Abstract
New organotin(IV) complexes of (2-hydroxyphenyl)(pyrrolidin-1-yl)methanone thiosemicarbazone [L1H2], (2-hydroxyphenyl)(pyrrolidin-1-yl)methanone phenylthiosemicarbazone [L2H2], and (2-hydroxyphenyl)(pyrrolidin-1-yl)methanone semicarbazone [L3H2] with formula [R2SnL] (where R = Bu and Me) have been synthesized. The ligands and their organotin(IV) complexes were characterized by elemental analyses, molar conductivity, molecular weight determination, electronic, Fourier-transform infrared, and 1H, 13C, and 119Sn nuclear magnetic resonance spectral studies. The ligands act as tridentate and coordinate with organotin(IV) atom through the thiolate sulfur, azomethine nitrogen, and phenoxide oxygen atoms. The low molar conductance values in dimethylformamide indicate that the metal complexes are nonelectrolytes. Theoretical calculation is provided in support of the structures. The in vitro antimicrobial activities have been evaluated against Klebsiella sp., Bacillus cereus, Staphylococcus sp., Escherichia coli, Rhizopus, Aspergillus, Alternaria, and Penicillium. The screening results show that the organotin(IV) complexes have better antibacterial activities and have potential as drugs. Furthermore, it has been shown that dibutyltin(IV) derivative exhibits significantly better activity than the other organotin(IV) derivatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interest in coordination chemistry is increasing continuously with the preparation of organic ligands containing a variety of donor groups, multiplied manyfold when the ligands have pharmaceutical and biological importance [1–5]. The number and diversity of nitrogen and sulfur chelating agents used to prepare new coordination and organometallic compounds have increased rapidly during the past few years [6–11]. Schiff base ligands play an important role in the development of coordination chemistry as they readily form stable complexes with most transition-metal ions [12–14]. Schiff bases are also important intermediates in synthesis of some biologically active compounds and are potent antibacterial, antifungal, anticancer, and antiviral compounds [15–19]. Semicarbazones and thiosemicarbazones are important and versatile types of ligands due to the potential donor atoms that they possess, among which sulfur is of paramount importance in the metal–ligand linkage. Moreover, their π delocalization and configurational flexibility create the possibility of a variety of coordination modes [20–23]. The coordination capacity of semi/thiosemicarbazones can be further increased if the parent aldehyde or ketone contains additional functional group in a position suitable for chelation.
Organotin(IV) compounds have a wide range of applications and are amongst the most widely used organometallic compounds. Organotin(IV) compounds possess potential applications in the fields of industrial and medicinal chemistry. Other major uses of these derivatives are well known, e.g., their versatile and important biological and pharmaceutical activities and as wood preservatives and pesticides [24–30]. Organotin(IV) complexes have been extensively studied due to their coordination geometries as well as structural diversity. To extend the knowledge in this research field, particularly with respect to the coordination properties of thiosemicarbazones and semicarbazones and the stereochemistry and molecular structure of the complexes, we undertook a study of organotin(IV) complexes derived from bibasic tridentate thiosemicarbazones and semicarbazones. Here we report on the synthesis, characterization, molecular structure, and biological studies of new organotin(IV) complexes derived from semicarbazone and thiosemicarbazones.
Experimental
All reagents were commercially available and used as supplied by Aldrich or Merck. Solvents were purified and dried according to standard methods, and moisture was excluded from the glass apparatus using CaCl2 drying tubes. Melting points were determined in open glass capillaries and were not corrected.
Analytical methods and spectral measurements
Tin was estimated gravimetrically as SnO2. Carbon and hydrogen analyses were carried out on a PerkinElmer 2400 elemental analyzer. Nitrogen and sulfur were estimated by Kjeldahl’s and Messenger’s methods, respectively. Spectral analysis of synthesized compounds was carried out at SAIF Punjab University, Chandigarh. Purity of compounds was checked on thin layers of silica gel in various nonaqueous solvent systems, e.g., benzene:ethylacetate (9:1), benzene:ethylacetate (8:2). Molecular weight determination was carried out by the Rast camphor method. Molar conductance measurement was carried out in anhydrous dimethylformamide at 25 ± 5 °C using a Systronics conductivity bride model 305. Electronic spectra were recorded in dimethyl sulfoxide on an Agilent Cary-60 uv–visible spectrophotometer. IR spectra were recorded on a PerkinElmer Spectrum SP-2 Fourier-transform spectrophotometer using KBr pellets. 1H and 13C NMR spectra were recorded on a Bruker Avance II (400 MHz) FTNMR spectrometer using DMSO-d6 as solvent at 400 and 100 MHz, respectively. Tetramethylsilane was used as internal reference for 1H NMR and 13C NMR. 119Sn NMR spectra with proton noise decoupling were recorded on a Bruker Avance II spectrometer using dry DMSO as solvent at 149.21 MHz and tetramethyltin as external standard. Geometry optimization of the compounds Bu2SnL1 and Me2SnL1 was performed with the Gaussian 03 software package and GaussView visualization program [31] to conduct the density functional theory calculations. B3LYP methods at 6.31G+(d,p) basis set calculation level were employed in investigation of the optimized molecular structures.
Synthesis of ligands
The ligands were prepared by condensation of (2-hydroxyphenyl)(pyrrolidin-1-yl)methanone with thiosemicarbazide, phenylthiosemicarbazide, and semicarbazide in 1:1 molar ratio using absolute ethanol as reaction medium. The contents were refluxed for 1–2 h, and solid thiosemicarbazone/semicarbazone separated out. Excess solvent was removed on a rotary evaporator, followed by drying and further purification by recrystallization from the same solvent and drying.
Compound (L 1 H 2 ) (2-hydroxyphenyl)(pyrrolidin-1-yl)methanone thiosemicarbazone; color, light brown; yield, 85.58 %; melting point, 156–157 °C; elemental analysis (%), calcd. for C12H16N4OS: C, 54.52; H, 6.10; N, 21.19; S, 12.13; found: C: 54.35; H, 6.04; N, 21.02; S, 12.18; molecular weight: found, 260.88, calcd. 264.35. Infrared (KBr, cm−1): ν(OH/NH), 3386–3060 (br); ν(C=N), 1615 (m); ν(C=S), 1383 (m); ν(C–O), 1290 (s); ν(C–S), 866 (w); ν(N–N), 945 (s); UV–Vis (λ max, nm): 214, 275, 340; 1H NMR (DMSO-d6, δ ppm, 400 MHz): 12.25 (s, 1H, Ph-OH), 8.60 (s, 1H, NH), 7.27–7.19 (m, 2H, aromatic), 7.48 (d, 1H, J = 7.0 Hz, aromatic), 6.91 (d, 1H, J = 8.2 Hz, aromatic), 3.51–3.12 and 1.97–1.87 (m, 8H, pyrrolidine); 3.51 (s, 2H, NH2); 13C NMR (DMSO, δ ppm, 100 MHz): 181.31 (–C=S), 155.16 (C–OH), 167.91 (C=N), 45.67, 44.63 (–CH2–CH2–, pyrrolidine); 25.67, 24.94 (–CH2–CH2–, pyrrolidine); 130.11, 127.68, 122.65, 118.42, 116.80 (aromatic carbons).
