, symmetrical 1,4-DHPs (16, 17) show antibacterial activity against organisms except against S. aureus (+). All tested organisms are resistant to Penicillin.
Our results indicated that the studied compounds can be used in the treatment of diseases caused by the bacteria tested. However, further studies are needed to evaluate the in vivo potential of these compounds in animal models. Inhibitory potency values of tested 1,4-DHPs and standard antibiotic discs are summarized in Tables 4 and 5.
Conclusions
In summary, we have developed facile and one-pot four-component synthesis of new substituted 1,4-dihydropyridine derivatives from various aldehydes, cyclic and acyclic 1,3-dicarbonyl compounds and ammonium acetate in the presence of TCBDA and PCBS as recoverable catalysts at room temperature under mild conditions. A simple procedure, good to high yields, easy purification and reusability of the catalyst are advantages of this process. All newly synthesized multisubstituted 1,4-DHP derivatives were evaluated for in vitro antibacterial and anti-oxidant activities. This might be helpful in preventing the progress of various diseases and the development of new therapeutic agents.
References
J.R. Liddell, Nat. Prod. Rep. 19, 773 (2002)
X.Q. Zhu, H.Y. Wang, J.S. Wang, Y.C. Liu, J. Org. Chem. 66, 344 (2001)
Q. Kang, Z.A. Zhao, S.L. You, Org. Lett. 10, 2031 (2008)
M. Rueping, J. Dufour, F.R. Schoepke, Green Chem. 13, 1084 (2011)
R. Lavilla, J. Chem. Soc. Perkin 1, 1141 (2002)
L.M. Yagupolskii, W. Antepohl, F. Artunc, R. Handrock, B.M. Klebanov, I.I. Maletina, B. Marxen, K.I. Petko, U. Quast, A. Vogt, C. Weiss, J. Zibold, S. Herzig, J. Med. Chem. 42, 5266 (1999)
K.C. Majumdar, S.K. Chattopadhyay, Heterocycles in natural product synthesis (Wiley-VCH Verlag & Co. KGaA, Weinheim, 2011), p. 91
L. Guerrier, J. Royer, D.S. Grierson, H.P. Husson, J. Am. Chem. Soc. 105, 7754 (1983)
R. Pena, S. Jimenez-Alonso, G. Feresin, A. Tapia, S. Méndez-Alvarez, F. Machín, A.G. Ravelo, A. Estevez-Braun, J. Org. Chem. 78, 7977 (2013)
C.O. Kappe, W.M.F. Fabian, M.A. Semones, Tetrahedron 53, 2803 (1997)
T. Yamamoto, S. Niwa, S. Ohno, M. Tokumasu, Y. Masuzawa, C. Nakanishi, A. Nakajo, T. Onishi, H. Koganei, S.I. Fujita, T. Takeda, M. Kito, Y. Ono, Y. Saitous, A. Takahara, S. Iwata, M. Shoji, Bioorg. Med. Chem. Lett. 18, 4813 (2008)
G. Swarralatha, G. Prasanthi, N. Sirisha, C. Madhusudhana Chetty, Int. J. ChemTech Res. 3, 75 (2011)
D. Mauzeral, F.H. Westheimer, J. Am. Chem. Soc. 77, 2261 (1955)
R.H. Boecker, F.P. Guengerich, J. Med. Chem. 29, 1596 (1986)
R. Surendra Kumar, A. Idhayadhulla, A. Jamal Abdul Nasser, J. Selvin, J. Serb. Chem. Soc. 76, 1 (2011)
G. Swarnalatha, G. Prasanthi, N. Sirisha, C. Madhusudhana Chetty, Int. J. ChemTech Res. 3, 75 (2011)
R. Surendra Kumar, A. Idhayadhulla, A. Jamal Abdul Nasser, J. Selvin, Eur. J. Med. Chem. 46, 804 (2011)