Compound (L 2 H 2 ) (2-hydroxyphenyl)(pyrrolidin-1-yl)methanone phenylthiosemicarbazone; color, brown; yield, 71.60 %; melting point, 118–120 °C; elemental analysis (%), calcd. for C18H20N4OS: C, 63.50; H, 5.92; N, 16.46; S, 9.42; found: C: 63.67; H, 5.95; N, 16.54; S, 9.40; molecular weight: found, 327.76, calcd. 340.44. Infrared (KBr, cm−1): ν(OH/NH), 3430–3058 br; ν(C=N), 1613 m; ν(C=S), 1380 w; ν(C–O), 1292 s; ν(C–S), 867 m; ν(N–N), 975 m; UV–Vis (λ max, nm): 216, 270, 348; 1H NMR (DMSO-d6, δ ppm, 400 MHz): 12.88 (s, 1H, Ph-OH), 9.15 (s, 1H, NH), 7.65–7.11 (m, 7H, aromatic), 7.75 (d, 1H, J = 6.8 Hz, aromatic), 6.86 (d, 1H, J = 8.0 Hz, aromatic), 3.54–3.12 and 1.94–1.80 (m, 8H, pyrrolidine); 4.55 (s, 2H, NH–Ph); 13C NMR (DMSO, δ ppm, 100 MHz): 181.54 (–C=O), 155.25 (C–OH), 164.45 (C=N), 44.85, 43.95 (–CH2–CH2–, pyrrolidine); 24.62, 23.78 (–CH2–CH2–, pyrrolidine); 131.99, 130.59, 130.18, 127.88, 122.65, 118.42, 116.80, 116.16, 115.80 (aromatic carbons).
Compound (L 3 H 2 ) (2-hydroxyphenyl)(pyrrolidin-1-yl)methanone semicarbazone; color, brown; yield, 68.66 %; melting point, 141–142 °C; elemental analysis (%), calcd. for C12H16N4O2: C, 58.05; H, 6.50; N, 22.57; found: C: 58.13; H, 6.48; N, 22.50; molecular weight: found, 245.12, calcd. 248.28. Infrared (KBr, cm−1): ν(OH/NH), 3254–3124 br; ν(C=N), 1625 m; ν(C=O) s, 1695; ν(C–O), 1252 m; ν(N–N), 870 m; UV–Vis (λ max, nm): 216, 278, 348; 1H NMR (DMSO-d6, δ ppm, 400 MHz): 13.18 (s, 1H, Ph-OH), 9.05 (s, 1H, NH), 7.57–7.19 (m, 2H, aromatic), 7.63 (d, 1H, J = 6.5 Hz, aromatic), 6.74 (d, 1H, J = 8.1 Hz, aromatic), 3.50–3.11 and 1.95–1.85 (m, 8H, pyrrolidine); 3.69 (s, 2H, NH2); 13C NMR (DMSO, δ ppm, 100 MHz): 179.37 (–C=O), 154.92 (C–OH), 167.74 (C=N), 45.63, 44.73 (–CH2–CH2–, pyrrolidine); 23.92, 23.73 (–CH2–CH2–, pyrrolidine); 130.53, 127.73, 123.19, 120.80, 116.90 (aromatic carbons).
Syntheses of dibutyltin(IV) complexes
The requisite amount of dibutyltin oxide was added to the calculated amount of the ligands in equimolar ratio in dry benzene medium. The contents were refluxed on a fractionating column for about 4–5 h. The water liberated in the reaction was removed azeotropically with benzene. On completion of the reaction, the resulting products were rendered free from solvent and then washed repeatedly with dry cyclohexane. The crystalline solids were separated out and purified by recrystallization from the same solvent. The products so formed were finally dried in vacuum at 40 ± 5 °C for 2 h. The purity of the complexes was checked by Thin-layer chromatography) using silica gel-G as adsorbent.
Compound Bu2SnL1 was prepared by reacting dibutyltin(IV) oxide with ligand (H2L1): color, creamy white; yield, 65.83 %; melting point, 194–196 °C (decomposed); elemental analysis (%), calcd. for C20H32N4OSSn: Sn, 23.97; C, 48.50; H, 6.51; N, 11.31; S, 6.47; found: Sn, 23.90, C, 48.32; H, 6.46; N, 11.23; S, 6.41; molecular weight: found, 488.96, calcd. 495.27. Molar conductance (DMF, 10−3, ohm−1 mol−1 cm2): 12.57; Infrared (KBr, cm−1): ν(C=N), 1592; ν(NH), 3370; ν(C–O), 1240; ν(C–S), 850; ν(N–N), 1330; ν(Sn ← N), 470; ν(Sn–O), 564; ν(Sn–C), 627. UV–Vis (λ max, nm): 232, 285, 370, 395; 1H NMR (DMSO-d6, δ ppm, 400 MHz): 7.35–7.18 (m, 2H, aromatic), 7.52 (d, 1H, J = 7.0 Hz, aromatic), 6.90 (d, 1H, J = 8.2 Hz, aromatic), 3.50–3.08 and 1.90–1.82 (m, 8H, pyrrolidine); 3.88 (s, 2H, NH2); 2.15 (t, 2H, J = 7.3 Hz, CH2(α), Sn–Bu), 2.14–1.73 (m, 2H, –CH2(β), Sn–Bu), 1.24–1.22 (m, 2H, –CH2(γ), Sn–Bu), 0.88 (t, 2H, J = 7.2 Hz, –CH3(δ), Sn–Bu). 13C NMR (DMSO, δ ppm, 100 MHz): 172.45 (–C=S), 156.42 (C–OH), 160.27 (C=N), 45.55, 44.74 (–CH2–CH2–, pyrrolidine); 25.70, 24.96 (–CH2–CH2–, pyrrolidine); 130.18, 127.60, 123.15, 120.18, 116.65 (aromatic carbons); 27.78, 26.91, 26.28, 14.67 (Sn–Bu); 119Sn NMR (DMSO, δ ppm, 149.21 MHz): –178.10.
Compound Bu2SnL2 was prepared by reacting dibutyltin(IV) oxide with ligand (H2L2): color, brown; yield, 70.41 %; melting point, 169–170 °C; elemental analysis (%), calcd. for C26H36N4OSSn: Sn, 20.78; C, 54.65; H, 6.35; N, 9.81; S, 5.61; found: Sn, 20.68, C, 54.60; H, 6.30; N, 9.67; S, 5.47; molecular weight: found, 573.56, calcd., 571.37. Molar conductance (DMF, 10−3, ohm−1 mol−1 cm2): 14.85; Infrared (KBr, cm−1): ν(C=N), 1598; ν(NH), 3267; ν m(C–O), 1248; ν(C–S), 851; ν(N–N), 1031; ν(Sn ← N), 465; ν(Sn–O), 560; ν(Sn–C), 632. UV–Vis (λ max, nm): 230, 285, 365, 390; 1H NMR (DMSO-d6, δ ppm, 400 MHz): 7.60–7.15 (m, 7H, aromatic), 7.72 (d, 1H, J = 6.9 Hz, aromatic), 6.95 (d, 1H, J = 7.9 Hz, aromatic), 3.50–3.15 and 1.90–1.78 (m, 8H, pyrrolidine); 4.50 (s, 2H, NH–Ph); 2.14 (t, 2H, J = 7.2 Hz, CH2(α), Sn–Bu), 2.10–1.73 (m, 2H, –CH2(β), Sn–Bu), 1.20–1.16 (m, 2H, –CH2(γ), Sn–Bu), 0.92 (t, 2H, J = 7.4 Hz, –CH3(δ), Sn–Bu). 13C NMR (DMSO, δ ppm, 100 MHz): 171.44 (–C=O), 155.95 (C–OH), 159.44 (C=N), 44.84, 44.10 (–CH2–CH2–, pyrrolidine); 24.68, 23.88 (–CH2–CH2–, pyrrolidine); 132.35, 130.89, 130.08, 128.12, 122.25, 119.22, 117.08, 116.56, 115.48 (aromatic carbons); 27.55, 26.65, 26.21, 14.86 (Sn–Bu); 119Sn NMR (DMSO, δ ppm, 149.21 MHz): –167.71.
Compound Bu2SnL3 was prepared by reacting dibutyltin(IV) oxide with ligand (H2L3): color, light brown; yield, 81.31 %; melting point, 216–218 °C; elemental analysis (%), calcd. for C20H32N4O2Sn: Sn, 24.77; C, 50.13; H, 6.73; N, 11.69; found: Sn, 24.65, C, 50.02; H, 6.75; N, 11.76; molecular weight: found, 469.78, calcd., 479.20. Molar conductance (DMF, 10−3, ohm−1 mol−1 cm2): 10.16; Infrared (KBr, cm−1): ν(C=N), 1605; ν(NH), 3390; ν(C–O), 1255; ν(N–N), 1042; ν(Sn ← N), 455; ν(Sn–O), 568; ν(Sn–C), 630. UV–Vis (λ max, nm): 225, 290, 370, 405; 1H NMR (DMSO-d6, δ ppm, 400 MHz): 7.50–7.14 (m, 2H, aromatic), 7.60 (d, 1H, J = 6.6 Hz, aromatic), 6.78 (d, 1H, J = 8.0 Hz, aromatic), 3.52–3.16 and 1.90–1.84 (m, 8H, pyrrolidine); 3.72 (s, 2H, NH2); 2.15 (t, 2H, J = 7.3 Hz, CH2(α), Sn–Bu), 2.10–1.72 (m, 2H, –CH2(β), Sn–Bu), 1.22–1.18 (m, 2H, –CH2(γ), Sn–Bu), 0.94 (t, 2H, J = 7.2 Hz, −CH3(δ), Sn–Bu). 13C NMR (DMSO, δ ppm, 100 MHz): 168.93 (–C=O), 155.02 (C–OH), 160.17 (C=N), 45.60, 44.72 (–CH2–CH2–, pyrrolidine); 23.98, 23.70 (–CH2–CH2–, pyrrolidine); 131.33, 127.55 123.28, 121.28, 116.74 (aromatic carbons); 28.78, 26.31, 26.18, 15.11 (Sn–Bu); 119Sn NMR (DMSO, δ ppm, 149.21 MHz): −163.09.
Syntheses of dimethyltin(IV) complexes
A weighed amount of dimethyltin dichloride was added to the calculated amount of the ligand and triethylamine in 1:1:2 molar ratio, using dry tetrahydrofuran as reaction medium in an oxygen-free atmosphere. The color of the contents immediately changed with precipitation of triethylamine hydrochloride. The solution was stirred for 2 h. The precipitate of triethylamine hydrochloride which separated out was filtered off and rejected. On completion of the reaction, excess solvent was removed under reduced pressure and the compound was dried in vacuum at 45 ± 5 °C after repeated washing with dry cyclohexane. The crystalline solids were purified by recrystallization from the same solvent. The products so formed were finally dried in vacuum at 40 ± 5 °C for 2 h. The purity of the complexes was checked by TLC using silica gel-G as adsorbent.
Compound Me2SnL1 was prepared by reacting dimethyltin(IV) dichloride with ligand (H2L1): color, light grey; yield, 85.55 %; melting point, 174–175 °C; elemental analysis (%), calcd. for C14H20N4OSSn: Sn, 28.88; C, 40.90; H, 4.90; N, 13.60; S, 7.80; found: Sn, 28.78, C, 40.78; H, 4.87; N, 13.46; S, 7.56; molecular weight: found, 405.74, calcd. 411.11. Molar conductance (DMF, 10−3, ohm−1 mol−1 cm2): 8.24; Infrared (KBr, cm−1): ν(C=N), 1600; ν(NH), 3262; ν(C–O), 1248; ν(C–S), 870; ν(N–N), 1036; ν(Sn ← N), 460; ν(Sn–O), 575; ν(Sn–C), 630. UV–Vis (λ max, nm): 232, 280, 360, 392; 1H NMR (DMSO-d6, δ ppm, 400 MHz): 7.28–7.16 (m, 2H, aromatic), 7.58 (d, 1H, J = 7.0 Hz, aromatic), 6.94 (d, 1H, J = 8.1 Hz, aromatic), 3.51–3.10 and 1.96–1.88 (m, 8H, pyrrolidine); 3.72 (s, 2H, NH2); 1.18 (s, 3H, Sn–CH3). 13C NMR (DMSO, δ ppm, 100 MHz): 170.25 (–C=S), 155.76 (C–OH), 157.19 (C=N), 45.68, 44.83 (–CH2–CH2–, pyrrolidine); 25.62, 24.96 (–CH2–CH2–, pyrrolidine); 131.58, 127.28, 123.35, 119.54, 116.15 (aromatic carbons); 13.86 (Sn-Me); 119Sn NMR (DMSO, δ ppm, 149.21 MHz): –137.98.