M. Miri, A. Mehdipour, Bioorg. Med. Chem. 16, 8329 (2008)
J.P. Wan, Y. Liu, RSC Adv. 2, 9763 (2012)
J.P. Wan, Y. Lin, Y. Jing, M. Xu, Y. Liu, Tetrahedron 70, 7874 (2014)
S. Ko, M.N.V. Sastry, C. Lin, C.F. Yao, Tetrahedron Lett. 46, 5771 (2005)
L.M. Wang, J. Sheng, L. Zhang, J.W. Han, Z.Y. Fan, H. Tian, C.T. Qian, Tetrahedron 61, 1539 (2005)
J.L. Donelson, R.A. Gibbs, S.K. De, J. Mol. Catal. A: Chem. 256, 309 (2006)
M. Maheswara, V. Siddaiah, G.L.V. Damu, C.V. Rao, ARKIVOC II, 201 (2006)
M. Maheswara, V. Siddaiah, Y. Koteswara Rao, Y.M. Tzeng, C. Sridhar, J. Mol. Catal. A: Chem. 260, 179 (2006)
H. Singh, J. Sindhu, J.M. Khurana, C. Sharma, K.R. Aneja, Aust. J. Chem. 66, 1088 (2013)
S. Tu, Q. Wei, H. Ma, D. Shi, Y. Gao, G. Cui, Synth. Commun. 31, 2657 (2001)
J.C. Legeay, J.J.V. Eynde, J.P. Bazureau, Tetrahedron 61, 12386 (2005)
N.K. Ladani, D.C. Mungra, M.P. Patel, R.G. Patel, Chin. Chem. Lett. 22, 1407 (2011)
S.H.S. Azzam, A. Siddekha, M.A. Pasha, Tetrahedron Lett. 53, 6306 (2012)
S. Balalaie, L. Baoosi, F. Tahoori, F. Rominger, H.R. Bijanzadeh, Tetrahedron 69, 738 (2013)
J. Shun-Jun, J. Zhao-Qin, L. Jun, L. Teck-Peng, Synlett 5, 831 (2004)
S. Pednekar, R. Bhalerao, N. Ghadge, J. Chem. Sci. 125, 615 (2013)
M. Nasr-Esfahani, S. Jafar Hoseini, M. Montazerozohori, R. Mehrabi, H. Nasrabadi, J. Mol. Catal. A: Chem. 382, 99 (2014)
J. Safari, S.H. Banitaba, S. Dehghan Khalili, Chin. J. Catal. 32, 1850 (2011)
A. Kumar, R.A. Maurya, Tetrahedron 63, 1946 (2007)
S.M. Baghbanian, S. Khaksar, S.M. Vahdat, M. Farhang, M. Tajbakhsh, Chin. Chem. Lett. 21, 563 (2010)
S. Cao, S. Zhong, C. Hu, J.P. Wan, C. Wen, Chin. J. Chem. 33, 568 (2015)
R. Ghorbani-Vaghei, R. Karimi-Nami, Z. Toghraei-Semiromi, M. Amiri, M. Ghavidel, Tetrahedron 67, 1930 (2011)
R. Ghorbani-Vaghei, S. Akbari-Dadamahaleh, Tetrahedron Lett. 50, 1055 (2009)
R. Ghorbani-Vaghei, H. Veisi, Mol. Diversity 14, 249 (2010)
R. Ghorbani-Vaghei, Z. Toghraei-Semiromi, M. Amiri, Mol. Diversity 17, 307 (2013)
R. Ghorbani-Vaghei, M. Amiri, R. Karimi-Nami, Z. Toghraei-Semiromi, M. Ghavidel, Mol. Diversity 17, 251 (2013)
R. Ghorbani-Vaghei, H. Veisi, Synthesis 6, 945 (2009)
H. Veisi, R. Ghorbani-Vaghei, J. Mahmmodi, Bull. Korean Chem. Soc. 32, 3692 (2011)
C.A. Rice-Evans, N.J. Miller, G. Paganga, Free Radic. Biol. Med. 20, 933 (1996)
S. Burt, Int. J. Food Microbiol. 94, 223 (2004)
A. Kumar Dutta, P. Gogoi, R. Borah, RSC Adv. 4, 41287 (2014)
Acknowledgment
We are grateful to Bu-Ali Sina University, Center of Excellence and Development of Chemical Methods (CEDCM) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghorbani-Vaghei, R., Malaekehpoor, S.M., Hasanein, P. et al. Synthesis and biological evaluation of new series 1,4-dihydropyridines. Res Chem Intermed 42, 4715–4731 (2016). https://doi.org/10.1007/s11164-015-2310-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2310-0