Compound Me2SnL2 was prepared by reacting dimethyltin(IV) dichloride with ligand (H2L2): color, reddish brown; yield, 72.85 %; melting point, 132–133 °C; elemental analysis (%), calcd. for C20H24N4OSSn: Sn, 24.37; C, 49.30; H, 4.97; N, 11.50; S, 6.58; found: Sn, 24.32, C, 49.39; H, 4.92; N, 11.40; S, 6.50; molecular weight: found, 485.15, calcd., 487.21. Molar conductance (DMF, 10−3, ohm−1 mol−1 cm2): 9.56; Infrared (KBr, cm−1): ν(C=N), 1608; ν(NH), 3283; ν(C–O), 1273; ν(C–S), 850; ν(N–N), 1037; ν(Sn ← N), 455; ν(Sn–O), 570; ν(Sn–C), 635. UV–Vis (λ max, nm): 235, 276, 362, 398; 1H NMR (DMSO-d6, δ ppm, 400 MHz): 7.70–7.14 (m, 7H, aromatic), 7.78 (d, 1H, J = 6.8 Hz, aromatic), 6.85 (d, 1H, J = 8.0 Hz, aromatic), 3.51–3.10 and 1.92–1.85 (m, 8H, pyrrolidine); 4.50 (s, 2H, NH–Ph); 1.22 (s, 3H, Sn–CH3). 13C NMR (DMSO, δ ppm, 100 MHz): 173.54 (–C=O), 156.355 (C–OH), 158.65 (C=N), 44.80, 43.98 (–CH2–CH2–, pyrrolidine); 24.66, 23.87 (–CH2–CH2–, pyrrolidine); 132.25, 131.38, 130.10, 128.18, 122.35, 119.44, 118.12, 116.06, 115.78 (aromatic carbons); 13.48 (Sn–Me); 119Sn NMR (DMSO, δ ppm, 149.21 MHz): –142.34.
Compound Me2SnL3 was prepared by reacting dimethyltin(IV) dichloride with ligand (H2L1): color, brown; yield, 70.15 %; melting point, 196–198 °C (decomposed); elemental analysis (%), calcd. for 14H20N4O2Sn: Sn, 30.05; C, 42.56; H, 5.10; N, 14.18; found: Sn, 30.00, C, 42.44; H, 5.06; N, 14.11; molecular weight: found, 388.58, calcd., 395.04. Molar conductance (DMF, 10−3, ohm−1 mol−1 cm2): 8.76; Infrared (KBr, cm−1): ν(C=N), 1594; ν(NH), 3248; ν(C–O), 1250; ν(N–N), 1042; ν(Sn ← N), 450; ν(Sn–O), 562; ν(Sn–C), 625. UV–Vis (λ max, nm): 230, 282, 372, 390; 1H NMR (DMSO-d6, δ ppm, 400 MHz): 7.50–7.12 (m, 2H, aromatic), 7.60 (d, 1H, J = 6.6 Hz, aromatic), 6.75 (d, 1H, J = 8.1 Hz, aromatic), 3.50–3.16 and 1.95–1.78 (m, 8H, pyrrolidine); 3.64 (s, 2H, NH2); 1.24 (s, 3H, Sn–CH3). 13C NMR (DMSO, δ ppm, 100 MHz): 170.17 (–C=O), 155.22 (C–OH), 160.64 (C=N), 45.64, 44.72 (–CH2–CH2–, pyrrolidine); 23.90, 23.78 (–CH2–CH2–, pyrrolidine); 130.96, 128.33, 123.09, 121.38, 116.54 (aromatic carbons); 13.44 (Sn-Me); 119Sn NMR (DMSO, δ ppm, 149.21 MHz): –125.85.
Antibacterial assay
Synthesized compounds were screened for their antibacterial activity against Bacillus cereus, Escherichia coli, Klebsiella sp., and Staphylococcus sp. at concentration of 200 μg/ml by agar well diffusion method [32]. The minimum inhibitory concentration (MIC) was determined using the serial dilution method. The lowest concentration that showed inhibition of growth of each organism was further diluted to different lower concentrations to determine the MIC. Aliquot of 5 ml of nutrient broth was inoculated into the test organisms and incubated at 30 °C for 24 h. Sterile nutrient agar plates were also prepared, and holes of 5 mm diameter were cut using a sterile cork borer ensuring proper distribution. The test organisms after 24 h of incubation were spread onto separate agar plates. The chemical compounds were dissolved in DMSO and were poured into appropriately labeled holes using a pipette in aseptic conditions. A hole containing DMSO served as control. Triplicate plate of each bacterial strain was prepared. The plates were incubated aerobically at 30 °C for 24 h. Antimicrobial activity was determined by measuring the diameter of the zone (mm) showing complete inhibition with respect to control (DMSO).
Antifungal assay
Besides antibacterial activity, the chemical compounds were also checked for their potential to inhibit growth of fungi Rhizopus, Aspergillus, Alternaria, and Penicillium at concentration of 100 μg/ml. The assay was prepared in triplicate. Fungal cells were grown in 5 ml of Sabouraud broth medium for 3 days at 28 °C. Sterile Sabouraud agar medium plates were prepared, and holes of 5 mm diameter were cut using a sterile cork borer ensuring proper distribution. The test organism after 72 h incubation was inoculated on Sabouraud agar medium plate. The inoculated plate was incubated at 28 °C for 7 days to develop a fungal mat. The synthesized compounds were dissolved in DMSO and were poured into appropriately labeled holes in separate plates. These plates were left at room temperature for 2 h to allow diffusion of the sample, followed by incubation for 7 days at 28 °C. Inhibition of growth was determined by measuring the growth of fungal mat towards the hole in the plate having compound with respect to DMSO and control plates.
Results and discussion
(2-Hydroxyphenyl)(pyrrolidin-1-yl)methanone thiosemicarbazone (H2L1), (2-hydroxyphenyl)(pyrrolidin-1-yl)methanone phenylthiosemicarbazone (H2L2), and (2-hydroxyphenyl)(pyrrolidin-1-yl)methanone semicarbazone (H2L3) were synthesized by condensation reaction of (2-hydroxyphenyl)(pyrrolidin-1-yl)methanone and thiosemicarbazide, phenylthiosemicarbazide, and semicarbazide in absolute ethanol in 1:1 molar ratio. There are two tautomers within the structure, existing as either thione or thiol tautomer (Scheme 1).
Dibutyltin(IV) complexes and dimethyltin(IV) complexes with formula Bu2SnL and Me2SnL were synthesized by interaction of dibutyltin oxide and dimethyltin dichloride with Schiff bases in 1:1 molar ratio according to Scheme 2.
The reactions proceeded easily, and all the complexes are colored solids. All the complexes are soluble in common organic solvents. The analytical data of the ligands and of their complexes, given in experimental section, are in good agreement with the proposed stoichiometry of the complexes. The compounds were dissolved in DMF, and molar conductance of 10−3 M solution was measured at 25 °C. The molar conductance values of the complexes fall in the range 8.20–15.14 Ohm−1 cm2 mol−1, indicating that these compounds are nonelectrolytes.
Electronic spectral study
The UV–Vis spectra of ligands and their organotin(IV) complexes were obtained in DMSO at room temperature. In the electronic spectra of the ligands, a band is observed at ~216 nm, which may be assigned to the 1B band of the phenyl ring. This shifts to longer wavelength on complexation, being observed at ~230 nm in the organotin(IV) complexes. Also, the ligands’ chromophore >C=N, which is observed at ~265 nm, shifts to higher wavelength and is observed at ~290 nm in the complexes [33]. In the spectra of ligands, a band is observed at ~340 nm due to the secondary band of benzene, being red-shifted due to the presence of >C=N–N=C<. However, this appears at higher side in the complexes, possibly due to polarization in C=N bond caused by the metal–ligand electron interaction. Furthermore, absorption bands were observed in the 340–385 nm region in the spectra of tin complexes, which could be assigned to charge-transfer transition from ligand → metal [34]. The shift of the λ max band from the ligand to the complexes is supported by the coordination of ligands to the tin(IV) ion.
IR spectral study
The infrared spectra of ligands [22] show a strong band in the 3400–3177 cm−1 region attributable to ν(NH), while the band at ~2575 cm−1 due to ν(SH) does not appear. However, it is observed in the solution spectra with NH frequency disappearing, indicating that there exists a tautomeric equilibrium [35] between the two forms as indicated in Scheme 1. The IR spectrum of free ligands showed absorption bands at 3370–3177 cm−1, which are due to the stretching vibrations of the OH and NH groups, respectively [36]. The absorption bands at 1610–1618, 1290–1295, 945–955, 1383, and 1695 cm−1 are due to ν(C=N), ν(C–O), ν(N–N), ν(C=S), and ν(C=O), respectively. Several significant changes on complexation with respect to the bands of free ligands suggest coordination through phenolic group, azomethine, sulfur of the thiolic and oxygen of the ketonic form of the ligands. The strong stretching band at ~3300 cm−1 that corresponds to the ν(OH) group in the spectrum of ligands disappeared in the spectra of complexes due to deprotonation, indicating coordination through the phenolic oxygen to tin(IV) atom. The free ligands showed a band at ~1290 cm−1, which is due to ν(C–O). This band is shifted to lower wavenumber at 1250 ± 10 cm−1 in the organotin(IV) complexes, indicating coordination of oxygen to the tin(IV) atom. The newly formed ν(C=N–N=C) bond showed medium to strong absorption peaks in the range of 1595 ± 5 cm−1 in the spectra of the complexes, indicating coordination of azomethine nitrogen to the tin(IV) atom [37]. A sharp band at ~950 cm−1 due to ν(N–N) for ligands is shifted to higher frequencies at 1035–1042 cm−1 in the spectra of organotin(IV) complexes. The increase in frequency of this band in the spectra of complexes due to an increase in bond length again confirms coordination via the azomethine nitrogen atom. The bands at ~1383 cm−1 in the free ligands due to ν(C=S) stretching vibrations are shifted to lower frequencies at 1347–1330 cm−1 in the spectra of the organotin(IV) complexes, suggesting coordination through the thiolate sulfur with tin(IV) atom. The IR bands observed in the range of ~564 cm−1 in the spectra of the complexes suggest the presence of Sn–O bonding in their structure [38]. The ν(Sn–C) and ν(Sn–N) bands are tentatively assigned to absorptions in the regions of ~627 and ~470 cm−1, respectively [39, 40]. Based on the infrared spectra analyses of ligands and their organotin(IV) complexes, it is suggested that ligand coordinated to the tin(IV) moiety through the phenoxide oxygen, azomethine nitrogen, and thiolato sulfur atoms.
1H NMR spectral study
The 1H NMR spectra of free ligands showed resonance signals at ~δ 12.82, 9.02, 7.31–7.25, 3.54, 1.92, and 1.21 ppm, due to OH, NH, aromatic protons, pyrrolidine protons, and SH, respectively. After complexation, the resonance signal of OH proton was absent from the spectra of the complexes, indicating deprotonation of the phenolic proton and supporting coordination of the phenolic oxygen atom with tin(IV) atom. The resonance signal of SH was not found in the spectra of complexes, suggesting deprotonation of the SH proton and confirming that the ligand coordinated to tin(IV) in thiolate form. The ligands give a complex multiplet signal in the region of δ 7.75–6.85 ppm for the aromatic protons, and these remained at almost the same position in the spectra of the metal complexes. The appearance of signals due to NH protons at the same positions in the ligand and its complex show the noninvolvement of this group in coordination. The complexes, however, show additional signals at δ 0.86–2.28 and 1.24 ppm owing to the butyl and methyl protons, respectively. The butyl groups attached to tin(IV) moiety in the organotin(IV) complexes gave four resonance signals, namely 2.28–2.15 ppm (t, 2H, J = 7.2 Hz, CH2(α), Sn–Bu), 2.14–1.73 ppm (m, 2H, –CH2(β), Sn–Bu), 1.24–1.22 ppm (m, 2H, –CH2(γ), Sn–Bu), and 0.99–0.86 ppm (t, 2H, J = 7.4 Hz, CH3(δ), Sn–Bu).
13C NMR spectral study
The 13C NMR spectral data along with the assignment of characteristic peaks of ligands and their diorganotin(IV) complexes are described in experimental section. The 13C–{1H} NMR spectrum of free ligands showed resonance signals at 180, 167, and 155–116 ppm, due to δ(NH–C=S) and δ(C=N), δ (aromatic ring carbon), respectively. After complexation, the carbon signals of the N=C–S group shifted to low frequency at 169–171 ppm in all the complexes compared with ligands, indicating participation of the N=C–S group in coordination to tin(IV) atom. The chemical shifts of carbon in C=N were observed at ~167 ppm in the free ligands, being shifted to lower frequency at 158–161 ppm in the organotin(IV) complexes. These results support that azomethine nitrogen is coordinated to the tin(IV) atom. After complexation, the δ value of carbon atoms in the aromatic ring did not show much change in the complexes as compared with the free ligands. Besides, the butyl group attached to the organotin(IV) moiety in complex gave four resonance signals at 28.78, 26.31, 26.18, and 15.11 ppm. In the 13C–{1H} NMR spectra of the organotin(IV) complexes, a sharp singlet resonance signal appeared at 15.11 [Sn–(CH3)2] ppm. In organotin(IV) compounds, the 1 J[119Sn, 13C] value is an important parameter to assess the coordination number of the tin atom. The calculated coupling constants for Bu2SnL1 and Me2SnL1 compounds were found to be 760 and 776 Hz, describing a penta-coordinate environment around the tin atom in these compounds [41].
119Sn NMR spectral study
119Sn NMR chemical shifts are very sensitive to the coordination number of tin and are generally shifted upfield on bonding to Lewis base. It has been reported that 119Sn chemical shifts in the ranges of 200 to −60, −90 to −190, and −210 to −400 ppm are associated with four-, five-, and six-coordinated alkyltin(IV) compounds, and these 119Sn shifts are higher with aryltin(IV) compounds. 119Sn NMR spectra can be used as an indicator of the coordination number of the tin atom. The 119Sn NMR spectra of all the complexes show only one resonance signal in the range of −125.85 to −178.10 ppm. The 119Sn NMR values are characteristic for five-coordinated tin atoms observed in organotin(IV) complexes [42–44].
Theoretical calculation
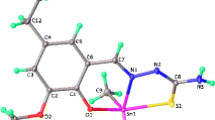
Molecular modeling was used to help demonstrate the significant features, i.e., molecular geometries, bond energies, and torsion angles, of the organometallic frameworks theoretically. Bond lengths, bond angles, and atomic coordinates depend on the hybridization of an atom and mode of bonding. Thus, molecular modeling provides a blueprint of the three-dimensional arrangements of atoms. Beside this, if deviations in bond distances, bond angles, or torsion angles are evidenced, specific electronic interactions can be detected and confirmed based on earlier spectral evidence [45]. The bonding capabilities of atoms impact on bond lengths and bond angles of concerned functional groups. Therefore, molecular models of complexes were demonstrated. The physical dimension of the molecules helped to demonstrate the changes that occurred during their topological assembly. The molecular structure of Me2SnL1 and Bu2SnL1 complexes were optimized by Gaussian 03 using the DFT/B3LYP method to support the spectroscopic data. The selected calculated geometric parameters, viz. bond lengths and bond angles, of the Me2SnL1 and Bu2SnL1 derivatives are summarized in Table 1. The optimized molecular structure of the most stable form of Me2SnL1 and Bu2SnL1 is shown in Figs. 1 and 2, respectively.
The deprotonated ligands are coordinated through phenolic oxygen, thiolic sulfur, and azomethine nitrogen atoms. The ligands act as tridentate with the ONS donors placed in the same side. Since the synthesized compounds are related and differ only in substituted groups, two compounds were theoretically studied. The Sn–O bond distance is nearly identical to values of reported tin complexes. The calculated Sn–O bond distances of 2.0472/2.0512 Å in Bu2SnL1/Me2SnL1, are also close to already reported [48] Sn–O distances in {CH2N(Me)CH(Me)CH(Ph)O}2Sn (2.048/2.078). The Sn–N distances of Sn(1)–N(5) in compounds Bu2SnL1/Me2SnL1 are 2.1063/2.0884 Å, similar to already reported [49] structures, i.e., SaleanH2Sn with 2.0535(9), 2.0369(8) Å. The Sn–S distances of Sn(1)–S(2) in compounds Bu2SnL1/Me2SnL1 are 2.4375/2.4337 Å, similar to the already reported [50] structure [nBu2Sn(dappt)]·(Me2CO)0.5, i.e., 2.6210 Å. The bond angles of C(21)–Sn(1)–C(20) of 94.92°, C(21)–Sn(1)–C(5) of 167.72°, C(20)–Sn(1)–O(19) of 114.39°, C(20)–Sn(1)–S(2) of 138.05°, and O(19)–Sn(1)–S(2) of 102.40°, in Me2SnL1, are in good agreement with values reported for tin complexes [49].
Molecular orbital calculations provide a detailed description of the orbitals, including the spatial characteristics, nodal patterns, and individual atom contributions. Contour plots of the frontier orbitals for the ground state of ligands are shown in Fig. 3 together with the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) [50]. It is interesting that both orbitals are substantially distributed over the conjugation plane. In addition, it can be observed in Fig. 3 that the HOMO orbitals are located on the substituted molecule, while the LUMO orbitals resemble those obtained for the unsubstituted molecule. Therefore, the substitution influenced the electron donation ability while imposing only a small impact on the electron acceptance ability. The orbital energy levels of the HOMO and LUMO of H2L1–3 are listed in Table 2. An electronic system with larger HOMO–LUMO gap should be less reactive than one having a smaller gap. In the present study, the HOMO–LUMO gap values of L1–3 are −3.901, −4.240, and −3.871 eV, respectively. The lower value of the HOMO–LUMO energy gap would explain the eventual charge-transfer interaction taking place within the molecules. The low HOMO values for L1–3 indicated that this molecule had low ionization energies, suggesting that it could lose electrons easily.
Antibacterial activities
In vitro antibacterial activity of the newly synthesized ligands and their corresponding organotin(IV) complexes was tested against Gram-positive and Gram-negative bacteria (B. cereus, E. coli, Klebsiella sp., and Staphylococcus sp.) using the agar well diffusion method. Streptomycin was used as reference drug. The screening results are presented in Fig. 4. The minimum inhibitory concentration (MIC) of complexes Me2Sn(L1,3) and Bu2Sn(L1,3) (which are soluble in DMSO) as well as the MIC values of Me2SnCl2 and Bu2SnO against a wide spectrum of bacteria are presented in Table 3. The microorganism against which compound 2c presented highest activity was Klebsiella sp., followed by E. coli and B. cereus. Compound 2a was active against all the bacteria tested, with the best activity of the compound being recorded against E. coli, followed by B. cereus and Staphylococcus sp. Compound 3a presented highest activity against B. cereus. Compound 2b was highly active against Gram-positive bacteria compared with Gram-negative bacteria.
The results show that all compounds exhibit antibacterial activity and organotin complexes are more potent bactericides than the free ligands. This can be explained in terms of the greater lipid solubility and cellular penetration of the complexes [51]. It is clear that the coordination enhances the antibacterial activity and that the newly synthesized complexes in the present study are more active against Gram-positive than Gram-negative bacteria. The preliminary results achieved lead us to conclude that the butyltin(IV) complexes should be studied in detail for their applications in diverse areas.
Antifungal activity
In vitro antifungal effects of the investigated compounds were tested against Rhizopus, Aspergillus, Alternaria, and Penicillium using the agar well diffusion method. The results are shown in Fig. 5. The minimum inhibitory concentrations of the compounds against respective fungal strains are summarized in Table 4. Newly synthesized compounds exhibit good antifungal activity against all the tested fungi especially against Alternaria. Compounds 2a and 3a recorded highest activity against Penicillium followed by compounds 2b and 3b. Furthermore, the antifungal activity of Bu2SnL1 (2a) was found to be significant against Rhizopus, Aspergillus, Alternaria, and Penicillium among other complexes. The investigation shows that all the synthesized organotin compounds are generally more active than the parent ligands. These studies indicate that the organotin complexes synthesized in the present study are highly active against all fungi tested.
Further, the organotin complexes are more active than the free ligands, indicating that metallation increases antifungal activity. Metal ions are adsorbed on the cell walls of the microorganisms, disturbing the respiration processes of the cells and thus blocking the protein synthesis that is required for further growth of the organisms. Hence, metal ions are essential for the growth-inhibitory effects [52]. According to Overtone’s concept of cell permeability, the lipid membrane that surrounds the cell favors the passage of only lipid-soluble materials, so lipophilicity is an important factor controlling the antifungal activity. Upon chelation, the polarity of the metal ion is reduced due to the overlapping of the ligand orbitals and partial sharing of the positive charge of the metal ion with donor groups. In addition, chelation allows for the delocalization of π-electrons over the entire chelate ring and enhances the lipophilicity of the complexes. This increased lipophilicity facilitates penetration of the complexes into lipid membranes, further restricting proliferation of the microorganisms. The variation in the effectiveness of different compounds against different organisms depends either on the impermeability of the microbial cells or on differences in the ribosomes of the cells [53]. All of the metal complexes possess higher antifungal activity than the ligand [54]. Although the exact biochemical mechanism is not completely understood, the mode of action of antimicrobials may involve various targets in the microorganisms. These targets include the following: (1) The higher activity of the metal complexes may be due to the different properties of the metal ions upon chelation. The polarity of the metal ions will be reduced due to the overlapping of the ligand orbitals and partial sharing of the positive charge of the metal ion with donor groups. Thus, chelation enhances the penetration of the complexes into lipid membranes and the blockage of metal binding sites in the enzymes of the microorganisms. (2) Tweedy’s chelation theory predicts that chelation reduces the polarity of the metal atom mainly because of partial sharing of its positive charge with donor groups and possible electron delocalization over the entire ring. This consequently increases the lipophilic character of the chelates, favoring their permeation through the lipid layers of the bacterial membrane [51]. (3) Interference with the synthesis of cellular walls, causing damage that can lead to altered cell permeability characteristics or disorganized lipoprotein arrangements, ultimately resulting in cell death. (4) Deactivation of various cellular enzymes that play a vital role in the metabolic pathways of these microorganisms. (5) Denaturation of one or more cellular proteins, causing the normal cellular processes to be impaired. (6) Formation of a hydrogen bond through the azomethine group with the active centers of various cellular constituents, resulting in interference with normal cellular processes.
The above studies indicate that the synthesized organotin complexes are highly active against all these microorganisms. The organotin complexes of thiosemicarbazones are more active for all organisms than corresponding semicarbazone complexes, and this also indicates that sulfur is a more effective antimicrobial agent than oxygen, as was suggested by Tandon [55]. The increase in the activity of organotin(lV) complexes as compared with the parent ligand may be due to the complex formation in which the ligand is coordinated to the central tin atom through the thioketonic sulfur and azomethine nitrogen, leading to increased biocidal action.
References
C. Andreini, I. Bertini, G. Cavallaro, G.L. Holliday, J.M. Thornton, J. Biol. Inorg. Chem. 13, 1205–1218 (2008)
A.K. Cheetham, C.N.R. Rao, R.K. Feller, Chem. Commun. 46, 4780–4795 (2006)
C. Rodrigues, A.A. Batista, J. Ellena, E.E. Castellano, D. Benítez, H. Cerecetto, M. González, L.R. Teixeira, H. Beraldo, Eur. J. Med. Chem. 45, 2847–2853 (2010)
S.L.A. Kumar, M.S. Kumar, P.B. Sreeja, A. Sreekanth, Spectrochim. Acta Part A 113, 123–129 (2013)
O.K. Farha, J.T. Hupp, Acc. Chem. Res. 43, 1166–1175 (2010)
D.F. Weng, Z.M. Wang, S. Gao, Chem. Soc. Rev. 40, 3157–3181 (2011)
J.Y. Lee, O.K. Farha, J. Roberts, K.A. Scheidt, S.T. Nguyen, J.T. Hupp, Chem. Soc. Rev. 38, 1450–1459 (2009)
P. Paul, P. Sengupta, S. Bhattacharya, J. Organomet. Chem. 724, 281–288 (2013)
P. Paul, S. Datta, S. Halder, R. Acharyya, F. Basuli, R.J. Butcher, S.M. Peng, G.H. Lee, A. Castineiras, M.G.B. Drew, S. Bhattacharya, J. Mol. Catal. A: Chem. 344, 62–73 (2011)
M. Nath, M. Vats, P. Roy, Eur. J. Med. Chem. 59, 310–321 (2013)
F. Shaheen, A. Badshah, M. Gielen, G. Croce, U. Florke, U.D. de-Vos, S. Ali, J. Organomet. Chem. 695, 315–322 (2010)
M.X. Li, L.Z. Zhang, M. Yang, J.Y. Niu, J. Zhou, Bioorg. Med. Chem. Lett. 22, 2418–2423 (2012)
A. Karaküçük-İyidoğan, D. Taşdemir, E.E. Oruç-Emre, J. Balzarini, Eur. J. Med. Chem. 46, 5616–5624 (2011)
V.B. Arion, M.A. Jakupec, M. Galanski, P. Unfried, B.K. Keppler, J. Inorg. Biochem. 91, 298–305 (2002)
H.L. Singh, Spectrochim. Acta Part A 76, 253–258 (2010)
H.L. Singh, Res. Chem. Intermed. 37, 1087–1101 (2011)
K.S.O. Ferraz, L. Ferandes, D. Carrilho, M.C.X. Pinto, M.D.F. Leite, E.M. Souza-Fagundes, N.L. Speziali, I.C. Mendes, H. Beraldo, Bioorg. Med. Chem. 17, 7138–7144 (2009)
D.K. Demertzi, M.A. Demertzis, J.R. Miller, C. Papadopoulou, C. Dodorou, G. Filousis, J. Inorg. Biochem. 86, 555–563 (2001)
Y.F. Win, S.G. Teoh, T.S. Tengku-Muhammad, Y. Sivasothy, S.T. Ha, Am. J. Appl. Sci. 7, 301–308 (2010)
J.S. Casas, M.S. Garcra-Tasende, J. Sordo, Coord. Chem. Rev. 209, 197–261 (2000)
J.S. Casas, M.C. Rodrίguez-Argüelles, U. Russo, A. Sánchez, J. Sordo, A. Vázquez-López, S. Pinelli, P. Lunghi, A. Bonati, J.S. Casas, R. Albertini, J. Inorg. Biochem. 69, 283–292 (1998)
H.L. Singh, A.K. Varshney, Appl. Organomet. Chem. 15, 762–768 (2001)
K.S. Prasad, L.S. Kumar, M. Prasad, H.D. Revanasiddappa, Bioinorg. Chem. Appl. (2010). doi:10.1155/2010/854514
K.E. Apple, Drug Metabol. Rev. 36, 763–786 (2004)
M. Jain, S. Manju, R.V. Singh, Appl. Organomet. Chem. 18, 471–479 (2004)
G.L. Parrilha, J.G. da Silva, L.E. Gouveia, A.K. Gasparoto, R.P. Dias, W.R. Rocha, D.A. Santos, N.L. Speziali, H. Beraldo, Eur. J. Med. Chem. 46, 1473–1482 (2011)
M.X. Li, D. Zhang, L.Z. Zhang, J.Y. Niu, B.S. Ji, J. Organomet. Chem. 696, 852–858 (2011)
I.C. Mendes, F.B. Costa, G.M. de Lima, J.D. Ardisson, I. Garcia-Santos, A. Castiñeiras, H. Beraldo, Polyhedron 28, 1179–1185 (2009)
I.C. Mendes, J.P. Moreira, J.D. Ardisson, R.G. dos Santos, P.R.O. da Silva, I. Garcia, A. Castiñeiras, H. Beraldo, Eur. J. Med. Chem. 43, 1454–1461 (2008)
A. Perez-Rebolledo, J.D. Ayala, G.M. de Lima, N. Marchini, G. Bombieri, C.L. Zani, E.M. Souza-Fagundes, H. Beraldo, Eur. J. Med. Chem. 40, 467–472 (2005)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, J.A. Montgomery, T. Vreven, K.N. Kudin, J.C. Burant, J.M. Millam, S.S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G.A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J.E. Knox, H.P. Hratchian, J.B. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, P.Y. Ayala, K. Morokuma, G.A. Voth, P. Salvador, J.J. Dannenberg, V.G. Zakrzewski, S. Dapprich, A.D. Daniels, M.C. Strain, O. Farkas, D.K. Malick, A.D. Rabuck, K. Raghavachari, J.B. Foresman, J.V. Ortiz, Q. Cui, A.G. Baboul, S. Clifford, J. Cioslowski, B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R.L. Martin, D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, M. Challacombe, P.M.W. Gill, B. Johnson, W. Chen, M.W. Wong, C. Gonzalez, J.A. Pople, Gaussian 03, Revision C.01. (Gaussian, Inc., Wallingford CT, 2004)
H.L. Singh, J.B. Singh, K.P. Sharma, Res. Chem. Intermed. 38, 53–65 (2012)
M. Das, S.E. Livingstone, Inorg. Chim. Acta 19, 5–10 (1976)
B. Clarke, N. Clarke, D. Cunningham, T. Higgins, P. McArdle, M.N. Cholchuin, M. O’Gara, J. Organomet. Chem. 559, 56–64 (1998)
A.K. Saxena, J.K. Koacher, J.P. Tandon, Inorg. Nucl. Chem. Lett. 17, 229–233 (1981)
G.K. Sandhu, R. Gupta, S.S. Sandhu, R.V. Parish, K. Brown, J. Organomet. Chem. 279, 373–384 (1985)
M.T.H. Tarafder, A.R. Khan, Polyhedron 10, 819–822 (1991)
H.L. Singh, J.B. Singh, A. Mukharjee, Bioinorg. Chem. Appl. (2013). doi:10.1155/2013/425832
M. Nath, P.K. Saini, A. Kumar, J. Organomet. Chem. 695, 1353–1362 (2010)
H.L. Singh, J.B. Singh, Nat. Sci. 4, 170–178 (2012)
T.P. Lockhart, W.F. Manders, J. Am. Chem. Soc. 109, 7015–7020 (1987)
K.C. Molloy, P.C. Waterfield, J. Organomet. Chem. 424, 281–287 (1992)
A. Saxena, J.P. Tandon, A.J. Crowe, Polyhedron 4, 1085–1089 (1985)
A. Lycka, J. Holecek, S. Angelika, T. Ivan, J. Organomet. Chem. 409, 331–339 (1991)
T. Zoller, L. Iovkova-Berends, T. Berends, C. Dietz, G. Bradtmoller, K. Jurkschat, Inorg. Chem. 50, 8645–8653 (2011)
D.A. Atwood, J.A. Jegier, K.J. Martin, D. Rutherford, J. Organomet. Chem. 503, C4–C7 (1995)
G.F. de Sousa, V.M. Deflon, E. Niquet, A. Abras, J. Braz. Chem. Soc. 12, 493–498 (2001)
A.A. Al-Amiery, R.I. Al-Bayati, K.Y. Saour, M.F. Radi, Res. Chem. Intermed. 38, 745–759 (2012)
T.D. Thangadurai, K. Natarajan, Transit. Met. Chem. 26, 500–504 (2001)
I. Pal, F. Basuli, S. Bhattacharya, Proc. Indian Acad. Sci. Chem. Sci. 114, 255–268 (2002)
Y. Anjaneyula, R.P. Rao, Synth. React. Inorg. Met. Org. Chem. 16, 257–272 (1986)
Z.H. Chohan, A. Scozzafava, C.T. Supuran, J. Enzy. Inhib. Med. Chem. 18, 259–263 (2003)
K.S. Prasad, L.S. Kumar, S.C. Shekar, M. Prasad, H.D. Revanasiddappa, Chem. Sci. J. 12, 1–10 (2001)
N. Dharmaraj, P. Viswanathamurthi, K. Natarajan, Transit. Met. Chem. 26, 105–109 (2001)
A. Saxena, J.P. Tandon, Polyhedron 2, 443–446 (1983)
Acknowledgments
The authors are grateful to the Dean of Faculty of Engineering and Technology, Mody University of Science and Technology, Lakshmangarh, Sikar, for providing facilities necessary to carry out this research work. They are also grateful to Dr. T. Dewa, Department of Microbiology, University of Delhi, for providing antimicrobial screening facilities. The authors are also grateful to Dr. Sangeeta Jhajharia for linguistic corrections.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, H.L., Singh, J.B. & Bhanuka, S. Synthesis, spectroscopic characterization, biological screening, and theoretical studies of organotin(IV) complexes of semicarbazone and thiosemicarbazones derived from (2-hydroxyphenyl)(pyrrolidin-1-yl)methanone. Res Chem Intermed 42, 997–1015 (2016). https://doi.org/10.1007/s11164-015-2069-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2069-3